Abstract
Objective(s):
The goal of this study was assessing the prophylactic effect of exercise and its role as an adjuvant therapy on level of cytokines involved in angiogenesis in estrogen-dependent breast cancer.
Materials and Methods:
Forty female BALB/c mice were randomly assigned to exercise-tumor-exercise (ETE), exercise-tumor-rest (ETR), rest-tumor-exercise (RTE) and rest-tumor-rest (RTR) groups. After orientation in the environment, two groups of mice performed continuous endurance exercise for 8 weeks, and thereafter estrogen-dependent MC4L2 cancer cells were injected to them. Then, one group of each of trained and non-trained mice performed endurance exercise 5 days per week for 6 weeks. Tumor volume was measured by a digital caliper weekly. Finally, the mice were sacrificed; tumor tissue was removed, immediately frozen and kept in -70°C. Tumor sample was homogenized; levels of cytokines were measured and quantified using ELISA.
Results:
There was significant reduction in the level of interlukin-6 (IL-6) (P=0.001), Vascular endothelial growth factor (VEGF) (P=0.0001) and tumor volume (P=0.0001) among the groups performing endurance exercise after malignancy (RTE and ETE) in comparison with groups not performing endurance exercise (ETR and RTR), and these results were in agreement with tumor growth rate.
Conclusion:
Exercise can cause reduction in levels of pro-inflammatory cytokines in tumor tissue. Decreased IL-6 production could reduce the generation of VEGF, resulting in reduced intra-tumor angiogenesis. Due to reduction of the level of these cytokines in groups doing exercise before and after malignancy, exercise is presumed to be an adjuvant therapy in estrogen-receptor dependent tumors in addition to its effective prophylactic role.
Keywords: Endurance training, Estrogen receptor depen-, dent cancer, IL-6, VEGF
Introduction
Nowadays, breast cancer is an important risk factor for women’s health worldwide, as it is the most common cancer in women and accounting for approximately one third of all cancers in women at western countries (1). Breast cancer is also an important health issue for Iranian women, and its incidence is increasing. Actual incidence of breast cancer has been estimated to be nearly 20 new cases for 100000 individuals per year, and one out of 10 Iranian women bears the risk of this disease. In Iran, young women are at relatively higher risk for developing breast cancer than their Western counterparts, make it important to diagnosis, assess and control it properly in Iran (2, 3). There are several major molecular subtypes of breast cancer, grouped in estrogen receptor dependent and independent breast cancers. The majority of breast cancers (known as estrogen receptor-α (ER-α positive) are epithelial tumors arising from the cells lining breast ducts or lobules. Animal models are important to understand the mechanisms related with physical activity and cancer, as epidemiological studies do not provide detailed information on initiation, progression or recovery from cancer associated with exercise (4). MC4-L2 cell line, a murine ER-α positive cell line, was used in our study (5).
Today, exercise training has been recognized as an effective factor in preventing chronic inflammatory diseases. Moderate physical activity plays an important role (with release of myokines from skeletal muscles) in prevention of inflammatory diseases including breast cancer (6). Exercise can decrease body fat, obesity and low-grade systemic inflammation. Since each of these factors plays a role in cancer pathogenesis, exercise is potentially involved in prevention of cancer (7).
In recent years, new research areas have been developed in Western countries with a therapeutic approach to exercise. There are lots of evidences suggesting that physical activity can reduce the risk of different types of malignant cancers such as colon, breast, prostate, endometrial and lung (8, 9). Friedenreich and Cust reviewed 250 epidemiological studies on the interaction between physical activity and cancer prevention, and showed that regular physical activity can act as a potent preventive agent against cancer, decreasing its incidence by 40% (10). In many studies, reduced tumor volume has been reported following regular exercise, but the exact mechanism has not been identified so far (11-13). Murphy et al reported that tumor volume was also reduced in exercise group that perform endurance training for 20 weeks. They attributed the reduction in tumor volume to the reduction of inflammatory factors.
In addition, intra-tumor angiogenesis and enhanced blood flow within the tumor is essential for its growth. Angiogenesis is the formation of new blood vessels, which is an important precondition for tumor growth and metastasis. According to one theory, during physical activity, a competition arises between tumor microenvironment and active muscles for supply of blood, oxygen and nutrients. As blood flow is decreased within the tumor, hypoxia in the middle part of the tumor increases the likelihood of apoptosis in it (14). No study has been performed up to now regarding to evaluate the factors involved in angiogenesis.
