Abstract
Objective(s):
In insulin resistance, the insulin action in liver, muscles and adipocytes decreases and result in hyperglycemia, dyslipidemia and hyperinsulinemia. In this study we evaluate the effect of Zataria multiflora extract on insulin sensitivity in high fructose fed insulin resistant rats, since this extract was shown antihyperglycemic effect in streptozotocin induced diabetes in rats.
Materials and Methods:
Experimental rats were fed with high fructose diet for 6 weeks and then were treated with Z. multiflora extract or a pioglitazone solution for 2 weeks. Blood and tissue samples were collected for analysis at the end of two weeks. Blood glucose, serum level of triglyceride and cholesterol were measured by auto analyzer. Insulin and adiponectin levels were assayed by enzyme-linked immunosorbent assay (ELISA) method. Plasma free fatty acids profile was studied by gas chromatography. Peroxisome proliferator activated receptor (PPAR.γ) and Glucose transporter type 4 (GLUT.4) gene expressions were assessed by real time polymerase chain reaction (PCR) and western blotting.
Results:
Animals were treated by Z. multiflora extract showed insulin (43±11pmol/l), adiponectin (5.3±0.5 μg/ml), glucose (144±9.8 mg/dl), and triglyceride (120±10 mg/dl) levels significantly improved as compare with the control group [insulin (137±34 pmol/l), adiponectin (3.9±0.15 μg/ml), glucose (187±15mg/dl), and triglycerides (217±18 mg/dl)]. PPARγ protein level, also significantly increased in Zataria multiflora treated group.
Conclusion:
This study demonstrates the beneficial effects of Zataria multiflora extract on insulin resistance in rats fed with a high-fructose diet through at least three mechanisms including direct insulin like effect, increasing in adiponectin and of PPARγ protein expression.
Keywords: Adiponectin, GLUT.4, Insulin resistance, PPARγ, Zataria multiflora
Introduction
Metabolic syndrome is characterized by insulin resistance, central obesity, hypertension and dyslipidemia and increases the risk of cardiovascular disease and type 2 diabetes mellitus (1). Insulin resistance is the main defect associated with the metabolic syndrome (2) and obesity is the critical factor that induces insulin resistance. In obesity, inflammation that initiates from adipose tissue, increases secretion of free fatty acids. Moreover, pattern of adipokines secretion is modified in obesity which can affect the insulin functions in the liver and muscles. Defects in glucose transporter 4 (GLUT.4) expression and translocation have been determined in the striated muscle cells and adipose tissues in insulin resistance (3).
In diabetes and pre-diabetes states, the plasma free fatty acids (FFAs) concentrations are increased, which could be due to the enlarged adipose tissue in obesity. These findings demonstrate that FFAs may be involved in the complications of diabetes (4). On the other hand, , FFAs level elevation inhibit insulin’s actions (5), including inhibition of lipolysis, which in turn will further elevate FFAs release into the circulation (6).
Adipose tissue not only releases FFAs, but also it is an active metabolic tissue that participates in insulin resistance occurrence in liver and muscles (7). Adipose tissue produces different inflammatory molecules, such as TNF-α and IL-6, which may affect other tissues. Overproduction of leptin, TNF-α and IL-6 and reduction of adiponectin were identified in obesity which are involved in insulin resistance (8).
Decline in adiponectin level which occurs in obesity has a positive correlation with insulin resistance and weight loss results in significant elevation of adiponectin level (9). Adiponectin after binding to its receptors can mediate phosphorylation of AMP-activated protein kinase (AMPK) and also peroxisome proliferator activated receptors (PPARs) and thereby increase fatty acid oxidation and glucose uptake. Thiazolidinediones (TZDs) (agonists of PPARs) also improve insulin sensitivity via increasing adiponectin concentration (9).
PPARs modulate expression of the genes involved in metabolism of lipids (9, 10). Its activation stimulates lipid oxidation and lipogenesis, induces differentiation of adipocytes and increases insulin sensitivity in mature adipocytes (11). Therefore, synthetic PPARγ ligands such as TZDs are applied clinically to control diabetes (12).
Zataria multiflora is a plant of Laminaceae family that grows only in Iran, Pakistan and Afghanistan (13, 14). This plant local name in Iran is Avishan-e-Shirazi (15). Z. multiflora is widely used in Iran as a flavor component in a wide variety of foods. The main constituents of this plant are phenolic compounds including carvacrol, thymol and gamma-terpinene (16). This plant has several traditional uses in Iran including as a carminative, stimulant, diuretic and antiseptic in (15). There is not any report on the antihyperglycemic effect of this plant in the literature. In our previous study, we demonstrated that water extract of Z. multiflora has a strong inhibitory effect on alpha glucosidase in vitro (17). This extract also significantly decreased the postprandial glucose level in diabetic rats after administration of 2 g/kg maltose, but no effect on postprandial glucose level in non-diabetic rats (18). Z. multiflora also decreases the postprandial glucose level after administration of glucose (unpublished data).
