Abstract
Objective(s):
This study investigated the effects of melatonin, famotidine, mirtazapine, and thiamine pyrophosphate on ischemia/reperfusion (I/R) injury in diabetic rats and evaluated oxidant and antioxidant marker measurement results. It also examined the effects of the drugs aimed at preventing infertility that may result from I/R injury.
Materials and Methods:
Diabetic rats were divided into a control group (IRC) to be exposed to I/R, an ovarian I/R + 2.2 mg/kg melatonin (IRML) group, an ovarian I/R + famotidine (IRFA) group, an ovarian I/R + 20 mg/kg mirtazapine (IRMR) group, an ovarian I/R + 20 mg/kg thiamine pyrophosphate (IRTP) group, and a sham operation (SO) group.
Results:
In the control group exposed to I/R, the levels of the oxidant parameters Malondialdehyde (MDA) and Myeloperoxidase (MPO) were significantly higher compared with the SO group, while the levels of the antioxidant parameters glutathione (GSH), Glutathione peroxidase (GPO), Glutathione reductase (GSHRd), Glutathione S - transferase (GST), and Superoxide dismutase (SOD) were significantly lower. Melatonin, famotidine, mirtazapine, and thiamin pyrophosphate prevented a rise in oxidant parameters and a decrease in antioxidants in ovarian tissue exposed to I/R. However, apart from thiamin pyrophosphate, none of the drugs were able to prevent infertility caused by I/R injury.
Conclusion:
Prevention of ovarian I/R injury-related infertility in rats with induced diabetes is not through antioxidant activity. Thiamine pyrophosphate prevents infertility through an as yet unknown mechanism. This study suggests that thiamine pyrophosphate may be useful in the prevention of I/R-related infertility in diabetics.
Keywords: Diabetes mellitus, Infertility, Injury, Ischemia/reperfusion, Ovary, Rat
Introduction
Diabetes mellitus is a pathology associated with various metabolic diseases with an elevated blood glucose level developing in association with insulin deficiency (1). Diabetes can lead to adverse long and short-term effects in several systems in the body (2). Macro and microvascular complications are known to be more frequent in diabetic patients compared with the normal population (3, 4). Delayed ovulation, delayed menarche, menstrual cycle irregularities, amenorrhea, and infertility are frequently seen in diabetic women (5, 6).
Previous studies have reported lower birth rates among insulin-dependent diabetic women (7). Similarly, a decrease in reproductive functions associated with ovarian function disorders has been shown in female rats with insulin deficiency in an induced diabetic model (8). Compensatory hyper-insulemia developing in association with insulin resistance is a significant risk factor for diabetes (9). It has also been reported that ovarian torsion is frequently seen, particularly in early ages, in polycystic ovary syndrome, shown to be associated with insulin resistance (10). Investigation of such ovarian diseases is recommended in ovarian torsions of unexplained cause, particularly in adolescent girls (10, 11). Ovarian ischemia develops if ovarian torsion is not treated, and this leads to infertility. Early diagnosis and treatment therefore plays an important role in the protection of ovarian functions (12). Treatment of ovarian torsion consists of re-establishment of ovarian blood flow, primarily by surgical methods (13). Ischemia developing in tissues and subsequent reperfusion leads to a rise in the formation of free oxygen radicals. Rising oxygen radicals cause oxidative damage (14). Several experimental studies have investigated the adverse effects of ischemia/reperfusion (I/R) on the ovaries, and different therapeutic agents have been used for the purpose of preventing adverse effects. Thiamin pyrophosphate, melatonin, carnitine, mirtazapine, and famotidine have been reported to prevent oxidative damage induced by I/R in rat ovaries (15–19). However, the effect of these drugs in preventing I/R injury-related infertility in diabetic rats have not been investigated. Oxidative stress plays an important role in the pathogenesis of early and late stage complications associated with diabetes. In diabetes, free radicals lead to complications resulting from an increase in I/R injury (20, 21)
However, studies have reported that antioxidant therapy has not been shown to have a positive effect on reproductive functions in diabetic rats (22). Antioxidant therapy has been reported to be incapable of preventing infertility arising from ovarian I/R injury (23, 24). This information from the literature shows that antioxidant activity is unimportant in the treatment of infertility occurring in diabetics, ovarian I/R, and pathologies in which both are seen together. It is therefore the most successful therapy in preventing infertility in diabetic or non-diabetic I/R injury.
The purpose of this study was to investigate whether melatonin, famotidine, mirtazapine, and thiamine pyrophosphate (MFMTP) affect ovarian I/R injury induced in diabetic rats and whether these effects prevent infertility arising in association with that injury. Another aim was to determine whether or not antioxidant activity is useful in preventing infertility.
Materials and Methods
Animals
All experiments were carried out at the Atatürk University, Faculty of Medicine, Department of Pharmacology. Ninety-six female albino Wistar rats weighing between 190 g and 200 g were used. Rats were obtained from the Atatürk University Medical Experimental Practice and Research Center and housed in randomly selected groups at normal room temperature (22 °C). All studies were performed in accordance with the ethical guidelines set out by the local ethical committee that are fully compatible with the NIH Guide for the Care and Use of Laboratory Animals.
Chemical materials
Thiopental sodium used in the experiment was obtained from IE Ulagay, Turkey; melatonin from Przedsiebiorstwo Farmaceutczne, Poland; famoti-dine from Mustafa Nevzat, Turkey; mirtazapine from Organon Pharmaceuticals, NJ, USA; and thiamine pyrophosphate from Biopharma, Russia.
Diabetic model induction in rats
In order to adapt to their environment, animals were fed in appropriate conditions and at normal room temperature (22°C) in the pharmacology laboratory for a week prior to the experiment. A diabetic model was established using alloxan, which was dissolved in distilled water and injected intraperitoneally for three days consecutively at a dosage of 120 mg/kg. Three days after administration of alloxan, fasting blood sugar was measured from blood samples collected from the tail vein. A commercially available device was used for blood sugar measurement. Animals with blood sugar levels of 250 mg/dL and above were included. Individuals with blood sugar levels above 250 mg/dl were regarded as diabetic (25).
