Abstract
Objective(s):
Regulatory T cells, including CD4+CD25+Fox3+ and CD8+CD28- cells play an important role in regulating the balance between immunity and tolerance. Since multiple sclerosis is an inflammatory autoimmune disease, regulatory T cells are considered to be involved in its pathogenesis. In this study, we investigated the circulatory numbers of the two mentioned types of regulatory T cells and also their association with different clinical characteristics in 84 multiple sclerosis patients.
Materials and Methods:
84 patients with multiple sclerosis and 75 normal individuals were studied. Demographic and clinical information of all participants were collected via questionnaire and clinical examination as well as MRI. The peripheral blood frequency of two different subgroups of regulatory T cells (CD4+ CD25+Foxp3+ and CD8+CD28- cells) were analyzed by flow cytometry using anti-human antibodies conjugated with CD4-FITC / CD25-PE/Foxp3-PE-Cy5, CD3-PE/CD8a-PE-Cy5/CD28-FITC.
Results:
The frequency of CD4+CD25+Foxp3+ cells in multiple sclerosis patients was significantly less than that in healthy controls (P=0.006) and in mild forms less than that in sever forms (P=0.003). There was not any correlation between the frequency of regulatory T cells and different clinical variables.
Conclusion:
Our results showed that the number of CD4+CD25+Foxp3+ cells decreases significantly in multiple sclerosis patients, which probably shows the regulatory role of these cells in multiple sclerosis.
Keywords: CD4+CD25+Foxp3+ -, Regulatory T cells, CD8+CD28- Regulatory T cells, Multiple sclerosis
Introduction
Multiple sclerosis (MS), as the most common inflammatory demyelinating disease of the central nervous system (CNS) and also the most common cause of neurological disability at young age, is an inflammatory demyelinating disease characterized by lymphocyte infiltration and inflammation of the CNS white matter. Such demyelinating process typically shows 4 clinical courses including: relapsing remitting (RRMS) which is the most common type (80%), characterized by unpredictable relapses followed by periods of months and years of relative quiet with no sings, and usually beginning with a clinical isolated syndrome (CIS) not fulfilling the criteria of MS; secondary progressive (SPMS) occurring in around 65% of those with initial RRMS, who eventually have progressive neurologic decline between acute attacks; primary progressive (PPMS) occurring in 10-20% of all, with no remission after the initial symptoms; and progressive relapsing (PRMS) describing a steady neurologic decline with superimposed attacks (1). The most widely used scale evaluating the severity of MS is the EDSS (expanded disability status scale) defined by a set of rules (2) and providing a numerical quantification of the neurological examination. The etiology of MS is unclear; however, it is thought that both genetic and environmental factors play a role in its pathogenesis (3). MS may result from the failure of tolerance mechanisms including CD4+CD25+ T regulatory cells (Treg) (4), which prevent expansion of pathogenic T cells directed against myelin determinants, or other self-tissue antigens. A lot of studies have reported numeric or functional deficiencies of Tregs in various human autoimmune diseases including inflammatory demyelinating disorders of the CNS (5). Although there is not yet a specific surface marker for Treg subsets, naturally occurring CD4+CD25+Treg (nTreg) cells can be characterized on the basis of their high expression of CD25 (in contrast to the intermediate expression in recently activated T cells) and the intracellular expression of the fork head transcription factor 3 (Foxp3) (6). Another Treg population, CD8+CD28- Treg (7) which lacks co-stimulatory molecule of CD28, is associated with some suppressor abilities and its failure has been recognized in numerous animal (8–10) as well as human autoimmunities (11–12).
The role of CD4+CD25+Foxp3+ and CD8+CD28- Treg cells (7, 13, 14) has been studied in MS. The present study has evaluated both of these Treg populations in all types of MS and has compared the results with healthy controls.
Materials and Methods
Study subjects
Peripheral blood samples were obtained from a total of 84 MS patients in remission phase, who were diagnosed according to clinical examination and MRI, and 75 sex and age-matched healthy controls. None of the patients and controls suffered from any other autoimmune or inflammatory states. The EDSS (Expanded Disability Status Scale) (15), the type of MS (CIS, RRMS, PPMS, SPMS, PRMS), duration as well as treatment of the illness, and the number of recurrences were determined for each patient before sampling. Blood samples of all subjects were taken after signing the informed consent form approved by the local ethical committee. The study protocol conformed to the ethical guidelines of the 1975 Helsinki Declaration.