These findings that mentioned above, demonstrate that Z. multiflora affects the blood glucose concentration through mechanism(s) independent of its inhibitory effect on alpha glucosidase. In this study we evaluated the effect of Z. multiflora on insulin resistant rats to assess the effect of this extract on muscle and hepatic insulin resistance and possible mechanism(s) involved. Skeletal muscle cells are the main site for insulin function and development of insulin resistance (5, 19) and the liver is another insulin sensitive organ that plays a critical role in energy homeostasis (8).
Materials and Methods
Preparation of extract
Z. multiflora was collected from Fars province, Iran, and authenticated by Department of Botany, Bahonar University, Kerman, Iran. The aerial parts of Z. multiflora were separated, washed and air dried. Plant tissues (300 g) were milled and extracted by maceration method in 1000 ml of distillated water at room temperature for 48 hr (20). After filtration water was evaporated at 40oC in an oven and dried extract was stored at -20oC.
Experimental animals and protocol
Male Wistar rats weighing 250–300 g were obtained from animal house of Kerman University of Medical Sciences. Rats were housed in animal room at 22±3oC for 12 hr light. Rats were fed with standard chow and fresh tap water for 2 weeks. After 2 weeks, rats were divided randomly into 4 groups (n=8). three groups was fed a 60% fructose diet and fourth group as the healthy control group (HCG) with standard diet (21). After 6 weeks insulin resistance in fructose fed rats was confirmed by oral glucose tolerance test (OGTT) and was compared with the healthy control group (results not shown). The fructose-treated groups were treated by:
-
1:
Z. multiflora extract group: a group who received intragastric injection of Zataria multiflora extract (1000 mg/kg) (18).
-
2:
Pioglitazone group (Pio): this group received intragastric injection of pioglitazone (10 mg/kg) (22).
-
3:
Control group (Con): used as insulin resistant control group and did not receive any injections.
Treatment was continued for 2 weeks. Water and food intake were measured daily in this period. Body weight was also measured weekly throughout the 8 weeks of treatment.
Blood and tissue collection
At the end of the treatment period, animals were fasted for 12 hr overnight, blood samples were collected from heart under ether anesthesia. Blood sample was divided into two vials with or without EDTA. Vials were centrifuged at 4000 rpm for 10 min at 4oC and Plasma (for FFA analysis) or serums (for other biochemical analysis) were separated immediately. Hind limb skeletal muscles and liver were excised. Tissue samples were immediately frozen in liquid nitrogen, and subsequently stored at −75oC until evaluation.
Measurement of serum parameters
Serum glucose, triglyceride, cholesterol and high density lipoprotein- cholesterol (HDL-c) concentrations were measured in an RA-1000 autoanalyser. Blood insulin and adiponectin levels were measured by ELISA using commercial assay kits according to the manufacturer’s directions (Mouse/Rat Adiponectin or insulin ELISA kit, USCN. China). Homeostasis Model Assessment of Insulin Resistance (HOMA-IR) was calculated using the equation: [(insulin (_U/ml)×glucose (mmol/l))/22.5].
Measurement of plasma free fatty acids
Plasma free fatty acids (FFAs) were extracted and analyzed by the method explained by Kangani et al with slight modifications (23). 500 μl of plasma was mixed with 20 μl of pentadecanoic acid (1 mg/ml) as an internal standard. Lipids were extracted from plasma by reagent which constituted from isopropanol–heptane–hydrochloric acid (1M) (40:10:1, v/v/v). FFAs were separated by TLC on silica gel plates using a heptane–ether–acetic acid [60:40:3] solvent system. FFAs were visualized by Iodine on TLC plates. FFA bands were scrapped and free fatty acid methyl esters (FAME) were prepared by a reaction with BF3 containing methanol (Sigma). FFA methyl esters were separated using an Agilent GC-7890A system equipped with a flame ionization detector. The injection volume was 1 μl in the split 30:1 injection mode. A capillary column (DB-225 20 m×0.1 mm I.D., 0.1 μm film thickness, J&W GC columns, USA) was employed.
Real time PCR
Total RNA from the skeletal muscle (for GLUT.4 assay) and liver (for PPARγ assay) tissues was extracted with RNeasy mini kit (Qiagen) and according to manufacturer’s guideline. The RNA concentration was determined by the ultraviolet (UV) light absorbency at 260 nm and 280 nm (ND-1000 nanodrop). The quality of the RNA was confirmed by ethidium bromide staining of 18S and 28S ribosomal RNA after electrophoresis on 2% agarose gel.
Then cDNA synthesis was performed by Quanti Tect Reverse Transcription Kit (Qiagen) and thoroughly according to the manufacture’s procedure. Relative Quantitative real time PCR was performed on a Qiagen Thermal Cycler (Rotor-Gene Q 5plex HRM System, Qiagen) using the corresponding QuantiFast SYBR Green PCR kit (Qiagen) according to manufacturer’s protocol. The primers that used in this study were shown in Table 1.