Interleukin-6 (IL-6) and vascular epithelial growth factor (VEGF) are important cytokines produced in tumor microenvironment, which play important roles in intra-tumor angiogenesis. IL-6 is a pleiotropic cytokine overexpressed in many human diseases such as arthritis, diabetes, obesity and different cancer types. IL-6 has a pro-inflammatory role in tumor microenvironment, and it is involved in tumor angiogenesis and metastasis (15). Evidences suggest that IL-6 is produced in high concentrations in estrogen receptor dependent human breast cancer cells (16) and breast tumor samples (17). The important role of IL-6 in breast cancer is stimulation of aromatase activity and production of VEGF. IL-6 up-regulates production of pro-angiogenic molecules such as VEGF (18). In addition, IL-6 stimulates conversion of estrogen precursors like estrone to estrogen (estradiol) (19, 20). So far, there has been no research on the effects of endurance exercise on IL-6 level in breast cancer samples.
VEGF is a potent angiogenic cytokine in normal tissue and tumor, stimulating endothelial cell proliferation (21). VEGF is overexpressed in breast cancer compared with normal breast tissue (22), and its level correlates with the density of small vessels, and is also significantly correlated with tumor grade and invasion of cancer cells (18, 23). No research has examined the effect of continuous endurance exercise on VEGF level in tumor tissue till now.
Regarding the fact, that only few studies have investigated the molecular mechanisms of the effect of exercise on treatment of cancer, in this research we seek to survey whether regular physical activity can have adjuvant effect in inhibition of tumor growth by modulating angiogenesis and whether physical exercise before the onset of cancer is involved in inhibition of tumor growth by evaluating the cytokines effects in angiogenesis.
Materials and Methods
The exercise protocol
40 BALB/c mice (3 to 5 weeks old weighing 14-15 g) was purchased from Pasteur Institute and transferred to the animal house in Tarbiat Modares University. After a week of orientation in the environment (light, temperature and humidity), the mice were divided into two groups randomly. One group underwent 8 weeks of endurance exercise on treadmill in accordance with Table 1 but the other group did not perform exercise. All mice were then injected with cancer cells and after tumor formation, half of the mice in trained group and half of them in untrained group randomly selected and they did endurance exercise on treadmill. Therefore, there are four groups of mice (10 mice in each group) in this research: exercise- tumor -exercise (ETE), exercise-tumor-rest (ETR), rest-tumor-exercise (RTE) and rest-tumor-rest (RTR). Frequency of training was 5 days per week.
Table 1.
Exercise protocol on treadmill in cancer groups
| Groups | Velocity (m/min) | Time (min) | |
|---|---|---|---|
| Orientation stage | 6-12 | 20 | |
| Before cancerous | |||
| First two weeks | 14 | 30 | |
| Second two weeks | ETR | 16 | 35 |
| Third two weeks | ETE | 18 | 35 |
| Fourth two weeks | 20 | 40 | |
| After cancerous | |||
| First two weeks | 14 | 25 | |
| Second two weeks | ETE RTE |
16 | 30 |
| Third two weeks | 18 | 30 |
Cell culture
Estrogen receptor-positive (ER+) breast duct carcinoma MC4-L2 cells were purchased for the first time by this research team from the University of Buenos Aires, Argentina (5), and was donated to Iranian Institute of Genetic Resources to be used by other researchers after proliferation and confirmation. MC4-L2 cells were cultured in T75 flask in DMEM/F-12 medium containing 15 mM HEPES, Glutamine, 100 μg/ml penicillin, 100 μg/ml Streptomycin and 10% FBS. After filling 90% of the flask by cells, the supernatant in the medium was decanted. The cells were detached from the bottom of flask using 0.025% trypsin, and after rinsing with PBS and enzyme neutralization using 10% FBS, all the flask contents were emptied into Falcon tubes and centrifuged in 1200 rpm for 3-5 min. The supernatant was decanted and the cell plate dissolved in the medium containing 10% FBS. After that, trypan blue and hemacytometer were used to determine cell viability and cell count, respectively.