Flow cytometric analysis
At least one million fresh peripheral blood mononuclear cells (PBMCs) were separated from 2 ml of anticoagulated blood by Ficoll-Hypaque (Lymphodex, Inno-Train, Germany) density gradient centrifugation. CD4+CD25+Foxp3+ Tregs were detected in one tube by staining with a cocktail of anti-human surface CD4-FITC / CD25-PE, and intracellular Foxp3-PE-Cy5 according to the manufacturer’s instructions (eBioscience, USA). In this procedure, CD4+-FITC/CD25+-PE-stained cells were fixed, permeabilized and washed twice. Then the cells were stained by 10 μl anti-human FoxP3 antibody and were incubated at 4oC for 30 min. Inducible CD8+CD28- Tregs were detected in another tube by staining with anti-human CD3-PE, CD8a-PE-Cy5, and CD28-FITC. All lymphocytes in each sample were gated on the basis of light scattering properties and then at least 20,000 events were obtained to count each subtype of Tregs. Having gated CD4+CD25+cells, we counted CD4+CD25+Foxp3+cells on the basis of simultaneous expression of CD25 and Foxp3. To count CD8+CD28- Tregs, gated CD3-expressing lymphocytes were counted on the basis of expression of CD8 and no expression of CD28 (Figure 1). All antibodies and their isotype-matched controls were purchased from eBioscience, USA. The percentages of CD4+CD25+Foxp3+ and CD8+CD28- cells were analyzed by a 3-color flow cytometry using BD FACSCalibur Flow Cytometer and CELLQuest software version 3 (BD, USA).
Figure 1.
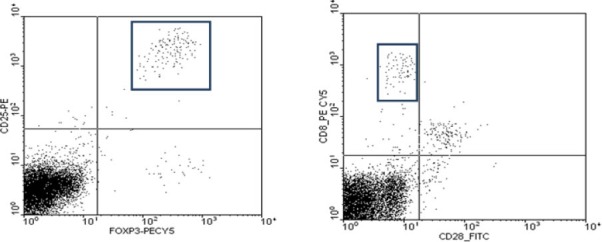
Flow cytometry results in a sample patient. A) Having gated on CD4+ cells, we counted CD4+CD25+Foxp3+ cells B) Having gated on CD3+ cells, we counted CD8+CD28- cells
Figure 2.
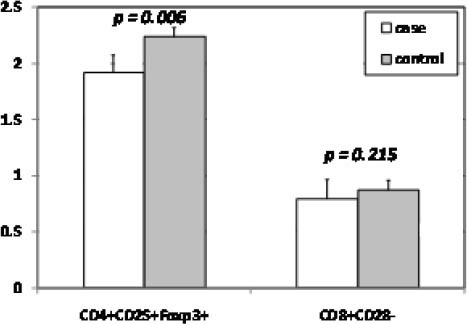
Numbers of CD4+CD25+Foxp3+ cells and CD8+CD28-cells in peripheral blood samples of 84 MS patients and 75 healthy controls
Statistical analysis
Data was expressed as mean±SD. The statistical indices of Tregs were analyzed using independent t and chi-square tests. Correlations between variables were calculated by Pearson’s correlation coefficient, and simultaneous effects of various factors on Tregs were analyzed by multiple linear regressions with backward method. Adjusted R Squared was determined as a criterion of goodness-of-fit test, and P<0.2 was considered for exclusion from the model. P-values < 0.05 were considered statistically significant. All analyses were performed using the SPSS 16 software.
Results
Patient characteristics and Treg frequencies
Basic and clinical characteristics of MS patients and controls enrolled in our study are shown in Table 1. The most affected ages were between 18 and 30. The least EDSS score was related to the most benign type of MS named CIS and then it raised in more sever types of MS. There were not any significant differences in the frequency of Tregs in terms of age and gender (Table 1). The frequency of CD4+CD25+Foxp3+Tregs in MS patients was significantly lower than that in healthy controls (P=0.006) (Table 1). The frequency of CD4+CD25+Foxp3+ Tregs was different in MS patients according to type of MS in such a way that the frequency of CD4+CD25+Foxp3+ Tregs in severe forms of MS (PPMS, SPMS, PRMS) was significantly higher than that in the mild forms (CIS, RRMS) (P=0.03) (Table 2).
Table 1.