Table 1.
Primers used in this study
| Gene | Primers | PCR product | Accession number |
|---|---|---|---|
| GLUT.4 | F: ACTGGCGCTTTCACTGAACT | 106 | NM_012751 |
| R: CGAGGCCAAGGCTAGATTTTG | |||
| PPARγ | F: CATGCTTGTGAAGGATGCAAG | 131 | NM_001145367 |
| R: TTCTGAAACCGACAGTACTGACAT | |||
| GAPDH | F: TGGAGTCTACTGGCGTCTT | 138 | NM_017008 |
| R: T GT CATATTTCTCGT GGTT CA |
Cycle of threshold (CT) for each sample was determined. ΔCT was calculated via formula: ΔCT = CT (target gene) – CT (endogenous reference gene (GAPDH))
Results were analyzed by 2-ΔΔCT method.
In the real time PCR all suggestions that published by Qiagen in “Critical Factors for Successful in Real Time PCR” were regarded.
Western blotting
Total protein was extracted from muscles or liver by homogenization in the RIPA (Radio Immuno Precipitation assay) buffer (sigma, cat number:
R0278). Homogenate was centrifugated at 14,000 rpm for 20 min and was removed supernatant that contain proteins. Total protein was estimated by Bradford method. Using SDS-PAGE, proteins were separated by loading of 120 μg/lane and transferred on PVDF membrane by overnight incubating with 5% non-fat skim milk in TBST buffer at 4oC. Non-specific binding sites were blocked. Then, the membrane was washed with TBST 3 times (each time for 20 min). The membrane was incubated with appropriate polyclonal primary antibodies for PPARγ (ab27649, Rabbit polyclonal to PPAR gamma, abcam) or GLUT.4 (ab33780, Rabbit polyclonal to GLUT4, abcam) antibody in TBST buffer for 1hr. The membrane was then washed with TBST, as mentioned above, followed by incubation with anti-rabbit secondary antibody (Goat polyclonal HRP conjugated Antibody to Rabbit IgG, , ab6112, abcam), for 1 hr at room temperature. Then PVDF was washed and incubated with substrate (western lightening plus ECL, Perkin-Elmer) for 1 min. Then, PVDF was exposed to Hyblot film (Denvill) for 30 sec, in a dark room. Density of bands was analyzed by image j software.
Statistical analysis
All data are presented as mean±SEM. Statistical analysis was performed by analysis of variance (ANOVA) and Post-hoc Tukey test; P-values of less than 0.05 were considered to be significant.
Results
Body weight gain and water and food intake
There was no significant difference in the mean body weight of groups at the start of treatment. Body weight gain that was calculated by subtracting initial body weight and weight at the end of 8th week, had significant difference between Z. multiflora and control groups (P<0.0001). There was also significant difference between Z. multiflora and pioglitazone groups weigh gain (P<0.05) and Z multiflora reduced weight gain in comparison with pioglitazone.
There was no significant difference in water or food intake between Z. multiflora group and pioglitazone or control group. (Results are shown in Table 2).
Table 2.
Effect of Zataria multiflora on body weight, water and food intake and other insulin resistance related parameters
| Parameter | Groups | P-value¥ | |||
|---|---|---|---|---|---|
| HCG | Con | Pio | ZME | ||
| initial weight (g) | 275±8 | 272±9 | 272±12 | 281±12 | 0.586 |
| weight after 8 week (g) | 285±9 | 307±8 | 294±16 | 282±15 | 0.832 |
| Weight gain (g) | 10±2* | 35±2# | 22±5# | 11±6* | <0.0001 |
| Water intake (ml) | 35±0.8* | 47±2# | 60±2#* | 54±3# | 0.221 |
| Food intake (g) | 21.6±0.7* | 13.4±0.4# | 13.5±0.3# | 13±0.2# | 0.779 |
| Insulin (pmol/L) | 50±4.8* | 137±34# | 40±2.7* | 43±11* | 0.007 |
| Adiponectin (μg/ml) | 2.9±0.16 | 3.9±0.15 | 5.6±0.4# * | 5.3±0.5# * | 0.029 |
| Glucose (mg/dl) | 132±4* | 187±15# | 129±5.8* | 144±9.8* | 0.029 |
| HOMA.IR | 2.7±0.37* | 9.7±2.1# | 2.1±0.12* | 2.6±0.74* | 0.001 |
| Cholesterol (mg/dl) | 71±12.1 | 59±3.2 | 63±4.2 | 54±2.9 | 0.955 |
| Triglyceride (mg/dl) | 85±13* | 217±18# | 200±51# | 120±10#* | 0.011 |
| HDL-c (mg/dl) | 24.86±1.4 | 29.25±1.8 | 29.38±2.3 | 26.35±1.2 | 0.668 |
HCG was fed standard chow and three other groups were fed 60% fructose diet for 8 weeks. In Pio and Z. multiflora groups after primary 6 weeks, animals were treated by pioglitazone (10 mg/kg/day) or Z. multiflora extract (1000 mg/kg/day) for 2 week. Biochemical parameters were assayed by auto analyzer and hormones by ELISA kits. HOMA-IR was calculated by the formula. All data are Mean± SEM. (n=8)
HCG: healthy control group, Con: control, Pio: pioglitazone, ZME: Zataria multiflora extract
¥ P-value demonstrates the difference significance between Zataria multiflora and control group.