Tumor formation
After cell culture and count, female BALB/c mice were anesthetized by using a suitable dose of ketamine and xyloxine, and then one million cells were injected subcutaneously into the right upper thigh of the mice. Approximately 10-14 days after injection of cancer cells, the tumor was palpable in the injected area.
Measurement of tumor volume
Tumor size was measured in two dimensions. The larger tumor dimension was considered as length (L), and the other (at 90 degrees) as width (W). After appearance of the tumor, the length and width of the tumor were measured by a digital caliper once a week. Tumor volume was then calculated using tumor volume formula of [V =π/6 (w × L 2] (24). The measured value of the last day was divided by that of the first day to determine final tumor volume for subsequent operations.
Measurement of IL-6 and VEGF
After sacrificing the mice, tumor tissue was removed immediately and the central necrotic part of it was eliminated. Superficial part of the tumor was frozen in liquid nitrogen at -70°C. In the laboratory, tumor tissue was homogenized with lyses solution and total protein extracted with Bradford method, and the supernatant was used for ELISA. The variables were measured and quantified using ELISA based on the kit instructions. Ab100713 ELISA kit and ab100752 kit manufactured by Abcam were used to measure IL-6 and VEGF, respectively.
Data analysis
SPSS software was used for data analysis. One way ANOVA and Tukey post hoc tests were used to determine the significance of differences between variables. Mean and standard deviation were used to report the values of measured variables.
Results
The levels of IL-6 and VEGF are shown in graph1 and 2. Based on our findings, the lowest levels of IL-6 and VEGF were related to ETE Group, and their levels were significantly lower in the two groups who performed endurance exercise.
Graph 1.
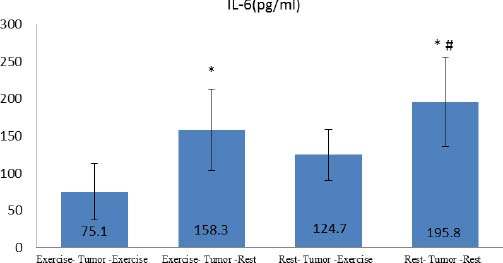
Tissue concentration of IL-6 in groups
*significant difference with Exercise- Tumor-Exercise
# significant difference with Rest- Tumor-Exercise
Graph 2.
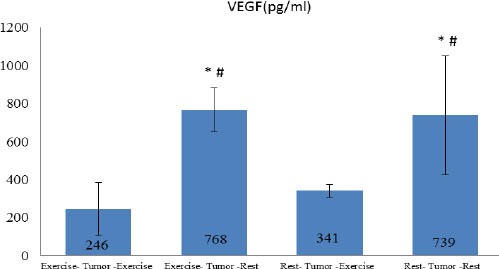
Tissue concentration of VEGF in groups
*significant difference with Exercise- Tumor-Exercise
# significant difference with Rest- Tumor-Exercise
The results of one-way ANOVA and Tukey post hoc test indicated a significant difference between groups in IL-6 (P=0.001), VEGF (P=0.0001) and tumor volume (P=0.0001) values. In fact, the exercise protocol was able to cause difference in rest values of measured variables between the group there was significant difference in research variables between ETE group with RTR and ETR groups. The significant difference was also observed between RTE and RTR groups, indicating the effect of endurance exercise as an adjuvant therapy. Interestingly, there was significant difference between RTE and ETR groups in VEGF (P=0.001) but not in IL-6 level, indicating the preventive effects of endurance exercise on tumor growth. Graph 3 shows the changes in tumor volume within 6 weeks in four groups. The results showed that there was significant difference between groups in progression of tumor volume (P=0.001). In fact, tumor volume increased in all the groups, but regression growth curve was less steep in ETE and RTE groups. Tumor volume was larger in RTE compared with ETR group. It should be noted that the mean volume of primary tumor was larger in RTE group than the others but regression of its growth was less than RTR and ETR groups. There were strong relationship between tumor volume (in the sixteenth week) and IL-6 (r=0.729 P=0.0001) and VEGF (r=0.45 P=0.01).
Diagram 1.