General procedure
Six experimental groups consisting of 16 diabetic rats were established. Basal weights and blood sugar levels of all animals in the study were similar. The groups consisted of a control group to be exposed to I/R (IRC, n : 16) and ovarian I/R + melatonin (IRML, n : 16), ovarian I/R + famotidine (IRFA, n : 16), ovarian I/R + mirtazapine (IRMR, n : 16), ovarian I/R + thiamin pyrophosphate (IRTP, n : 16), and healthy sham operation (SO, n : 16) groups. The procedures were all performed in daytime. Surgical procedures were performed under infertile conditions, in a suitable laboratory environment with intraperitoneal (IP) thiopental sodium anesthesia at 25 mg/kg.
Surgical and Pharmacological Procedures on Rats
Following anesthesia, ovaries in all rats were accessed through a vertical incision, 2-2.5 cm in length, in the lower abdomen. Ischemia was subsequently established with the attachment of arterial clamps for 3 hr to the inferior parts (where the ovary joins the uterus) of the right and left ovaries of the rats in the IRC, IRML, IRFA, IRMR, and IRTP groups. No ischemia was applied to SO group ovaries. Reperfusion was established by releasing the clamps at the end of that period, and the inferior part of the abdomen was closed with sutures. The IRML group was injected with melatonin at 2.5 mg/kg, the IRFA group with famotidine at 20 mg/kg, the IRMR group with mirtazapine at 20 mg/kg and the IRTP group with thiamine pyrophosphate at 20 mg/kg, once a day IP for 10 days before anesthesia and after the I/R procedure. The IRC and SO group rats were administered distilled water in equal volumes by the same route. At the end of the treatment (after 10 days), six rats from all groups were sacrificed with high-dose anesthesia, their ovaries extracted and biochemical examinations performed. Blood sugar levels were measured again before sacrifice. The remaining rats (IRC n : 10, IRML n : 10, IRFA n : 10, IRMR n : 10, IRTP n : 10, SO n : 10) were kept in appropriate laboratory conditions for 3 months in order to breed. Blood sugar levels were measured again at the end of this period; rats that did not become pregnant during this time were regarded as infertile.
Biochemical Analysis
Biochemical analysis of ovarian tissue in rats
In this part, 0.2 mg of whole ovarian tissue was weighed for each ovary. The samples were homogenized in ice with 2-ml buffers (consisting of 0.5% HDTMAB [0.5% hexa desil tri methyl ammonium bromide])[y4] pH: 6 potassium phosphate buffer for myeloperoxidase analyze, consisting of 1.15% potassium chloride solution for thiobarbituric acid reactions (TBARS) analysis and pH: 7.5 phosphate buffer for the superoxide dismutase total glutathione analysis. They were then centrifuged at 4 °C, 10,000 rpm for 15 min. The supernatant part was used as the analysis sample.
Malondialdehyde (MDA) analysis
Concentrations of ovarian lipid peroxidation were determined by estimating MDA using the thiobarbituric acid test (26). The rat ovaries were rinsed with cold saline. The corpus mucosa was scraped, weighed, and homogenized in 10 ml of 100 g/l KCl. The homogenate (0.5 ml) was added to a solution containing 2-thiobarbiturate (1.5 ml of 8 g/l), acetic acid (1.5 ml of 200 g/l), sodium lauryl sulfate (0.2 ml of 80 g/l), and distilled water (0.3 ml). The mixture was incubated at 98°C for 1 hr. n-butanol:pyridine 5 ml (ratio:15:l) was then added. The mixture was vortexed for 1 min and centrifuged for 30 min at 4000 rpm. The absorbance of the supernatant was measured at 532 nm using a spectrophotometer. The standard curve was obtained by using 1,1,3,3- tetramethoxypropane.
Myeloperoxidase (MPO) analysis
The activity of MPO in the total homogenate was measured according to previously described methods (27). The sample was weighed and homogenized in 2 ml of 50 mmol/l phosphate buffer containing 0.5% hexadecyltrimethyl ammonium bromide (HDTMAB) and centrifuged at 3500 rpm for 60 min at 4°C. The supernatant was used to determine MPO activity using 1.3 ml 4-aminoantipyrine-2% phenol (25 mM) solution. 25 mmol/l 4-aminoantipyrine–2% phenol solution and 0.0005% 1.5 ml H2O2 were added and equilibrated for 3–4 min. After establishing the basal rate, a sample suspension (0.2 ml) was added and mixed. Increases in absorbance at 510 nm for 4 min at 0.1-min intervals were recorded. Absorbance was measured at 412 nm.
Total glutathione (tGSH) analysis
The amount of GSH in the total homogenate was measured according to the previously described methods with some modifications (28). The sample was homogenized at pH 7.5, in Tris–HCl buffer (2 ml of 50 mmol/l). The homogenate was precipitated with trichloroacetic acid (0.1 ml of 25%), and the precipitate was removed after centrifugation at 4200 rpm at 4°C for 40 min, and the supernatant was used to measure GSH level. A total of 1500 μl of measurement buffer (200 mmol/l Tris–HCl buffer containing 0.2 mmol/L EDTA at pH 7.5), 500 μl supernatant, 100 μl DTNB (10 mmol/l) and 7900 μl methanol were added to a tube and vortexed and incubated for 30 min in 37 °C. 5,5-Dithiobis (2- nitrobenzoic acid) (DTNB) was used as a chromogen; it formed a yellow-colored complex with sulfhydryl groups. The absorbance was measured at 412 nm using a spectrophotometer (Beckman DU 500, USA). The standard curve was obtained by using reduced glutathione.
Glutathione peroxidase (GPO) analysis
GPO activity was determined according to the method of Lawrence and Burk (29). After tissue homogenization, supernatant was used for GPO measurement. Following the addition of KH2PO4, EDTA, GSH, B-NADPH, NaN3, and GR, the mixture was incubated. As soon as H2O2 was added, chronometer was on, and the absorbance at 340 nm was recorded for 5 min every 15 sec.
Glutathione reductase (GSHRd) analysis
GR activity was determined spectrophotometri-cally by measuring the rate of NADPH oxidation at 340 nm according to Carlberg and Mannervik method (30). After tissue homogenization, supernatant was used for GR measurement. After the NADPH and GSSG addition, chronometer was on and absorbance was measured for 5 min in 30 min intervals at 340 nm spectrophotometrically.