* Significant difference with control group P<0.05)
# Significant difference with HCG group (P<0.05)
Blood glucose, triglyceride and cholesterol
Blood glucose in Z. multiflora group (144±9.8 mg/dl) had significant difference with control group (187±15 mg/dl) (P<0.05). Moreover, Z. multiflora significantly decreased serum triglyceride concentration (120±10 mg/dl) as compare with control group (217±18 mg/dl) (P= 0.011). Pioglitazone did not have any effect on triglyceride in this study (200±51 mg/dl). There is nonsignificant difference in cholesterol and LDL-c between Z multiflora and other groups (all results are summarized in Table 2).
Serum level of insulin and adiponectin
Serum level of insulin (43±11 pmol/l) in Z. multiflora group was significantly lower in comparison with the control group (137±34 pmol/l) (P<0.01). However, adiponectin level in Z. multiflora group (5.3±0.5 μg/ml) significantly increased in comparison with control group (3.9±0.15 μg/ml) (P<0.05). There was no significant difference in insulin and adiponectin level between Z. multiflora and pioglitazone treated groups (all results are summarized in Table 2).
Homeostasis model assessment of insulin resistance
There was a significant difference in HOMA-IR between Z. multiflora (2.6±0.74) and control groups (9.7±2.1) and treatment with Z. multiflora decreased the HOMA-IR as compared with control group (P=0.001). Also HOMA.IR in Z. multiflora group did not have significant difference with pioglitazone treated group (Table 2).
Plasma free fatty acids
There was no significant difference in total free fatty acids or free fatty acids fractions between Z multiflora treated and control group, butpioglitazone significantly decreased the level of total free fatty acids, oleic acid, palmitic acid and palmitoleic acid as compare with control and Z multiflora group (P<0.05) (all results are presented in Table 3).
Table 3.
Effect of Zataria multiflora on plasma free fatty acids profiles
| Parameter | Groups | *P- value | |||
|---|---|---|---|---|---|
| HCG | Con | Pio | ZME | ||
| myristic acid (μmol/l) | 1.07±0.01 | 1.12±0.06 | 1.2±0.06 | 1.3±0.08 | 0.109 |
| palmitic acid (μmol/l) | 5.9±0.57 | 11.1±2.6 | 5.1±0.18 | 7.8±0.18 | 0.438 |
| palmitoleic acid (μmol/l) | 1.06±0.04 | 1.4±0.12 | 1.1±0.01 | 1.6±0.03 | 0.2 |
| stearic acid (μmol/l) | 1.3±0.12 | 2.7±0.12 | 2.06±0.11 | 2.6±0.07 | 0.961 |
| oleic acid (μmol/l) | 2.2±0.21 | 2.9±0.22 | 1.7±0.08 | 3.6±0.31 | 0.122 |
| total free fatty acids (μmol/l) | 12.53±1.95 | 22±2.7 | 15±2.3 | 19.44±2.8 | 0.791 |
HCG was fed standard chow and three other groups were fed 60% fructose diet for 8 weeks. In Pio and ZME groups after primary 6 weeks, animals were treated by pioglitazone (10 mg/kg/day) or Zataria multiflora extract (1000 mg/kg/day) for 2 weeks. FFA was analyzed by Gas Chromatography as free fatty acid methyl ester after extraction by TLC. All data were shown Mean± SEM (n=8)
HCG: healthy control group, Con: control, Pio: pioglitazone, ZME: Zataria multiflora extract
* P-value demonstrate the difference between Zataria multiflora and control group
GLUT.4 and PPARγ gene expression
Our result demonstrated that Z. multiflora did not have any effect on PPARγ and GLUT.4 gene expression at mRNA level (P=0.928 and P=0.995, respectively). However pioglitazone significantly increased the mRNA level of PPAR.γ. (Figure 1).
Figure 1.