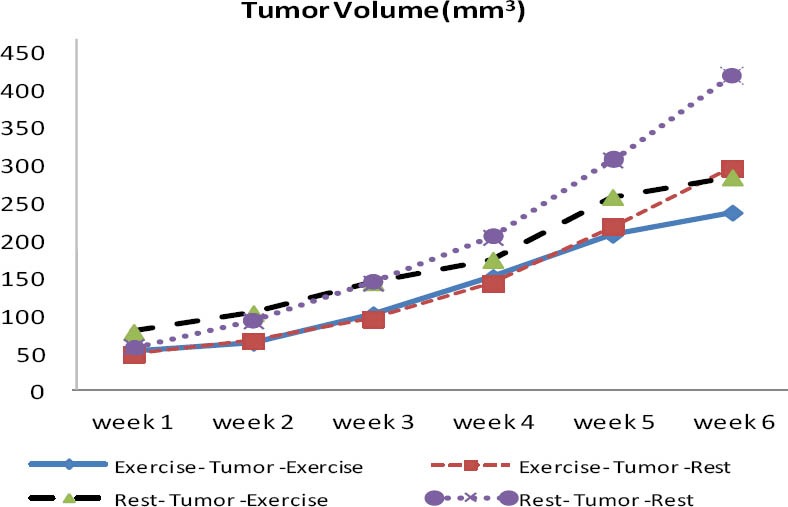
Changes in tumor volume between groups. Tumor growth rate is higher in RTR and ETR groups, initial tumor volume in RTE group is higher than the other groups but final growth rate has been reduced. ETE (exercise-tumor-exercise group), ETR (exercise-tumor-rest group), RTE (rest-tumor-exercise group) and RTR (rest-tumor-rest group). Unit of Tumor volume is cmm3
Discussion
The aim of this study was to examine the effects of endurance exercise on level of cytokines involved in intra-tumor angiogenesis in order to evaluate its role as an adjuvant therapy for breast cancer and its effects on the body’s resistance against cancer. The results showed that the level of IL-6 and VEGF were significantly different between groups performing and not performing exercise. Given the smaller tumor volume in early weeks in ETE and ETR groups compared with the others and the difference in the level of measured cytokines after training protocol, endurance exercise seems to play a preventive role and be an effective adjuvant therapy in estrogen hormone dependent breast cancer.
Angiogenesis is a prerequisite for continued growth and spread of tumors. After reaching a specific extent, solid tumors develop several hypoxic areas in tumor center to which oxygen and nutrients are not supplied to provide for metabolic needs because of their low number. Tumor tissue can express and produce several protein factors during hypoxic and necrotic stress. These factors are in fact a series of angiogenic peptides produced by tumor and other cells within it. Cytokines such as IL-6 and IL-8 activate a wide range of signal transduction pathways that regulate the process of angiogenesis. Studies show that these cytokines lead to activation of NF-κB, JNK, ERK and P38 signaling pathways (25), which ultimately stimulate vascular endothelial cells to produce VEGF. IL-6 can increase tumor growth by up-regulating angiogenic and anti-apoptotic proteins in tumor cells. IL-6 plays a key role along with other cytokines in the angiogenic process, stimulating VEGF production in vascular endothelial cells. VEGF is not only a potent mitogen for vascular endothelial cells but also a vascular permeability factor causing continuous production of fibrin and fibronectin to produce tissue matrix (26). In the present study, we observed that the level of IL-6 and VEGF in tumor tissue was significantly reduced in groups performing endurance exercise after tumor induction in comparison with other groups. Regular exercise reduces the level of pro-inflammatory cytokines such as IL-6 in tumor tissue. Since this cytokine has an effective role in producing VEGF by stimulating the expression of hypoxia inducible factor- α (HIF-α) (23), reduced level of IL-6 in groups doing exercise indicated apparent role of regular physical activity in reduction of angiogenesis in tumor by suppressing the production of pro-inflammatory cytokines. These results were in agreement with tumor growth rate, i.e. regression of tumor growth was lower in exercise groups.
