Abstract
Objective(s):
Consumption of high-fat foods is one of the major causes of obesity. Physical exercise is a strategy used to counteract obesity. The aim of this study was to investigate the effect of eight weeks endurance training and high-fat diet (HFD) on appetite-regulating hormones in rat plasma.
Materials and Methods:
Twenty eight male Wistar rats were randomly divided into four groups: Control group with standard diet (CSD), endurance training with a standard diet (ESD), control group with high-fat diet (CHFD) and endurance training with high-fat diet (EHFD). Twenty-four hr after the last training session, the blood samples were obtained and analyzed for hormones levels.
Results:
The significant increased weight gain and food intake and decreased plasma nesfatin-1 and PYY3-36 levels were observed in CHFD group, while exercise under the HFD antagonized these effects. There were no significant changes in ghrelin, insulin and leptin levels in different groups.
Conclusion:
These results suggest that exercise can prevent fattening effect of HFD. Probably, performing exercise makes a reduction of food intake and weight gain in rat via the increase in nesfatin-1 and PYY levels. However, further studies are necessary to understand the exact mechanisms involved in this field.
Keywords: Endurance training, Food intake, High fat diet, Peptide hormones
Introduction
Obesity results from a complex interaction of genetic, behavioural and environmental factors causing an imbalance between energy intake and expenditure. It is well known that diet and exercise are the two main ways to lose weight. Physical exercise can enhance weight loss because it lowers the energetic balance by increasing energy expenditure and reducing food intake. Increase in energy intake is likely to result from changes in the appetite control system toward anorexigenic environment (1-3).
Few studies have measured how exercise impacts on both orexigenic and anorexigenic peptides. In this regard, recent studies have sought to examine how changes in hormones and neuro- peptides regulate appetite and food intake after exercise (4-6). Nesfatin-1, ghrelin and peptide YY (PYY) are peptides that appear to regulate food intake and release in response to changes in energy homeostasis (7, 8). Nesfatin-1 is a potent anorexigenic peptide inducing satiety and strongly inhibits food and water intake and thereby reduces body weight (8, 9). Ghanbari-Niaki and coworkers reported no significant effects of interval or circuit anaerobic exercise on plasma Nesfatin-1 level, but they recommended that probably, a longer exercise protocol with lower intensity which elicited greater total caloric expenditure would have affected nesfatin-1 plasma concentrations (10).
Ghrelin predominantly secreted by endocrine cells in the gastrointestinal tract, transfers information from the stomach to the hypothalamus, and influences growth hormone (GH) release in response to changes in energy homeostasis (11). Plasma ghrelin concentrations rise before meals and decrease following meals, suggesting that ghrelin is orexigenic (appetite stimulating) (4). Ghrelin appears in two major acylated and nonacylated forms. It correlates negatively with body fat mass (12), and is responsive to diet- and exercise induced changes in body mass (13). Broom and colleague, show that plasma acylated ghrelin is suppressed during vigorous treadmill running (5), while another study has reported increases in acylated ghrelin after five consecutive days of aerobic exercise (1 hr/day) (14). The gut hormone PYY has strong appetite-suppressing effects (6). Among the members of the PYY peptide family, PYY3–36 in particular, has been shown to play a major role in appetite control (15). Several studies have reported that plasma PYY concentrations are increased during aerobic exercise both in lean (5, 16) and obese (17), participants. A study found that increase in blood PYY3–36 levels depends on exercise intensity (16).
Studies examining the effects of acute bouts of exercise are informative, but exercise training studies are required to determine the long-term effects of exercise on appetite, food intake and weight control. The objective of this study was to investigate the responses of the main regulatory hormones of food intake to eight weeks endurance training. Thus, we explored whether a HFD paired with endurance training had any effects on food intake, body weight and the plasma levels of nesfatin-1, ghrelin, PYY, leptin, insulin, glucose and lipids profiles in rat.
Materials and Methods
Animals
Male Wistar rats (10 weeks old, 160±10 g body weight) were obtained from Pasteur Institute (Tehran, Iran) and acclimated for at least 1 week prior to experimental use. The animals were housed in normalized light-polyethylene cages, each one in a cage and the exposure to light was regulated in 12 hr intervals; 12 hr (7:00-19:00) of darkness, and 12 hr of light (19:00-7:00), and they were kept in the Animal House. Temperature and humidity were maintained at 25±2°C and 60%±5.0%, respectively. All experiments involving the animals were conducted according to the policy of the Iranian Conversion for the Protection of Vertebrate Animals used for experimental and other scientific purposes and in accordance with the international principles for biomedical research involving animals, revised in 1985.