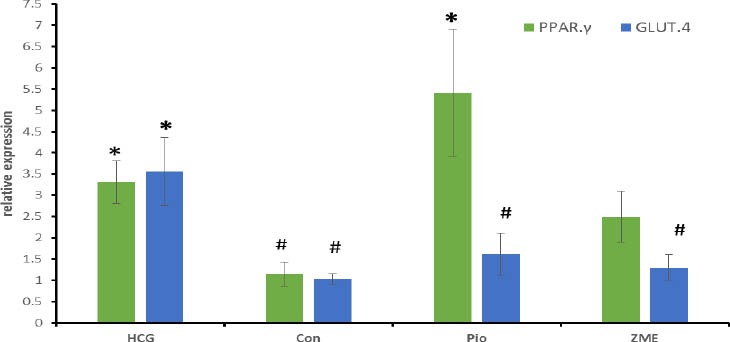
Effect of Zataria multiflora on mRNA level of liver PPARγ and muscle GLUT.4GLUT.4 and PPARγ gene expression
Our result demonstrated that Z. multiflora did not have any effect on PPARγ and GLUT.4 gene expression at mRNA level (P=0.928 and P=0.995, respectively). However pioglitazone significantly increased the mRNA level of PPARγ (Figure 1)
HCG was fed standard chow and three other groups were fed 60% fructose diet for 8 weeks. In Pio and Z multiflora groups after primary 6 weeks, animals were treated by pioglitazone (10 mg/kg/day) or Zataria multiflora extract (1000 mg/kg/day) for 2 weeks. PPARγ and GLUT.4 mRNA level in liver and muscle, respectively, were quantified by real time PCR. All data are Mean±SEM (n=8)
HCG: healthy control group, Con: control, Pio: pioglitazone, ZME: Zataria multiflora extract
* Significant difference with control group (P<0.05)
# Significant difference with HCG group (P<0.05)
Effect of Zataria multiflora on protein level of liver, PPARγ and muscle GLUT.4
This study showed that, there was a significant difference in PPARγ protein level between Z. multiflora and control group. Moreover, pioglitazone significantly increased the level of PPARγ protein as compare with control group. There was no significant difference in GLUT.4 protein level between Z. multiflora and control or pioglitazone groups (Figure 2).
Figure 2.
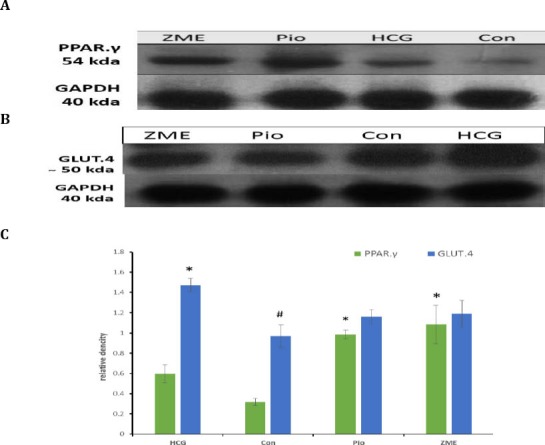
Effect of Zataria multiflora on protein level of liver, PPARγ and muscle GLUT.4
HCG was fed standard chow and three other groups were fed 60% fructose diet for 8 weeks. In Pio and Z multiflora groups after primary 6 weeks, animals were treated by pioglitazone (10 mg/kg/day) or Zataria multiflora extract (1000 mg/kg/day) for 2 weeks. PPARγ and GLUT.4 protein level in liver and muscle, respectively, were assayed by Western blotting
HCG: healthy control group, Con: control, Pio: pioglitazone, ZME: Zataria multiflora extract
* Significant difference with control group (P<0.0001)
# Significant difference with HCG group (P<0.05)
Discussion
In this study we found that Z. multiflora has beneficial effect on insulin resistance and this extract increased the insulin sensitivity in fructose fed insulin resistant rats. Z. multiflora significantly decreased glucose, insulin and HOMA.IR in insulin resistant animal model. The effect of Z. multiflora on these parameters was comparable with pioglitazone and there was no significant difference between Z. multiflora and pioglitazone treated groups. Z. multiflora also decreased the weight gain of animals in comparison with control group. To the best of our knowledge, there was not any study about Z multiflora effects on insulin resistance in the literature but these results clearly suggest that Z. multiflora improves insulin resistance. Reduced HOMA-IR and glucose level along with reduction in insulin level, suppose that antihyperglycemic effect arises from improving insulin action rather than insulin secretion (24) and decrease in insulin and glucose level propose that Z. multiflora has a direct insulin like effect (25).
Based on our results, TFG showed lowering effect on triglyceride level in insulin resistant rats but Z. multiflora did not have any effect on total cholesterol, HDL-C and water and food intake. In recent years, ATPIII guidelines suggest therapies that lower triglycerides in patients with metabolic syndrome (26). Therefore, TFG is more suitable than pioglitazone for this purpose.