Glutathione S - transferase (GST) activity
GST activity was determined by Habig and Jakoby (31). Enzyme activity was determined in a 4-ml cuvette containing 30 mM GSH, 30 mM 1-chloro-2,6-dinitrobenzene, 0.1 M PBS (pH: 6.5), and tissue homogenate at 340 nm using a spectrophotometer.
Superoxide dismutase (SOD) analysis
Measurements were performed according to the method described by Sun et al (32); when xanthine is converted into uric acid by xanthine oxidase, SOD forms. If nitro blue tetrazolium (NBT) is added to this reaction, SOD reacts with NBT and a purple-colored formazan dye appears. The sample was weighed and homogenized in 2 ml of 20 mmol/l phosphate buffer containing 10 mmol/l EDTA at pH 7.8. The sample was centrifuged at 6000 rpm for 10 min, and the resulting brilliant supernatant was used as assay sample. The measurement mixture containing 2450 μL measurement mixture (0.3 mmol/l xanthine, 0.6 mmol/l EDTA, 150 μmol/l NBT, 0.4 mol/l Na2CO3, 1 g/l bovine serum albumin), 500 μL supernatant, and 50 μl xanthine oxidase (167 U/l) was vortexed. It was then incubated for 10 min. At the end of the reaction, formazan appeared. The absorbance of the purple-colored formazan was measured at 560 nm. The more enzyme present, the less O2− radical that reacts with NBT appears.
Statistical Analyses
Experiment results are expressed as “mean ± standard deviation” (x ± SD). All data were subjected to one-way analysis of variance using Statistical Package for Social Sciences 18.0 (Armonk, NY, USA) software. Differences among groups were obtained using the least significant difference option, and significance was set at P ≤ 0.01.
Results
Blood sugar measurement results
Mean blood sugar levels in the IRC, IRML, IRFA, IRMR, IRTP, and SO groups with a diabetic model induced with alloxan were 275.7 ± 13.5, 278.7 ± 13.5, 286.5 ± 13.9, 268.6 ± 11.2, 274.1 ± 15.4, and 279 ± 13.9 mg/dl, respectively.
Blood sugar levels after 10-day therapy in the IRC, IRML, IRFA, IRMR, IRTP, and SO groups were 279.6 ± 11.6, 284.7 ± 14.2, 289.5 ± 12.9, 271.6 ± 13.3, 281.1 ± 12.8, and 280 ± 12.3 mg/dl, respectively. Blood sugar levels at the end of 3 months were 252.1 ± 12.2, 244.4 ± 12.2, 254.8 ± 11.7, 252.9 ± 12.4, 246.1 ± 10.3, and 249 ± 13.7 mg/dl, respectively.
MDA, MPO, GSH, GPO, GSHRd, GST, and SOD measurement results
Our results showed an MDA concentration of 15.1 ± 1.3 µmol/g protein in ovarian tissue in the I/R control (IRC) group. MDA levels in the IRML, IRFA, IRMR, IRTP, and SO groups were 3.4 ± 0.4, 6.3 ± 0.9, 5.2 ± 0.9, 6.5 ± 1, and 3.2 ± 0.5 µmol/g protein, respectively (Figure 1). The difference between these groups’ MDA levels and that of the IRC group was significant (P<0.01). MPO activity in IRC, IRML, IRFA, IRMR, IRTP, and SO ovarian tissues was 10.5 ± 0.9, 2.4 ± 0.8, 5.2 ± 0.9, 3.9 ± 0.8, 5.6 ± 0.7, and 2.3 ± 0.5 u/g protein, respectively (Figure 2). The difference between these groups’ MPO activities and that of the IRC group was statistically significant (P < 0.01). GSH values for these groups were 1.5 ± 0.4, 11.9 ± 1, 5.3 ± 1, 6.9 ± 2, 5.1 ± 0.7, and 13 ± 1.3 nmol/g protein, respectively (Figure 3), while GPO activity values were 14.1 ± 0.8, 50.9 ± 1.7, 26.1 ± 1.5, 36.8 ± 2.3, 25.5 ± 1.8, and 56.7 ± 1.9 u/g protein (Figure 4). The differences between the groups’ GSH levels and GPO activities and those of the IRC group were again statistically significant (P < 0.01).
Figure 1.
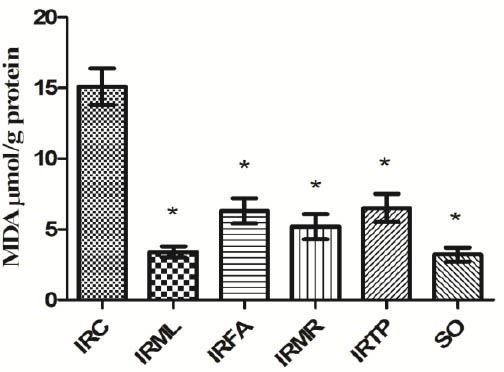
Comparison of the IRC, IRML, IRFA, IRMR, IRTP and SO groups in terms of MDA levels. Differences among groups were obtained using ANOVA post-hoc with the least significant difference option. Bars are means ± SD. MDA levels defined in µmol/g protein. IRC, control group; IRML, ovarian ischemia reperfusion + melatonin; IRFA, ovarian ischemia reperfusion + famotidine; IRMR, ovarian ischemia reperfusion + mirtazapine; IRTP, ovarian ischemia reperfusion + thiamin pyrophosphate groups and SO, healthy sham operation groups.
* P ≤ 0.01 was significant
Figure 2.