Various studies showed tumor volume reduced following regular exercise (11-13,27). In the present study, following endurance training, tumor volume and tumor growth rate was decreased. In another study, Murphy et al reported reduction in tumor volume after 20 weeks of exercise in mice with cancer, which was attributed to reduced level of intra-tumor inflammatory cytokines, and showed a direct relationship between inflammatory cytokines and tumor volume (12). Reduced volume of tumor in our study was observed in exercise groups, and considering the strong correlation between the level of IL-6 and tumor volume (r= 0.729), reduction in tumor volume in exercise groups was attributed to decreased level of intra-tumor inflammatory factors. In this regard, findings of our study can better justify the relationship between inflammatory cytokines and tumor volume changes, since it seems that local cytokine levels in tumor microenvironment is more effective than systemic and circulating levels of cytokines. In another study, Donatto et al evaluated the effect of 6 weeks of endurance exercise on the levels of IL-6, tumor necrotizing factor- α (TNF-α) and IL-10 in obese rats with tumor. The results showed that IL-6 and TNF-α was decreased in tumor-exercise group compared with control tumor groups. In addition, the level of IL-10 as an antitumor cytokine, was higher relative to TNF-α in the exercise group (28). Most of the studies in this field support the positive impact of exercise in reducing chronic inflammation, and exercise has been introduced as an effective measure to reduce inflammation.
Zielinski et al showed that endurance exercise led to a delay in tumor growth. They showed that performing regular endurance exercise decreases intra-tumor macrophage and neutrophil density especially in early stage of the tumor (13). Since immune cells, in addition to the destruction of tumor cells play an effective role in production of growth and angiogenesis factors such as IL-6 and IL-8, they accelerate tumor growth and proliferation. IL-6 plays a dual role in progression and destruction of tumor cells. It has the ability to stimulate natural killer cells and T-cytotoxic lymphocytes, and is involved in their destruction in the early stages of tumor formation, but when IL-6 production is chronically increased or when immune cells are persistently present within the tumor, they become important in tumor progression through mechanisms involved in angiogenesis and anti-apoptotic activity. IL-6 increases the growth of tumor by up-regulating angiogenic and anti-apoptotic proteins in tumor cells. In estrogen receptor dependent breast cancer, estrogen can activate NF-κB when it binds to its receptor. It leads to increase IL-6 levels, which results in suppression of programmed cell death 4 (PDCD4) and tropomyosin 1 (TPM 1) and up-regulation B-cell lymphoma protein (BCL2), in addition to stimulation of induction VEGF production for angiogenesis, through IL-6/STAT3 pathway (25). So IL-6 play key role in anti-apoptotic activity in breast cancer. This study show that endurance training lowering IL-6 and VEGF levels in tumor of breast cancer at two groups ETE and RTE. Since suppression of IL-6 (29) and VEGF expression (30) within the tumor tissue is a therapeutic target to prevent tumor growth, and as regular exercise has such capacity, it can be argued that endurance training has an adjuvant effect in inhibiting tumor growth at least in estrogen-receptor dependent cancers.
An interesting finding in this study was that tumor volume in RTE group was larger than ETR. As endurance exercise has been claimed to act as an adjuvant therapy, there seems to be a contradiction between this point and tumor growth in this group. There are two reasons for this: First, the regression of growth was lower in this group, i.e. primary tumor volume was larger than ETR group, indicating the role of exercise in inhibition and formation of tumor in these groups. This means that the groups that did exercise had smaller tumors during tumor formation but tumor growth regression was lower in RTE group after exercise. Another point is that central part of the tumor becomes usually necrotic because of low supply of blood and oxygen. We observed that the central necrotic part of the tumor was larger in this group than other groups, and endurance exercises seem to have increased necrotic stress within the tumor. The theory seems to be true that there is a competition between active muscles and tumor tissue in blood and nutrients supply, during which blood flow is directed towards active muscles, and tumor tissue probably undergoes higher necrotic and apoptotic stress by receiving less blood flow and nutrients, as nutrients and tropic factors are consumed in active muscles.
Data show that ETE group was the best group in terms of preventing cancer progression because of performing endurance exercise before and after cancer. Regular exercise contributes to the prevention and treatment of cancer due to anti-inflammatory, psychological and metabolic effects and boosting the immune system. By comparing ETR and RTE groups in terms of the measured factors, preventive and adjuvant role of exercise is clearly indicated. Overall, data in this study proved the efficacy of moderate-intensity endurance exercise in decreasing the level of inflammatory and angiogenic intra-tumor cytokine levels and subsequent tumor volume. Considering these data, it can be claimed that in addition to preventive role, exercise has the role of adjuvant role in estrogen-receptor-positive cancers.