Basic and clinical characteristic of MS patients and controls
| MS patients | Healthy controls | P- value | ||
|---|---|---|---|---|
| Number of subjects | 84 | 75 | - | |
| Male/female | 15/69 | 15/60 | 0.73 | |
| Age (years) mean±SD | 34.2±8.8 | 31.4±10 | 0.4 | |
| Family history (%) | Positive | 77 (91.7%) | - | - |
| Negative | 7 (7.3%) | |||
| Disease duration (years) mean±SD | 5.8±4.4 | - | - | |
| Treatment duration (years) mean±SD | 3.1±3.31 | - | - | |
| Number of recurrences mean±SD | 3.7±4 | - | - | |
| Number of patients in different types of MS (%) | CIS | 5(6.1) | ||
| RRMS | 69(82.1) | |||
| PPMS | 1(1.2) | - | - | |
| SPMS | 7(8.5) | |||
| PRMS | 2(2.4) | |||
| Number of patients using different kinds of drugs (%) | Cinovex | 63(76.8) | ||
| Rebief | 10(12.2) | |||
| Betaferon | 2(2.4) | - | - | |
| Others | 2(2.4) | |||
| No drug | 5(6.1) | |||
| EDSS (mean±SD) | CIS | 0.5±1.1 | ||
| RRMS | 2±1.44 | |||
| PPMS | 6 | - | - | |
| SPMS | 5±1.42 | |||
| PRMS | 6.25±0.35 | |||
| Tregs±SD | CD4+CD25+Foxp3+ | 1.91±0.71 | 2.24±0.74 | 0.006 |
| CD8+CD28- | 0.79±0.37 | 0.87±0.38 | 0.215 | |
CIS: clinical isolated syndrome, RRMS: relapsing remitting multiple sclerosis, PPMS: primary progressive multiple sclerosis, SPMS: secondary progressive multiple sclerosis, PRMS: progressive relapsing multiple sclerosis, EDSS: expanded disability status scale, Tregs: regulatory T cells
Table 2.
Values of Tregs according to different variates in MS patients
| Tregs | |||||
|---|---|---|---|---|---|
| CD4+CD25+Foxp3+ | P- value | CD8+CD28- | P- value | ||
| EDSS | Mild (0.5-4.5) | 1.83±0.7 | 0.08 | 0.75±0.4 | 0.48 |
| Sever (5-9.5) | 2.23±0.7 | 0.83±0.23 | |||
| Type | Mild (CIS, RRMS) | 1.86±0.72 | 0.8±0.4 | ||
| Sever (PPMS, SPMS, PRMS) | 2.25±0.57 | 0.03 | 0.7±0.26 | 0.72 | |
| sex | Male | 2.24±0.8 | 0.054 | 0.71±0.34 | 0.36 |
| Female | 1.84±0.68 | 0.81±0.38 | |||
| Family history | Positive | 1.85±0.78 | 0.764 | 0.84±0.2 | 0.323 |
| Negative | 1.92±0.72 | 0.79±0.39 | |||
CIS: clinical isolated syndrome, RRMS: relapsing remitting multiple sclerosis, PPMS: primary progressive multiple sclerosis, SPMS: secondary progressive multiple sclerosis, PRMS: progressive relapsing multiple sclerosis, EDSS: expanded disability status scale
Correlation of Treg frequencies with different parameters
There was not any significant correlation between the frequency of Tregs with age and various clinical variables including EDSS scores, number of recurrences, duration of the disease, and duration of the treatment (Table 3). Also, no correlation was found between the frequencies of Tregs with age in healthy group (data not shown). Using linear multiple regression analysis, we found a significant goodness-of-fit for both subsets of Treg frequencies (CD4+CD25+FoxP3+Treg: Adjusted R2=0.845, P<0.001; CD8+CD28-Treg: Adjusted R2=0.759, P<0.001). CD4+CD25+FoxP3+ Treg frequencies remained not significant in the presence of factors of positive family history (P=0.519), number of recurrences (P=0.656), and EDSS score (P=0.939). Although the factors of age, sex, disease duration, and treatment duration stayed in the model, the frequency of such cells turned significant in the presence of age (P<0.001) and treatment duration (P=0.016). The model also revealed that CD8+CD28-Treg frequencies remain insignificant in the presence of factors of positive family history (0.315), EDSS score (0.908), disease duration (0.466), and treatment duration (0.409). Although the factors of age, sex, and number of recurrences stayed in the model, the frequency of such cells turned significant only in the presence of age (P<0.001) (Table 4).