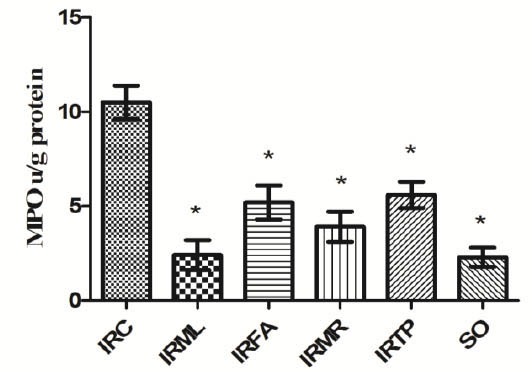
Comparison of the IRC, IRML, IRFA, IRMR, IRTP and SO groups in terms of MPO levels. Differences among groups were obtained using ANOVA post-hoc with the least significant difference option. Bars are means ± SD. MPO levels defined in u/g protein. IRC, control group; IRML, ovarian ischemia reperfusion + melatonin; IRFA, ovarian ischemia reperfusion + famotidine; IRMR, ovarian ischemia reperfusion + mirtazapine; IRTP, ovarian ischemia reperfusion + thiamin pyrophosphate groups and SO, healthy sham operation groups.
* P ≤ 0.01 was significant
Figure 3.
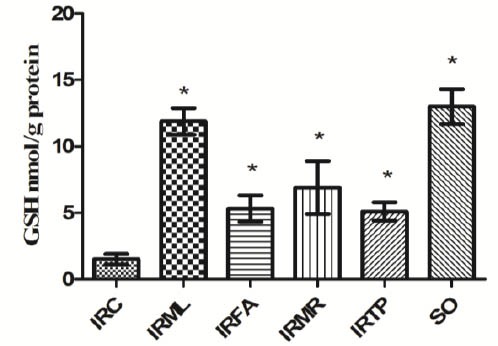
Comparison of the IRC, IRML, IRFA, IRMR, IRTP and SO groups in terms of GSH levels. Differences among groups were obtained using ANOVA post hoc with the least significant difference option. Bars are means ± SD. GSH levels defined in nmol/g protein. IRC, control group; IRML, ovarian ischemia reperfusion + melatonin; IRFA, ovarian ischemia reperfusion + famotidine; IRMR, ovarian ischemia reperfusion + mirtazapine; IRTP, ovarian ischemia reperfusion + thiamin pyrophosphate groups and SO, healthy sham operation groups
* P ≤ 0.01 was significant
Figure 4.
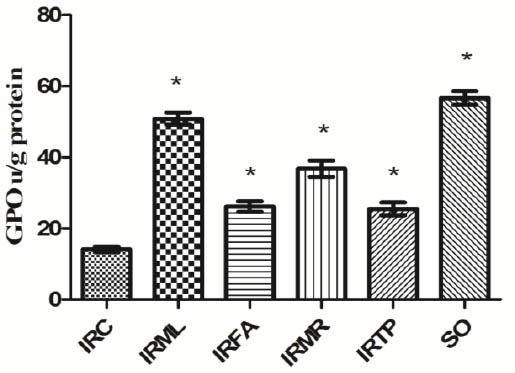
Comparison of the IRC, IRML, IRFA, IRMR, IRTP and SO groups in terms of GPO levels. Differences among groups were obtained using ANOVA post hoc with the least significant difference option. Bars are means ± SD. GPO levels defined in u/g protein. IRC, control group; IRML, ovarian ischemia reperfusion + melatonin; IRFA, ovarian ischemia reperfusion + famotidine; IRMR, ovarian ischemia reperfusion + mirtazapine; IRTP, ovarian ischemia reperfusion + thiamin pyrophosphate groups and SO, healthy sham operation groups
* P ≤ 0.01 was significant
As shown in Figure 5, GSHRd activities in ovarian tissue in the IRC, IRML, IRFA, IRMR, IRTP, and SO group rats were 11.1 ± 0.4, 47.8 ± 0.4, 22.9 ± 3.6, 32.8 ± 1.7, 20.1 ± 1.7, and 50.7 ± 1.6 u/g protein, respectively. The differences between these groups’ GSHRd activities and those of the IRC group were statistically significant (P < 0.01).
Figure 5.
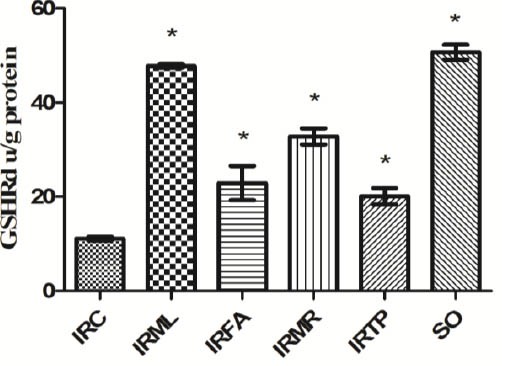
Comparison of the IRC, IRML, IRFA, IRMR, IRTP and SO groups in terms of GSHRd levels. Differences among groups were obtained using ANOVA post hoc with the least significant difference option. Bars are means ± SD. GSHRd levels defined in u/g protein. IRC, control group; IRML, ovarian ischemia reperfusion + melatonin; IRFA, ovarian ischemia reperfusion + famotidine; IRMR, ovarian ischemia reperfusion + mirtazapine; IRTP, ovarian ischemia reperfusion + thiamin pyrophosphate groups and SO, healthy sham operation groups
GST activity in the groups listed was 8.0 ± 1.4, 27.5 ± 1.9, 15.3 ± 2.5, 19.1 ± 2.8, 13.3 ± 2.6, and 31.8 ± 2.6 u/g protein (Figure 6), and SOD activity was 22 ± 2.2, 63.4 ± 3, 35.8 ± 3.2, 48 ± 4.5, 36.5 ± 3.4, and 66.2 ± 2.6 u/g (Figure 7). There were significant differences between the groups’ GST and SOD activities and the IRC group values (P < 0.01).
Figure 6.
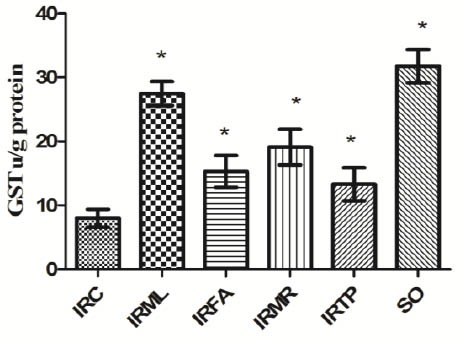
Comparison of the IRC, IRML, IRFA, IRMR, IRTP and SO groups in terms of GST levels. Differences among groups were obtained using ANOVA post hoc with the least significant difference option. Bars are means ± SD. GST levels defined in u/g protein. IRC, control group; IRML, ovarian ischemia reperfusion + melatonin; IRFA, ovarian ischemia reperfusion + famotidine; IRMR, ovarian ischemia reperfusion + mirtazapine; IRTP, ovarian ischemia reperfusion + thiamin pyrophosphate groups and SO, healthy sham operation groups
Figure 7.