Z. multiflora significantly increased the level of serum adiponectin concentration. Adiponectin level is modulated by a number of hormones and factors that participated in regulation of metabolic processes. Insulin diminishes the adiponectin expression in mice and humans (27) and thiazolidinediones, as mentioned above, increase the expression of adiponectin (28). Adiponectin inhibits gluconeogenesis in the liver and increases β-oxidation of free fatty acids in muscles and therefore increases insulin sensitivity and energy homeostasis (29). These findings are sufficient to conclude that antihyperglycemic effect of Z. multiflora may be due to the enhancing of adiponectin production.
In molecular level, Z. multiflora increases the protein of PPARγ in the liver without rising PPARγ mRNA level, and it also has no effect on GLUT.4 protein and mRNA in the striated muscles. PPAR-γ agonists exert their antidiabetic effect by PPAR-γ activation resulting to increase the sensitivity of insulin receptors (30). Thiazolidinediones significantly increase insulin sensitivity and adiponectin concentration in diabetic patients (30, 31). In Yadav et al study, it was shown that PPARγ mRNA level increased in liver of insulin resistant rats that treated with rosiglitazone (32). PPARγ protein decreased in fructose fed rats and increased after treatment with pioglitazone and Z. multiflora. Based on the role of PPARγ activity in improvement of insulin resistance, it is possible that increasing the PPARγ protein level could be another mechanism for antihyperglycemic effect of Z. multiflora.
PPARγ activation increases fatty acid uptake, adipogenesis and fat deposition (12). Decrease of serum triglycerides that induced by Z. multiflora can be correlate with triglyceride storage in insulin-sensitive tissues resulting from PPARγ over expression (33).
PPARγ increase the expression and translocation of GLUT4 in adipose tissue, and increase the catabolism of glucose in the liver along with the reduction in hepatic glucose output (34). We did not find any difference between Z. multiflora and control group in GLUT.4 levels. Besides, pioglitazone did not have any effect on GLUT.4 protein and mRNA levels. We evaluated in this study, total GLUT.4 in striated muscle cells. It is possible that translocation of GLUT.4 to the cell membrane is increased after treatment with Z multiflora and pioglitazone despite the total GLUT.4 protein is not significantly different between two groups (3). In shih et al study that was assayed membrane GLUT.4 instead of total GLUT.4, showed increase in GLUT.4 simultaneously with elevation of PPARγ gene expression after treatment with Momordica charantia (21).
Total FFA or FFA fraction did not significantly change with Z. multiflora in insulin resistant rat. However there was a significant difference in serum total free fatty acids and its fractions between fructose and control groups. Increase of FFA levels in the blood is an important sign of diabetes and there is an association between increased plasma FFA levels and reduction of glucose catabolism in skeletal muscles (12). PPARγ agonists as mentioned above enhance insulin sensitivity in adipose tissue and reduce serum FFA levels (11). PPARγ agonists play their insulin sensitizing activity by increasing FFA uptake and storage in adipose tissues (35). But in our study, FFA level has no significant difference between two groups whereas PPARγ protein increased. We evaluated PPARγ gene expression in the liver to evaluate hepatic insulin resistance and it is possible that PPARγ gene expression in adipocytes did not increase.
Conclusion
The findings explained in this study clearly demonstrated that Zataria multiflora water extract can improve insulin resistance in insulin resistant animal model. This antihyperglycemic effect and increase in insulin sensitivity may be exerted by at least three mechanisms including direct insulin like effect, increase in adiponectin and increase of PPARγ protein expression.
Acknowledgment
We would like to appreciate the Vice Chancellor for Research of Kerman University of Medical Sciences, Kerman, Iran for their financial support of this project.