Table 3.
Correlation between the number of Treg subtypes with age and different clinical variates in MS patients
| Tregs | ||||
|---|---|---|---|---|
| CD4+CD25+Foxp3+ | CD8+CD28- | |||
| Correlation coefficient | P- value | Correlation coefficient | P- value | |
| Age | -0.043 | 0.7 | -0.07 | 0.5 |
| EDSS | 0.15 | 0.2 | 0.09 | 0.45 |
| Number of recurrence | -0.03 | 0.8 | 0.16 | 0.13 |
| Disease duration | 0.008 | 0.94 | 0.2 | 0.08 |
| Treatment duration | 0.17 | 0.12 | 0.14 | 0.2 |
Table 4.
Linear multiple regression analysis evaluating the effect of different factors on the frequency of Tregs in MS patients
| Variables | Coefficients | t | Sig. P | Adjusted R Squared | ||
|---|---|---|---|---|---|---|
| B | Std. error | |||||
| CD4+CD25+Foxp3+Tregs | Age | 0.0489 | 0.005 | 9.1 | <0.001 | 0.845 |
| Sex | 0.458 | 0.252 | 1.8 | 0.07 | ||
| Disease duration | -0.041 | 0.025 | -1.63 | 0.10 | ||
| Treatment duration | 0.072 | 0.029 | 2.47 | 0.016 | ||
| CD8+CD28-Tregs | Age | 0.018 | 0.002 | 8.64 | <0.001 | 0.759 |
| Sex | -0.178 | 0.134 | -1.32 | 0.18 | ||
| Number of recurrences | 0.021 | 0.012 | 1.79 | 0.07 | ||
Discussion
We evaluated the frequency of two subtypes of Tregs and showed that the frequency of CD4+CD25+Foxp3+ cells is significantly lower in MS patients than in healthy controls; it is also significantly lower in mild forms of MS than in sever forms.
Although inconsistent with some other studies (16–19) that showed no significant differences in the circulatory number of CD4+CD25+ cells in patients with MS compared to that in healthy controls, our study showed lower circulatory number of CD4+CD25+Foxp3+ cells in MS patients, which may be due to their disturbed thymic development (20) and/or their recruitment into the inflamed organ i.e. CNS. It may be considered that the reduction in the percentage of CD4+CD25+Foxp3+ cells could be one probable reason (or at least a predisposing factor) of MS. Restoring the frequency as well as function of regulatory cells in MS patients after taking INF-β, strengthens the likelihood of this idea (21). Evaluating the percentage of Tregs in different types of MS, we found that the number of CD4+CD25+Foxp3+ cells in mild forms of MS (CIS and RRMS) was significantly lower than in severe forms (SPMS, PPMS, and PRMS), yet we found a study inconsistent with ours (16). It is likely that in more sever types of MS, more de novo or induced production of CD4+CD25+Foxp3+Tregs, as a manifestation of their immunomodulatory function, constitutes a circulatory reservoir that replaces the CNS-redistributed ones. Such observation has also been made in rejection episodes of organ transplantation, in which more circulatory number of Tregs, as a demonstration of their generation, tries to compensate both the residing ones in lymphoid tissues (to block initiation of aggressive immune responses) and migrating ones to graft sites (to inhibit the aggressive cells that have escaped from the regulation) (22). However, despite several studies which have demonstrated that rather functional defects of Tregs are present in MS patients (7, 16–19, 23), we showed that numbers of such cells may also be important in determining the severity of the disease. In other words, according to their immunomodulatory roles, naturally increasing numbers of Tregs in sever forms, in line with therapeutic procedures which increase the number of Tregs such as INF-β (21) and inhibitors of Histone Deacetylase (22), may delay the progression of the process to more severe types.
Although not statistically significant, the circulatory frequency of CD8+CD28-Tregs was lower in our MS patients than in healthy controls. However, some other studies found a significant decrease (7, 24–26). According to our knowledge, the exact pathophysiologic basis of such reduction is not clear, but it may simply be a demonstration of tolerance failure in MS patients.
We did not find any correlation between the frequencies of Tregs with different parameters. However, adjusting the effects of different factors, we showed that age affects the frequency of both subsets of Tregs and treatment duration affects the frequency of CD4+CD25+Foxp3+Tregs. The effect of age also has been shown in other studies (17, 20).