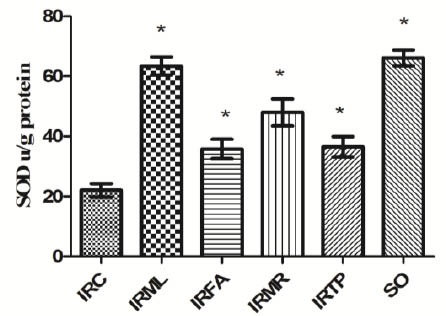
Comparison of the IRC, IRML, IRFA, IRMR, IRTP and SO groups in terms of SOD levels. Differences among groups were obtained using ANOVA post hoc with the least significant difference option. Bars are means ± SD. SOD levels defined in u/g protein. IRC, control group; IRML, ovarian ischemia reperfusion + melatonin; IRFA, ovarian ischemia reperfusion + famotidine; IRMR, ovarian ischemia reperfusion + mirtazapine; IRTP, ovarian ischemia reperfusion + thiamin pyrophosphate groups and SO, healthy sham operation groups
While IRML group MDA and GSH concentrations and MPO, GSHRd, GST, and SOD activities did not differ significantly from those of the SO group (P > 0.01); the differences between the IRFA, IRMR, and IRTP groups were significant (P < 0.01). A significant difference was determined between the IRML group and the other groups in terms of GPO activity (P < 0.01). According to ANOVA, the differences between groups in terms of all oxidant and antioxidant parameters were significant (P < 0.01).
Reproduction results
Reproduction took place in 8 of the 10 animals set aside for that purpose in the IRTP group, compared with 4 out of 10 in the SO group. Reproduction results for the rats in the other groups are given in the Table.
Table.
Reproduction results for rat groups used in the experiment
| Animal groups | Number of animals taken for reproduction | Number of animals giving birth | Number of infertile animals | ||
|---|---|---|---|---|---|
| N | % | N | % | ||
| IRC | 10 | 0 | 0 | 10 | 100 |
| IRML | 10 | 2 | 20 | 8 | 80 |
| IRFA | 10 | 1 | 10 | 9 | 90 |
| IRMR | 10 | 2 | 30 | 8 | 80 |
| IRTP | 10 | 8 | 80 | 2 | 20 |
| SO | 10 | 4 | 40 | 6 | 60 |
Notes: N, number of animals; IRC, control group; IRML, ovarian ischemia reperfusion + melatonin; IRFA, ovarian ischemia reperfusion + famotidine; IRMR, ovarian ischemia reperfusion + mirtazapine; IRTP, ovarian ischemia reperfusion + thiamin pyrophosphate groups and SO, healthy sham operation groups.
Discussion
This study investigated the effects on I/R injury in diabetic rats of MFMTPs which were tested and found to be efficacious in ovarian I/R injury[p5]. It also examined their effects in terms of preventing infertility caused by ovarian I/R injury in diabetic rats. As the study results show, MDA concentration in the IRC group diabetic rat ovarian tissue was significantly higher compared with that in the SO group. MDA is the final product of lipid peroxidation. Free oxygen radicals whose production rises excessively for various reasons, affect membrane lipids containing unsaturated fatty acids more than other biomolecules. Interaction with membrane lipids leads to a rise in membrane permeability and severe cell damage (33, 34). The elevated MDA concentration in the IRC group shows that oxidative stress developed. Ingec M et al reported that more I/R tissue MDA formed compared with healthy tissue (35). Oxidative stress was more pronounced in rats with an induced diabetic model (33, 36). Greater oxidative damage would therefore be expected to develop in a diabetic rat after ovarian I/R (37). The melatonin tested in our study prevented a rise in MDA concentration in ovarian tissue in rats with diabetes induced with I/R in a more statistically significant manner than famotidine, mirtazapine, or thiamine pyrophosphate. Melatonin also suppressed a rise in MPO activity, an oxidant parameter, significantly better than famotidine, mirtazapine, or thiamine pyrophosphate. Production of MPO by neutrophils and macrophages rises in areas of damage established with various agents. MPO catalyzes the reaction between hydrogen peroxide and chlorine, and gives rise to the toxic compound hypochlorous acid. Hypochlorous acid is involved in the formation of the hydroxyl radical (OH) (38, 39). Studies have shown that MPO activity increases more in damaged ovarian tissue with induced I/R compared with healthy tissue (40). These data from the literature are compatible with our own results.
Ovarian tissue GSH levels in the IRC group were statistically significantly lower, compared with those in the SO group. Melatonin best prevented a fall in GSH levels in ovarian tissue injured with induced I/R. GSH is a tripeptide consisting of L-glutamate, cysteine, and glycine. It is also a powerful antioxidant with known involvement in the establishment of cell redox balance (41). A high level of GSH in cells shows cell vitality, while a fall in GSH levels shows weakening of and damage to the cellular defense system (42). In addition to GSH, a significant decrease in GPO activity was observed in IRC group ovarian tissue. There are studies showing that GPO activity decreases significantly in tissue with oxidative stress-related injury compared with healthy tissue (43). GPO detoxifies the hydrogen peroxide radical that forms in the cell by converting it to water and prevents the formation of more toxic products from hydrogen peroxide radical (44). GSH is oxidized during the detoxification of hydrogen peroxide radical. In order for the antioxidant effectiveness of GSH to be maintained, oxidized glutathione (GSSG) must again be converted to reduced (GSH) glutathione. This process is carried out by GSHRd, a NADPH-dependent enzyme (45). GSHRd is known to exhibit higher activity in healthy tissue compared with damaged tissue. GSHRd activity has been shown to decrease in parallel with tissue damage (46).