Conclusion
The results of this study suggest that continuous moderate endurance exercise has an effective role in reduction of inflammatory and angiogenic cytokines within the tumor, and these reduced levels are in line with decreased tumor volume and tumor growth regression. Therefore, we can claim that exercise training has an adjuvant role in estrogen-receptor dependent cancers in addition to prophylactic effect.
Acknowledgment
The results presented in this article, were part of doctoral dissertation of two PhD students in Tarbiat Modares University, Tehran, Iran.
References
- 1.Siegel R, Naishadham D, Jemal A. Cancer statistics 2013. Cancer J Clin. 2013;63:11–30. doi: 10.3322/caac.21166. [DOI] [PubMed] [Google Scholar]
- 2.Ebrahimi M, Vahdaninia M, Montazeri A. Risk factors for breast cancer in Iran: a case-control study. Breast Cancer Res. 2002;4:R10. doi: 10.1186/bcr454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Harirchi I, Ebrahimi M, Zamani N, Jarvandi S, Montazeri A. Breast cancer in Iran: a review of 903 case records. Public Health. 2000;114:143–145. doi: 10.1038/sj.ph.1900623. [DOI] [PubMed] [Google Scholar]
- 4.Hoffman-Goetz L. Physical activity and cancer prevention: animal-tumor models. Med Sci Sports Exerc. 2003;35:1828–1833. doi: 10.1249/01.MSS.0000093621.09328.70. [DOI] [PubMed] [Google Scholar]
- 5.Lanari C, Lüthy I, Lamb CA, Fabris V, Pagano E, Helguero LA, et al. Five novel hormone-responsive cell lines derived from murine mammary ductal carcinomas: in vivo and in vitro effects of estrogens and progestins. Cancer Res. 2001;61:293–302. [PubMed] [Google Scholar]
- 6.Pedersen BK. Exercise-induced myokines and their role in chronic diseases. Brain Behav Immun. 2011;25:811–816. doi: 10.1016/j.bbi.2011.02.010. [DOI] [PubMed] [Google Scholar]
- 7.Lee IM. Physical activity and cancer prevention-data from epidemiologic studies. Med Sci Sports Exerc. 2003;35:1823–1827. doi: 10.1249/01.MSS.0000093620.27893.23. [DOI] [PubMed] [Google Scholar]
- 8.Na HK, Oliynyk S. Effects of physical activity on cancer prevention. Ann N Y Acad Sci. 2011;1229:176–183. doi: 10.1111/j.1749-6632.2011.06105.x. [DOI] [PubMed] [Google Scholar]
- 9.Montaruli A, Patrini P, Roveda E, Carandente F. Physical activity and breast cancer. Sport Sci Health. 2012;8:1–13. [Google Scholar]
- 10.Friedenreich CM, Cust AE. Physical activity and breast cancer risk: impact of timing, type and dose of activity and population subgroup effects. Br J Sports Med. 2008;42:636–647. doi: 10.1136/bjsm.2006.029132. [DOI] [PubMed] [Google Scholar]
- 11.Jones LW, Antonelli J, Masko EM, Broadwater G, Lascola CD, Fels D, et al. Exercise modulation of the host-tumor interaction in an orthotopic model of murine prostate cancer. J Appl Physiol. 2012;113:263–272. doi: 10.1152/japplphysiol.01575.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Murphy EA, Davis JM, Barrilleaux T, McClellan J, Steiner J, Carmichael M, et al. Benefits of exercise training on breast cancer progression and inflammation in C3 (1) SV40Tag mice. Cytokine. 2011;55:274–279. doi: 10.1016/j.cyto.2011.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Zielinski MR, Muenchow M, Wallig MA, Horn PL, Woods JA. Exercise delays allogeneic tumor growth and reduces intratumoral inflammation and vascularization. J Appl Physiol. 2004;96:2249–2256. doi: 10.1152/japplphysiol.01210.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thompson HJ, Jiang W, Zhu Z. Candidate mechanisms accounting for effects of physical activity on breast carcinogenesis. IUBMB Life. 2009;61:895–901. doi: 10.1002/iub.233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong DS, Angelo LS, Kurzrock R. Interleukin-6 and its receptor in cancer. Cancer. 2007;110:1911–1928. doi: 10.1002/cncr.22999. [DOI] [PubMed] [Google Scholar]