Experimental design
Twenty-eight male Wistar rats were randomly divided into four groups, each comprising of seven animals: The control group with standard diet (CSD), endurance training group with a standard diet (ESD), control group with High-Fat Diet (CHFD) and endurance training group with high-fat diet (EHFD). The rats in the standard diet group were daily fed with a laboratory chow (containing 407 kcal/100 g total energy, 69.9% carbohydrate, 16.1% protein, and 14% fat based on percentage of total calories; Razi Institute, Iran). The rats in the high-fat diet group were fed with HFD (containing 457kcal/100 g total energy, 49.9% carbohydrate, 13.1% protein, and 37% fat based on percentage of total calories; Razi Institute, Iran) (18). Water was available ad libitum. Each trained rat was exercised between 8 and 10 a.m., 5 days a week for 8 weeks. The rats progressively ran on a motor-driven rodent treadmill from 15 min/day at 15 m/min speed, 0% slope, up to 50 min/day at 25 m/min speed, 0% slope and the control rats were placed on the non-moving treadmill.
Body weight and the food intake
Body weight of the rats in each group was measured and recorded every day. Food intake was estimated daily by differential weighting for each group of seven rats and summed.
Blood samples
Twenty-four hr after the last training session, animals were killed after anesthetizing by ether after an overnight fasting. Blood samples were collected by cardiac puncture in EDTA (3 mg per 1 ml of blood) as the anticoagulant and immediately centrifuged at 1000 rpm for 10 min at 4°C. Plasma were removed and stored in 0.5 ml aliquots at –70oC freezer until biochemical analysis.
Determination of hormonal parameters
Levels of insulin and cortisol were determined by enzyme-linked immunosorbent assay (ELISA) kit from Mercodia Company (Uppsala- Sweden) and Diagnostic Biochem Company (Canada), respectively. Nesfatin-1, acylated ghrelin and leptin plasma levels were measured using Cusabio Company ELISA kit (China) and PYY3-36 level by Phoenix Pharmaceuticals Company ELISA kit (California).
Biochemical parameters assays
Plasma glucose, cholesterol (CS), triglyceride (TG), and high-density-lipoprotein-cholesterol (HDL-C) concentrations were measured using Pars Azmoun Company kit (Tehran-Iran). The procedure of Friedewald et al (19), was used to estimate low-density-lipoprotein-cholesterol (LDL-C) concentration. It was calculated as the difference between CS, HDL-C and TG/5. Interlukin-6 (IL-6) was measured by Diaclone Company ELISA kit (France).
Statistical analysis
All calculations were performed using the Statistical Package for Social Sciences version 18 (SPSS Inc., Chicago, IL, USA). For comparing between groups analysis of variance (ANOVA) test was used following by Tukey post hoc multiple comparison test. P-values less than 0.05 were considered as statistically significant. Data were expressed as mean±SD.
Results
The body weight
Figure 1 displays the weekly body weight of rats in the control and the experimental groups. Our analysis with repeated measures shows that the body weight in all groups increased significantly during the experimental period. The body weight in the EHFD groups significantly decreased between weeks 4 to 8 compared to the CHFD group. Moreover, the body weight in the ESD and EHFD groups significantly decreased at weeks 5 and 6 compared to the CSD group (P<0.05).
Figure 1.
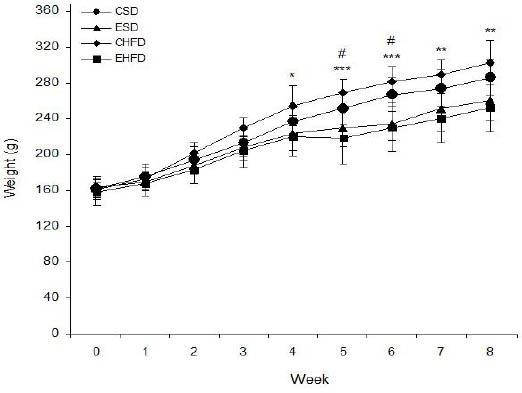
Effect of High-Fat Diet and eight weeks endurance training on the weekly body weight in control and trained rats. Values are expressed as mean±SD (n=7)
* P <0.05, ** P <0.01 and *** P <0.001, CHFD group vs. EHFD group
# P <0.05, ESD and EHFD groups vs. CSD group
CSD: control group with standard diet; ESD: endurance training with a standard diet; CHFD: control group with high-fat diet; EHFD: endurance training with high-fat diet
The total food intake
Figure 2 displays the effect of HFD and eight weeks endurance training on the total food intake in the control and the experimental groups. ANOVA test shows that the food intake in the CHFD group significantly increased compared to the CSD group but significantly decreased in the EHFD group compared to the CHFD group (P<0.05).
Figure 2.