In our study, GST activity in ovarian tissue exposed to IR was significantly lower than those in the melatonin, famotidine, mirtazapine, thiamine pyrophosphate, and healthy groups. GST binds foreign substances to the –SH group of cysteine in glutathione, thus neutralizing their electrophilic regions and protecting the cells from the harmful effects of foreign substance regions (47). GST activity has been shown to decrease in oxidative tissue injury induced by ischemia (48).
SOD activity in the IRC group subjected to I/R, decreased markedly compared with the control group. SOD is an important enzyme that catalyzes the conversion of superoxide radical into hydrogen peroxide (49). SOD activity in uterine tissue has been reported to decrease in parallel with a rise in DNA injury (50). These data from the literature are compatible with our own results.
The fact that GSH, GPO, GST, GSHRd, and SOD levels in IRML group ovarian tissues were significantly higher compared with the IURC group shows that melatonin possesses powerful antioxidant activity. Melatonin is thought to prevent I/R injury by scavenging hydroxyl, superoxide anion, and peroxyl radicals, directly neutralizing singlet oxygen and raising the levels of the antioxidant parameters GSH, GST, SOD, GPO, and GSHRd (16, 51, 52). Famotidine has been reported to protect against oxidative damage by preventing rising leukocyte infiltration in ovarian tissue with induced I/R (19). Polymorph nuclear leukocytes in the damaged region synthesize toxic oxygen derivatives (53). Mirtazapine has also been shown to alter the oxidant/antioxidant balance in damaged tissue against oxidants (18). Thiamine pyrophosphate has been reported to prevent an increase in MDA concentrations and a decrease in GSH caused by I/R damage in ovarian tissue (54). Our study results and the findings in the literature show that MFMTPs are effective antioxidant agents in the prevention of I/R-associated oxidative damage. However, as discussed above, there are also studies in which antioxidant therapy in diabetic rats has not been shown to have a positive effect on reproductive functions (22). Melatonin can be used for the stimulation of follicular development in animals, to improve oocyte quality, or against damage caused by oxidative stress during pregnancy (54). The inability of the melatonin used in our study and other drugs shown to exhibit antioxidant activities (famotidine, mirtazapine), to prevent ovarian I/R injury-associated infertility in rats with induced diabetes, shows that antioxidant therapy does not have a positive effect on reproductive functions and that our findings are compatible with those in the literature.
Conclusion
The I/R procedure performed in rats with induced diabetes led to oxidative stress in the ovaries and to infertility. The melatonin, famotidine, and mirtazapine prevented I/R damage-related oxidative stress, but not infertility. Although thiamine pyrophosphate exhibited lower antioxidant activity than melatonin and mirtazapine, it prevented infertility caused by oxidative damage. This indicates that the prevention of I/R-related infertility in diabetics is not the result of antioxidant activity and reveals that thiamine pyrophosphate prevents infertility through an as yet unknown mechanism. This study shows that thiamine pyrophosphate may be useful in the prevention of ovarian I/R-related infertility in diabetics. We think that thiamine pyrophosphate should be administered for at least 10 days for that purpose.
Acknowledgement
This research received no specific grants from any funding agency in the public, commercial, or not-for-profit sectors.
Footnotes
Conflict of Interests
All authors declare that they have no conflicts of interest.
References
- 1.Yamane S, Inagaki N, Nihon Rinsho. JPN J Clin Med. 2012;70:846–851. [PubMed] [Google Scholar]
- 2.Nathan DM. Prevention of long-term complications of non-insulin-dependent diabetes mellitus. Clin Invest Med. 1995;18:332–339. [PubMed] [Google Scholar]
- 3.Steiner G. Dyslipoproteinemias in diabetes. Clin Invest Med. 1995;18:282–287. [PubMed] [Google Scholar]
- 4.Tattersall R. Targets of therapy for NIDDM. Diabetes Res Clin Pract. 1995;28:S49–55. doi: 10.1016/0168-8227(95)01086-s. [DOI] [PubMed] [Google Scholar]
- 5.Yeshaya A, Orvieto R, Dicker D, Karp M, Ben-Rafael Z. Menstrual characteristics of women suffering from insulin-dependent diabetes mellitus. Int J Fertil Menopausal Stud. 1995;40:269–273. [PubMed] [Google Scholar]
- 6.Steel JM, Johnstone SD, Corrie JE. Early assessment of gestation in diabetics. Lancet. 1984;2:975–976. doi: 10.1016/s0140-6736(84)91183-8. [DOI] [PubMed] [Google Scholar]
- 7.Pedersen KK, Hagen C, Sando-Pedersen SH, Eshoj O. Ugeskr Laeger. 1994;156:6196–6200. [PubMed] [Google Scholar]
- 8.Tesone M, Ladenheim RG, Oliveira-Filho RM, Chiauzzi VA, Foglia VG, Charreau EH. Ovarian dysfunction in streptozotocin-induced diabetic rats. Proc Soc Exp Biol Med. 1983;174:123–130. doi: 10.3181/00379727-174-41714. [DOI] [PubMed] [Google Scholar]
- 9.Legro RS, Kunselman AR, Dodson WC, Dunaif A. Prevalence and predictors of risk for type 2 diabetes mellitus and impaired glucose tolerance in polycystic ovary syndrome: a prospective, controlled study in 254 affected women. J Clin Endocrinol Metab. 1999;84:165–169. doi: 10.1210/jcem.84.1.5393. [DOI] [PubMed] [Google Scholar]