The limitation of our study was that we did not monitor the changes of Tregs longitudinally. This limitation allowed just a cross-sectional analysis of Treg profiles of only limited robustness. Secondly, functional assays which provide further information on the immunoregulatory status of the patients were not performed. It should also be mentioned that we studied a greater number of MS patients in comparison with others working on numerical status of Tregs in MS patients.
Conclusion
Our study shows that although the number of CD4+CD25+Foxp3+ cells is reduced in MS patients, there is no statistical correlation between the number of such cells and the type/severity of MS according to EDSS scale. According to our results it seems that Treg cells may play an important role in the pathogenesis of MS.
Acknowledgment
This study was funded and supported by Deputy of Research, Kashan University of Medical Sciences (kaums), Grant number 9091.
The authors have no conflicts of interest to declare.
References
- 1.Compston A, Coles A. Multiple sclerosis. Lancet. 2008;372:1502–1517. doi: 10.1016/S0140-6736(08)61620-7. [DOI] [PubMed] [Google Scholar]
- 2.Trapp B, Peterson J, Ransohoff R, Rudick R, Mork S, Bo L. Axonal transection in the lesions of multiple sclerosis. N Engl J Med. 1998;338:278–285. doi: 10.1056/NEJM199801293380502. [DOI] [PubMed] [Google Scholar]
- 3.Hafler DA, Slavik JM, Anderson DE, O'Connor KC, De Jager P, Baecher-Allan C. Multiple sclerosis Immunol Rev. 2005;204:208–231. doi: 10.1111/j.0105-2896.2005.00240.x. [DOI] [PubMed] [Google Scholar]
- 4.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic selftolerance maintained by activated T cells expressing IL-2 receptor alpha-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J Immunol. 1995;155:1151–1164. [PubMed] [Google Scholar]
- 5.Fritzsching B, Korporal M, Haas J, Krammer PH, Suri-Payer E, Wildemann B. Similar sensitivity of regulatory T cells toward CD95Lmediated apoptosis in patients with multiple sclerosis and healthy individuals. J Neurol Sci. 2006;251:91–97. doi: 10.1016/j.jns.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Kukreja A, Cost G, Marker J, Zhang C, Sun Z, Lin-Su K, et al. Multiple immuno-regulatory defects in type-1 diabetes. J Clin Invest. 2002;109:131–140. doi: 10.1172/JCI13605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Crucian B, Dunne P, Friedman H, Ragsdale R, Pross S, Widen R. Alterations in levels of CD28-/CD8+ suppressor cell precursor and CD45RO+/ CD4+ memory T lymphocytes in the peripheral blood of multiple sclerosis patients. Clin Diagn Lab Immunol. 1995;2:249–252. doi: 10.1128/cdli.2.2.249-252.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Najafian N, Chitnis T, Salama AD, Zhu B, Benou C, Yuan X, et al. Regulatory functions of CD8+CD28- T cells in an autoimmune disease model. J Clin Invest. 2003;112:1037–1048. doi: 10.1172/JCI17935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ménager-Marcq I, Pomié C, Romagnoli P, Van Meerwijk JP. CD8+CD28- regulatory T lymphocytes prevent experimental inflammatory bowel disease in mice. Gastroenterology. 2006;131:1775–1785. doi: 10.1053/j.gastro.2006.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ben-David H, Sharabi A, Dayan M, Sela M, Mozes E. The role of CD8+CD28-regulatory cells in suppressing myasthenia gravis-associated responses by a dual altered peptide ligand. Proc Natl Acad Sci USA. 2007;104:17459–17464. doi: 10.1073/pnas.0708577104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tulunay A, Yavuz S, Direskeneli H, Eksioglu-Demiralp E. CD8+CD28-, suppressive T cells in systemic lupus erythematosus. Lupus. 2008;17:630–637. doi: 10.1177/0961203308089400. [DOI] [PubMed] [Google Scholar]
- 12.North ME, Webster AD, Farrant J. Primary defect in CD8+ lymphocytes in the antibody deficiency disease (common variable immunodeficiency): abnormalities in intracellular production of interferon-gamma (IFN-c) in CD28+ (“cytotoxic”) and CD28- (“suppressor”) CD8+ subsets. Clin Exp Immunol. 1998;11:70–75. doi: 10.1046/j.1365-2249.1998.00479.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Liu W, Putnam AL, Xu-yu Z, Szot GL, Lee MR, Zhu S, et al. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2009;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Michalek J, Vrabelova Z, Hrotekova Z, Kyr M, Pejchlova M, Kolouskova S, et al. Immune regulatory T cells in siblings of children suffering from type 1 diabetes mellitus. Scand J Immunol. 2006;64:531–535. doi: 10.1111/j.1365-3083.2006.01837.x. [DOI] [PubMed] [Google Scholar]
- 15.Expanded Disability Status Scale. 2008. Available at: http://www mult-sclerosis. org/expandeddisabilitystatusscale. html .