References
- 1.Chen CH, Chang MY, Lin YS, Lin DG, Chen SW, Chao PM. A herbal extract with acetyl-coenzyme A carboxylase inhibitory activity and its potential for treating metabolic syndrome. Metabolism. 2009;58:1297–1305. doi: 10.1016/j.metabol.2009.04.012. [DOI] [PubMed] [Google Scholar]
- 2.Anagnostis P, Katsiki N, Adamidou F, Athyros VG, Karagiannis A, Kita M, et al. 11Beta-Hydroxysteroid dehydrogenase type 1 inhibitors: novel agents for the treatment of metabolic syndrome and obesity-related disorders? Metabolism. 2013;62:21–33. doi: 10.1016/j.metabol.2012.05.002. [DOI] [PubMed] [Google Scholar]
- 3.Kim S, Jwa H, Yanagawa Y, Park T. Extract from Dioscorea batatas ameliorates insulin resistance in mice fed a high-fat diet. J Med Food. 2012;15:527–534. doi: 10.1089/jmf.2011.2008. [DOI] [PubMed] [Google Scholar]
- 4.Hong-Liang L, Wen-Ying Y, Jian-Zhong X, Rui-Qin D, Jing H, Lin P, et al. Do free fatty acids induce insulin resistance in alpha cells? Biosci Hypotheses. 2009;2:19–23. [Google Scholar]
- 5.Ragheb R, Shanab GML, Medhat AM, Seoudi DM, Adeli K, Fantus IG. Free fatty acid-induced muscle insulin resistance and glucose uptake dysfunction: Evidence for PKC activation and oxidative stress-activated signaling pathways. Biochem Biophys Res Commun. 2009;389:211–216. doi: 10.1016/j.bbrc.2009.08.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boden G. Obesity, insulin resistance and free fatty acids. Curr Opin Endocrinol Diabetes Obes. 2011;18:139–143. doi: 10.1097/MED.0b013e3283444b09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ragheb R, Medhat AM, Shanab GML, Seoudi DM, IG Fantus. Links between enhanced fatty acid flux, protein kinase C and NFкB activation, and apoB–lipoprotein production in the fructose-fed hamster model of insulin resistance. Biochem Biophys Res Commun. 2008;370:134–139. doi: 10.1016/j.bbrc.2008.03.044. [DOI] [PubMed] [Google Scholar]
- 8.Meshkani R, Adeli K. Hepatic insulin resistance, metabolic syndrome and cardiovascular disease. Clin Biochem. 2009;42:1331–1346. doi: 10.1016/j.clinbiochem.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 9.Yadav A, Kataria MA, Saini V. Role of leptin and adiponectin in insulin resistance. Clin Chim Acta. 2013;417:80–84. doi: 10.1016/j.cca.2012.12.007. [DOI] [PubMed] [Google Scholar]
- 10.Kim H-S, Hwang Y-C, Koo S-H, Park KS, Lee M-S, Kim K-W, et al. PPAR-Y activation increases insulin secretion through the up-regulation of the free fatty acid receptor GPR40 in pancreatic b-Cells. PLoS One. 2013;8:1–12. doi: 10.1371/journal.pone.0050128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li RW, Theriault AG, Au K, Douglas TD, Casaschi A, Kurowska EM, et al. Citrus polymethoxylated flavones improve lipid and glucose homeostasis and modulate adipocytokines in fructose-induced insulin resistant hamsters. Life Sci. 2006;79:365–373. doi: 10.1016/j.lfs.2006.01.023. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Li Y, Wang Y, Wen Y, Sun C. Berberine improves free-fatty-acid-induced insulin resistance in L6 myotubes through inhibiting peroxisome proliferator-activated receptor gamma and fatty acid transferase expressions. Metabolism. 2009;58:1694–1702. doi: 10.1016/j.metabol.2009.06.009. [DOI] [PubMed] [Google Scholar]
- 13.Misaghi A, Basti AA. Effects of Zataria multiflora Boiss. essential oil and nisin on Bacillus cereus ATCC 11778. Food Control. 2007;18:1043–1049. [Google Scholar]
- 14.Azizkhani M, Misaghi A, Basti AA, Gandomi H, Hosseini H. Effects of Zataria multiflora Boiss essential oil on growth and gene expression of enterotoxins A, C and E in Staphylococcus aureus ATCC 29213. Int J Food Microbiol. 2013;163:159–165. doi: 10.1016/j.ijfoodmicro.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 15.Sajed H, Sahebkar A, Iranshahi M. Zataria multiflora Boiss. (Shirazi thyme)--an ancient condiment with modern pharmaceutical uses. J Ethnopharmacol. 2013;145:686–698. doi: 10.1016/j.jep.2012.12.018. [DOI] [PubMed] [Google Scholar]
- 16.Azizkhani M, Misaghi A, Basti AA, Gandomi H, Hosseini H. Effects of Zataria multiflora Boiss. essential oil on growth and gene expression of enterotoxins A, C and E in Staphylococcus aureus. Int J Food Microbiol. 2013;163:159–165. doi: 10.1016/j.ijfoodmicro.2013.02.020. [DOI] [PubMed] [Google Scholar]
- 17.Gholamhoseinian A, Fallah H, Sharififar F, Mirtajaddini M. The inhibitory effect of some Iranian plants extracts on the alpha glucosidase. Iran J Basic Med Sci. 2008;11:1–9. [Google Scholar]
- 18.Gholamhoseinian A, Fallah H, Sharififar F. Anti-hyperglycemic activity of four plants extracts effective against alpha glucosidase in normal and diabetic rats. J Kerman Univ Med Sci. 2009;16:35–44. [Google Scholar]