- 10.Shah AA, Likes CE, Price TM. Early polycystic ovary syndrome as a possible etiology of unexplained premenarcheal ovarian torsion. J Pediatr Adolesc Gynecol. 2009;22:265–269. doi: 10.1016/j.jpag.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 11.Arian A. Acute female pelvic pain. Int J Fertil Steril. 2012;6:26. [Google Scholar]
- 12.Krishnan S, Kaur H, Bali J, Rao K. Ovarian torsion in infertility management - Missing the diagnosis means losing the ovary: A high price to pay. J Hum Reprod Sci. 2011;4:39–42. doi: 10.4103/0974-1208.82359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gaied F, Emil S, Lo A, Baird R, Laberge JM. Laparoscopic treatment of isolated salpingeal torsion in children: case series and a 20-year review of the literature. J Laparoendosc Adv Surg Tech A. 2012;22:941–947. doi: 10.1089/lap.2011.0530. [DOI] [PubMed] [Google Scholar]
- 14.Yılmaz M, Isaoglu U, Cetin N, Turan MI, Suleyman B, Gocer F, et al. Effects of adrenalin on ovarian injury formed by ischemia reperfusion in rats. Lat Am J Pharm. 2012;31:1032–1037. [Google Scholar]
- 15.Cosar E, Sahin FK, Koken G, Toy H, Basarali K, Buyukbas S. The protective effect of alpha-lipoic acid in experimental ovarian ischaemia-reperfusion injury. Aust N Z J Obstet Gynaecol. 2007;47:499–503. doi: 10.1111/j.1479-828X.2007.00787.x. [DOI] [PubMed] [Google Scholar]
- 16.Turkoz Y, Celik O, Hascalik S, Cigremis Y, Hascalik M, Mizrak B, et al. Melatonin reduces torsion-detorsion injury in rat ovary: biochemical and histopathologic evaluation. J Pineal Res. 2004;37:137–141. doi: 10.1111/j.1600-079X.2004.00146.x. [DOI] [PubMed] [Google Scholar]
- 17.Guan Y, Zheng XM, Yang ZW, Li SW. Protective effects of L-carnitine upon testicular ischemia-reperfusion damage in rats. Zhonghua Yi Xue Za Zhi. 2009;89:1858–1861. [PubMed] [Google Scholar]
- 18.Tok A, Sener E, Albayrak A, Cetin N, Polat B, Suleyman B, et al. Effect of mirtazapine on oxidative stress created in rat kidneys by ischemia-reperfusion. Ren Fail. 2012;34:103–110. doi: 10.3109/0886022X.2011.623499. [DOI] [PubMed] [Google Scholar]
- 19.Kurt A, Isaoglu U, Yilmaz M, Calik M, Polat B, Hakan H, et al. Biochemical and histological investigation of famotidine effect on postischemic reperfusion injury in the rat ovary. J Pediatr Surg. 2011;46:1817–1823. doi: 10.1016/j.jpedsurg.2011.04.092. [DOI] [PubMed] [Google Scholar]
- 20.Baynes JW. Role of oxidative stress in development of complications in diabetes. Diabetes. 1991;40:405–412. doi: 10.2337/diab.40.4.405. [DOI] [PubMed] [Google Scholar]
- 21.Baynes JW, Thorpe SR. Role of oxidative stress in diabetic complications: a new perspective on an old paradigm. Diabetes. 1999;48:1–9. doi: 10.2337/diabetes.48.1.1. [DOI] [PubMed] [Google Scholar]
- 22.Tsounapi P, Saito M, Dimitriadis F, Koukos S, Shimizu S, Satoh K, et al. Antioxidant treatment with edaravone or taurine ameliorates diabetes-induced testicular dysfunction in the rat. Mol Cell Biochem. 2012;369:195–204. doi: 10.1007/s11010-012-1382-z. [DOI] [PubMed] [Google Scholar]
- 23.Sugino N, Nakamura Y, Okuno N, Ishimatu M, Teyama T, Kato H. Effects of ovarian ischemia-reperfusion on luteal function in pregnant rats. Biol Reprod. 1993;49:354–358. doi: 10.1095/biolreprod49.2.354. [DOI] [PubMed] [Google Scholar]
- 24.Ruder EH, Hartman TJ, Blumberg J, Goldman MB. Oxidative stress and antioxidants: exposure and impact on female fertility. Hum Reprod Update. 2008;14:345–357. doi: 10.1093/humupd/dmn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jaouhari JT, Lazrek HB, Jana M. The hypoglycemic activity of Zygophyllum gaetulum extracts in alloxan-induced hyperglycemic rats. J Ethnopharmacol. 2000;69:17–20. doi: 10.1016/s0378-8741(99)00064-1. [DOI] [PubMed] [Google Scholar]
- 26.Ohkawa H, Ohishi N, Yagi K. Assay for lipid peroxides in animal tissues by thiobarbituric acid reaction. Anal Biochem. 1979;95:351–358. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 27.Wei H, Frenkel K. In vivoformation of oxidized DNA bases in tumor promoter-treated mouse skin. Cancer Res. 1991;51:4443–4449. [PubMed] [Google Scholar]
- 28.Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Anal Biochem. 1968;25:192–205. doi: 10.1016/0003-2697(68)90092-4. [DOI] [PubMed] [Google Scholar]
- 29.Lawrence RA, Burk RF. Glutathione peroxidase activity in selenium-deficient rat liver. Biochem Biophys Res Commun. 1976;71:952–958. doi: 10.1016/0006-291x(76)90747-6. [DOI] [PubMed] [Google Scholar]
- 30.Carlberg I, Mannervik B. Glutathione reductase. Methods Enzymol. 1985;113:484–490. doi: 10.1016/s0076-6879(85)13062-4. [DOI] [PubMed] [Google Scholar]
- 31.Habig WH, Jakoby WB. Assays for differentiation of glutathione S-transferases. Methods Enzymol. 1981;77:398–405. doi: 10.1016/s0076-6879(81)77053-8. [DOI] [PubMed] [Google Scholar]
- 32.Sun Y, Oberley LW, Li Y. A simple method for clinical assay of superoxide dismutase. Clin Chem. 1988;34:497–500. [PubMed] [Google Scholar]