- 16.Feger U, Luther C, Poeschel S, Melms A, Tolosa E, Wiendl H. Increased frequency of CD4+ CD25+ regulatory T cells in the cerebrospinal fluid but not in the blood of multiple sclerosis patients. Clin Exp Immunol. 2007;147:412–418. doi: 10.1111/j.1365-2249.2006.03271.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Haas J, Hug A, Viehöver A, Fritzsching B, Falk CS, Filser A, et al. Reduced suppressive effect of CD4+CD25high regulatory T cells on the T cell immune response against myelin oligodendrocyte glycoprotein in patients with multiple sclerosis. Eur J Immunol. 2005;35:3343–3352. doi: 10.1002/eji.200526065. [DOI] [PubMed] [Google Scholar]
- 18.Viglietta V, Baecher-Allan C, Howard LW, Hafler D. Loss of functional suppression by CD4+CD25+ regulatory T cells in patients with multiple sclerosis. J Exp Med. 2004;199:971–979. doi: 10.1084/jem.20031579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Putheti P, Pettersson A, Soderstrom M, Link H, Huang YM, et al. Circulating CD4+CD25+T regulatory cells are not altered in multiple sclerosis and unaffected by disease-modulating drugs. J Clin Immunol. 2004;24:155–161. doi: 10.1023/B:JOCI.0000019780.93817.82. [DOI] [PubMed] [Google Scholar]
- 20.Venken K, Hellings N, Broekmans T, Hensen K, Rummens JL, Stinissen P. Natural naive CD4+CD25+CD127low regulatory T cell (Treg) development and function are disturbed in multiple sclerosis patients: recovery of memory Treg homeostasis during disease progression. J Immunol. 2008;180:6411–6420. doi: 10.4049/jimmunol.180.9.6411. [DOI] [PubMed] [Google Scholar]
- 21.Namdar A, Nikbin B, Ghabaee M, Bayati A, Izad M. Effect of IFN-ß therapy on the frequency and function of CD4+CD25+ regulatory T cells and Foxp3 gene expression in relapsing–remitting multiple sclerosis (RRMS):A preliminary study. J Neuroimmunol. 2010;218:120–124. doi: 10.1016/j.jneuroim.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 22.Nikoueinejad H, Sharif MR, Amirzargar A, Mirshafiey A, Einollahi B. Regulatory T cells as a therapeutic tool to induce solid-organ transplant olerance: current clinical experiences. Exp Clin Transplant. 2013;11:379–387. doi: 10.6002/ect.2013.0004. [DOI] [PubMed] [Google Scholar]
- 23.Huan J, Culbertson N, Spencer L, Bartholomew R, Burrows GG, Chou YK. Decreased FOXP3 levels in multiple sclerosis patients. J Neurosci Res. 2005;81:45–52. doi: 10.1002/jnr.20522. [DOI] [PubMed] [Google Scholar]
- 24.Correale J, Villa A. Role of CD8+CD25+Foxp3+ regulatory T Cells in multiple sclerosis. Ann Neurol. 2010;67:625–638. doi: 10.1002/ana.21944. [DOI] [PubMed] [Google Scholar]
- 25.Mikulkova Z, Praksova P, Stourac P, Bednarik J, Strajtova L, Pacasova R, et al. Numerical defects in CD8+CD28- T-suppressor lymphocyte population in patients with type 1 diabetes mellitus and multiple sclerosis. Cell Immunol. 2010;262:75–79. doi: 10.1016/j.cellimm.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 26.Frisullo G, Nociti V, Iorio R, Plantone D, Patanella AK, Tonali PA, et al. CD8(+)Foxp3(+) T cells in peripheral blood of relapsing-remitting multiple sclerosis patients. Hum Immunol. 2010;71:437–441. doi: 10.1016/j.humimm.2010.01.024. [DOI] [PubMed] [Google Scholar]