- 19.Kim KCaY-B. Molecular mechanism of insulin resistance in obesity and Type 2 diabetes. Korean J Intern Med. 2010;25 doi: 10.3904/kjim.2010.25.2.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gholamhoseinian A, Fallah H, Sharifi far F. Inhibitory effect of methanol extract of Rosa damascena Mill flowers on alpha-glucosidase activity and postprandial hyperglycemia in normal and diabetic rats. Phytomedicine. 2009;16:935–941. doi: 10.1016/j.phymed.2009.02.020. [DOI] [PubMed] [Google Scholar]
- 21.Shih C-C, Lin C-H, Lin W-L, Jin-Bin Wu. Momordica charantia extract on insulin resistance and the skeletal muscle GLUT4 protein in fructose-fed rats. J Ethnopharmacol. 2009;123:82–90. doi: 10.1016/j.jep.2009.02.039. [DOI] [PubMed] [Google Scholar]
- 22.Elrashidy RA, Asker ME, Mohamed HE. Pioglitazone attenuates cardiac fibrosis and hypertrophy in a rat model of diabetic nephropathy. J Cardiovasc Pharmacol Ther. 2012;17:324–333. doi: 10.1177/1074248411431581. [DOI] [PubMed] [Google Scholar]
- 23.Kangani CO, Kelley DE, Delany JP. New method for GC/FID and GC-C-IRMS analysis of plasma free fatty acid concentration and isotopic enrichment. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873:95–101. doi: 10.1016/j.jchromb.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Abdin AA, Baalash AA, Hamooda HE. Effects of rosiglitazone and aspirin on experimental model of induced type 2 diabetes in rats: focus on insulin resistance and inflammatory markers. J Diabetes Complications. 2010;24:168–178. doi: 10.1016/j.jdiacomp.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 25.Ohnogi H, Hayami S, Kudo Y, Deguchi S, Mizutani S, Enoki T, et al. Angelica keiskei extract improves insulin resistance and hypertriglyceridemia in rats fed a high-fructose drink. Biosci Biotechnol Biochem. 2012;76:928–932. doi: 10.1271/bbb.110927. [DOI] [PubMed] [Google Scholar]
- 26.Naples M, Federico LM, Xu E, Nelken J, Adeli K. Effect of rosuvastatin on insulin sensitivity in an animal model of insulin resistance: evidence for statin-induced hepatic insulin sensitization. Atherosclerosis. 2008;198:94–103. doi: 10.1016/j.atherosclerosis.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 27.Han SH, Sakuma I, Shin EK, Koh KK. Antiatherosclerotic and anti-insulin resistance effects of adiponectin: basic and clinical studies. Prog Cardiovasc Dis. 2009;52:126–140. doi: 10.1016/j.pcad.2009.06.004. [DOI] [PubMed] [Google Scholar]
- 28.Ziemke F, Mantzoros CS. Adiponectin in insulin resistance: lessons from translational research. Am J Clin Nutr. 2010;91:258S–261S. doi: 10.3945/ajcn.2009.28449C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee CY, Lee CH, Tsai S, Huang CT, Wu MT, Tai SY, et al. Association between serum leptin and adiponectin levels with risk of insulin resistance and impaired glucose tolerance inon-diabetic women. Kaohsiung J Med Sci. 2009;25:116–125. doi: 10.1016/S1607-551X(09)70050-6. [DOI] [PubMed] [Google Scholar]
- 30.Monte SMdl, Pang M, Chaudhry R, Duan K, Lisa Longato, Carter J, et al. Peroxisome proliferator-activated receptor agonist treatment of alcohol-induced hepatic insulin resistance. Hepatol Res. 2011;41:386–398. doi: 10.1111/j.1872-034X.2011.00775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JH, Lee SH, Chung M, Park1§ Y. Sorghum extract exerts an anti-diabetic effect by improving insulin sensitivity via PPAR-γ in mice fed a high-fat diet. Nutr Res Pract. 2012;6:322–327. doi: 10.4162/nrp.2012.6.4.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Yadav H, Jain S, Yadavb M, Sinha PR, Prasad GBKS, Marotta F. Epigenomic derangement of hepatic glucose metabolism by feeding of high fructose diet and its prevention by rosiglitazone in rats. Dig Liver Dis. 2009;41:500–508. doi: 10.1016/j.dld.2008.11.012. [DOI] [PubMed] [Google Scholar]
- 33.Li RW, Douglas TD, Maiyoh GK, Adeli K, Theriault AG. Green tea leaf extract improves lipid and glucose homeostasis in a fructose-fed insulin-resistant hamster model. J Ethnopharmacol. 2006;104:24–31. doi: 10.1016/j.jep.2005.08.045. [DOI] [PubMed] [Google Scholar]
- 34.Elmazar MM, El-Abhar HS, Schaalan MF, Farag NA. Phytol/Phytanic Acid and insulin resistance: potential role of phytanic Acid proven by docking simulation and modulation of biochemical alterations. PLoS One. 2013;8:e45638. doi: 10.1371/journal.pone.0045638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Sharma AK, Bharti S, Kumar R, Krishnamurthy B, Bhatia J, Kumari S, et al. Syzygium cumini ameliorates insulin resistance and beta-cell dysfunction via modulation of PPAR, dyslipidemia, oxidative stress, and TNF-alpha in type 2 diabetic rats. J Pharmacol Sci. 2012;119:205–213. doi: 10.1254/jphs.11184fp. [DOI] [PubMed] [Google Scholar]