- 16.Sullivan NJ. Gunduz M, Gunduz E, editors. Interleukin-6 in the Breast Tumor Microenvironment. Breast Cancer - Focusing Tumor Microenvironment, Stem cells and Metastasis: InTech. 2011:584. [Google Scholar]
- 17.Honma S, Shimodaira K, Shimizu Y, Tsuchiya N, Saito H, Yanaihara T, et al. The influence of inflammatory cytokines on estrogen production and cell proliferation in human breast cancer cells. Endocrin J. 2002;49:371–377. doi: 10.1507/endocrj.49.371. [DOI] [PubMed] [Google Scholar]
- 18.Longatto Filho A, Lopes J, Schmitt FC. Angiogenesis and breast cancer. J Oncol. 2010:2010. doi: 10.1155/2010/576384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Purohit A, Newman SP, Reed MJ. The role of cytokines in regulating estrogen synthesis: implications for the etiology of breast cancer. Breast Cancer Res. 2002;4:65–69. doi: 10.1186/bcr425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dethlefsen C, Højfeldt G, Hojman P. The role of intratumoral and systemic IL-6 in breast cancer. Breast Cancer Res Treat. 2013:1–8. doi: 10.1007/s10549-013-2488-z. [DOI] [PubMed] [Google Scholar]
- 21.Schneider BP, Miller KD. Angiogenesis of breast cancer. J Clin Oncol. 2005;23:1782–1790. doi: 10.1200/JCO.2005.12.017. [DOI] [PubMed] [Google Scholar]
- 22.Ghaderi A, Hosseini A, Jaberipour M, Razmkhah M. Os-13: VEGF, IL-8 and MMP2 expression profile in adipose-derived stem cells (ASCs) of breast cancer patients. Cell J (Yakhteh) 2010;1-2 [Google Scholar]
- 23.Lee K, Qian DZ, Rey S, Wei H, Liu JO, Semenza GL. Anthracycline chemotherapy inhibits HIF-1 transcriptional activity and tumor-induced mobilization of circulating angiogenic cells. Proc Natl Acad Sci. 2009;106:2353–2358. doi: 10.1073/pnas.0812801106. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 24.Jones LW, Viglianti BL, Tashjian JA, Kothadia SM, Keir ST, Freedland SJ, et al. Effect of aerobic exercise on tumor physiology in an animal model of human breast cancer. J Appl Physiol. 2010;108:343–348. doi: 10.1152/japplphysiol.00424.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Iliopoulos D, Hirsch HA, Struhl K. An epigenetic switch involving NF-κB, Lin28, Let-7 MicroRNA, and IL6 Links inflammation to cell transformation. Cell. 2009;139:693–706. doi: 10.1016/j.cell.2009.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Balkwill F, Mantovani A. Inflammation and cancer: back to Virchow? Lancet. 2001;357:539–545. doi: 10.1016/S0140-6736(00)04046-0. [DOI] [PubMed] [Google Scholar]
- 27.Betof AS, Dewhirst MW, Jones LW. Effects and potential mechanisms of exercise training on cancer progression: a translational perspective. Brain Behav Immun. 2013;30:S75–87. doi: 10.1016/j.bbi.2012.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Donatto F, Neves R, Rosa F, Camargo R, Ribeiro H, Matos-Neto E, et al. Resistance exercise modulates lipid plasma profile and cytokine content in the adipose tissue of tumour-bearing rats. Cytokine. 2013;61:426–432. doi: 10.1016/j.cyto.2012.10.021. [DOI] [PubMed] [Google Scholar]
- 29.Paul-Pletzer K. Tocilizumab: blockade of interleukin-6 signaling pathway as a therapeutic strategy for inflammatory disorders. Drugs Today. 2006;42:559–576. doi: 10.1358/dot.2006.42.9.1025692. [DOI] [PubMed] [Google Scholar]
- 30.Benoy I, Salgado R, Colpaert C, Weytjens R, Vermeulen PB, Dirix LY. Serum interleukin 6, plasma VEGF, serum VEGF, and VEGF platelet load in breast cancer patients. Clin Breast Cancer. 2002;2:311–315. doi: 10.3816/cbc.2002.n.008. [DOI] [PubMed] [Google Scholar]