- 33.Tamaddonfard E, Farshid AA, Asri-Rezaee S, Javadi S, Khosravi V, Rahman B, et al. Crocin improved learning and memory impairments in streptozotocin-induced diabetic rats. Iran J Basic Med Sci. 2013;16:91–100. [PMC free article] [PubMed] [Google Scholar]
- 34.Razavi M, Hosseinzadeh H, Abnous K, Motamedshariaty VS, Imenshahidi M. Crocin restores hypotensive effect of subchronic administration of diazinon in rats. Iran J Basic Med Sci. 2013;16:64–72. [PMC free article] [PubMed] [Google Scholar]
- 35.Ingec M, Calik M, Gundogdu C, Kurt A, Yilmaz M, Isaoglu U, et al. Biological and histopathological investigations of moclobemide on injured ovarian tissue following induction of ischemia-reperfusion in rats. Int J Fertil Steril. 2012;6:19–26. [PMC free article] [PubMed] [Google Scholar]
- 36.Okazaki T, Otani H, Shimazu T, Yoshioka K, Fujita M, Iwasaka T. Ascorbic acid and N-acetyl cysteine prevent uncoupling of nitric oxide synthase and increase tolerance to ischemia/reperfusion injury in diabetic rat heart. Free Radic Res. 2011;45:1173–1183. doi: 10.3109/10715762.2011.605361. [DOI] [PubMed] [Google Scholar]
- 37.Jawerbaum A, Gonzalez ET, Carolina P, Debora S, Christian P, Gimeno MA. Diminished levels of prostaglandin E in type I diabetic oocyte-cumulus complexes. Influence of nitric oxide and superoxide dismutase. Reprod Fertil Dev. 1999;11:105–110. doi: 10.1071/rd99033. [DOI] [PubMed] [Google Scholar]
- 38.Ximenes VF, Paino IM, Faria-Oliveira OM, Fonseca LM, Brunetti IL. Indole ring oxidation by activated leukocytes prevents the production of hypochlorous acid. Braz J Med Biol Res. 2005;38:1575–1583. doi: 10.1590/s0100-879x2005001100003. [DOI] [PubMed] [Google Scholar]
- 39.Van Antwerpen P, Boudjeltia KZ, Babar S, Legssyer I, Moreau P, Moguilevsky N, et al. Thiol-containing molecules interact with the myeloperoxidase/H2O2/chloride system to inhibit LDL oxidation. Biochem Biophys Res Commun. 2005;337:82–88. doi: 10.1016/j.bbrc.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 40.Isaoglu U, Yılmaz M, Sener E, Cetin N, Altuner D, Bilen H, et al. The impaired balances of oxidant/antioxidant and COX-1/COX-2 in ovarian ischemia-reperfusion injury and prevention by nimesulide. Lat Am J Pharm. 2012;31:1481–1488. [Google Scholar]
- 41.Ault JG, Lawrence DA. Glutathione distribution in normal and oxidatively stressed cells. Exp Cell Res. 2003;285:9–14. doi: 10.1016/s0014-4827(03)00012-0. [DOI] [PubMed] [Google Scholar]
- 42.Ballatori N, Hammond CL, Cunningham JB, Krance SM, Marchan R. Molecular mechanisms of reduced glutathione transport: role of the MRP/CFTR/ABCC and OATP/SLC21A families of membrane proteins. Toxicol Appl Pharmacol. 2005;204:238–255. doi: 10.1016/j.taap.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 43.Celebı F, Akbas A, Saglam MB, Kısaoglu A, Alp HH. Effect of sertraline in indomethacin-induced gastric mucosal damage. Asian J Chem. 2012;24:1966–1970. [Google Scholar]
- 44.Valko M, Rhodes CJ, Moncol J, Izakovic M, Mazur M. Free radicals, metals and antioxidants in oxidative stress-induced cancer. Chem Biol Interact. 2006;160:1–40. doi: 10.1016/j.cbi.2005.12.009. [DOI] [PubMed] [Google Scholar]
- 45.Buege JA, Aust SD. Microsomal lipid peroxidation. Methods Enzymol. 1978;52:302–310. doi: 10.1016/s0076-6879(78)52032-6. [DOI] [PubMed] [Google Scholar]
- 46.Polat B, Suleyman H, Alp HH. Adaptation of rat gastric tissue against indomethacin toxicity. Chem Biol Interact. 2010;186:82–89. doi: 10.1016/j.cbi.2010.03.041. [DOI] [PubMed] [Google Scholar]
- 47.Pickett CB, Lu AY. Glutathione S-transferases: gene structure, regulation, and biological function. Annu Rev Biochem. 1989;58:743–764. doi: 10.1146/annurev.bi.58.070189.003523. [DOI] [PubMed] [Google Scholar]
- 48.Mansoorali KP, Prakash T, Kotresha D, Prabhu K, Rama Rao N. Cerebroprotective effect of Eclipta alba against global model of cerebral ischemia induced oxidative stress in rats. Phytomedicine. 2012;19:1108–1116. doi: 10.1016/j.phymed.2012.07.004. [DOI] [PubMed] [Google Scholar]
- 49.Fridovich I. Mitochondria: are they the seat of senescence? Aging Cell. 2004;3:13–16. doi: 10.1046/j.1474-9728.2003.00075.x. [DOI] [PubMed] [Google Scholar]
- 50.Salman S, Kumbasar S, Gursan N, Kumtepe Y, Borekci B, Polat B, et al. Investigation of the relationship of some antihypertensive drugs with oxidant/antioxidant parameters and DNA damage on rat uterus tissue. Int J Fertıl Sterıl. 2011;5:96–103. [PMC free article] [PubMed] [Google Scholar]
- 51.Reiter RJ, Tan DX. Melatonin: a novel protective agent against oxidative injury of the ischemic/reperfused heart. Cardiovasc Res. 2003;58:10–19. doi: 10.1016/s0008-6363(02)00827-1. [DOI] [PubMed] [Google Scholar]
- 52.Reiter RJ, Tan DX, Kim SJ, Qi W. Melatonin as a pharmacological agent against oxidative damage to lipids and DNA. Proc West Pharmacol Soc. 1998;41:229–36. [PubMed] [Google Scholar]
- 53.Grace PA. Ischaemia-reperfusion injury. Br J Surg. 1994;81:637–647. doi: 10.1002/bjs.1800810504. [DOI] [PubMed] [Google Scholar]
- 54.Berlinguer F, Leoni GG, Succu S, Spezzigu A, Madeddu M, Satta V, et al. Exogenous melatonin positively influences follicular dynamics, oocyte developmental competence and blastocyst output in a goat model. J Pineal Res. 2009;46:383–391. doi: 10.1111/j.1600-079X.2009.00674.x. [DOI] [PubMed] [Google Scholar]