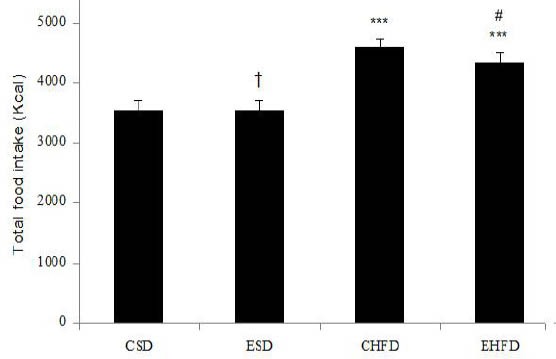
Effect of high-fat diet and eight weeks endurance training on the total food intake (Kcal) in control and trained rats. Values are expressed as mean±SD (n=7). ***P<0.001 vs. CSD group;
# P <0.05 vs. CHFD group; † P <0.05 vs. CHFD and EHFD groups
CSD: control group with standard diet; ESD: endurance training with a standard diet; CHFD: control group with high-fat diet; EHFD: endurance training with high-fat diet
The effect of HFD and eight weeks endurance training on hormonal parameters in different groups is presented in Figure 3. Plasma nesfatin-1 level in the CHFD group (14.32±1.06 pg/ml) significantly decreased compared to the CSD (22.91±3.81 pg/ml) and ESD (24.67±4.19 pg/ml) groups (P<0.01), while it did not significantly differ in the EHFD group (19.37±3.57 pg/ml) from that of the standard diet group. Nesfatin-1 level in the EHFD groups significantly increased in comparison with the CHFD group (P<0.05). Plasma PYY3-36 level in CHFD (0.55±0.11 ng/ml) and EHFD (0.7±0.1 ng/ml) groups were lower than CSD (0.85±0.06 ng/ml) and ESD (0.89±0.05 ng/ml) groups. PYY3-36 level in EHFD group was higher than CHFD group (P<0.05). Cortisol concentration in CHFD (15.83±2.09 µg/dl) and EHFD (13.93±3.57 µg/dl) groups were significantly higher than CSD (8.52±1.57 µg/dl) and ESD (8.77±1.97 µg/dl) groups. No significant differences in acylated ghrelin, insulin and leptin levels were observed between groups.
Figure 3.
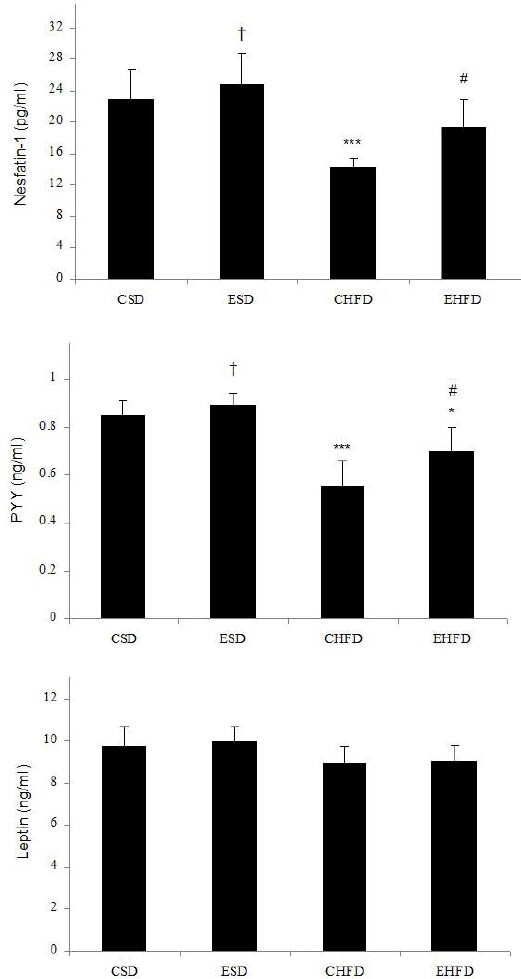
Effect of High-Fat Diet and eight weeks endurance training on hormonal parameters in control and trained rats. Values are expressed as mean±SD (n=7). * P <0.05, ** P <0.01 and *** P <0.001 vs. CSD group; # P<0.05 vs. CHFD group; † P <0.05 vs. CHFD and EHFD groups
CSD: control group with standard diet; ESD: endurance training with a standard diet; CHFD: control group with high-fat diet; EHFD: endurance training with high-fat diet
Plasma biochemical parameters
The effect of HFD and eight weeks endurance training on biochemical parameters in different groups are summarized in Table 1. Glucose, TG, Cholesterol and LDL-C levels in CHFD group were higher than CSD group. These parameters in EHFD group were significantly lower than CHFD group. There were no significant changes in HDL and IL-6 levels in different groups.
Table 1.
Effect of high-fat diet and eight weeks endurance training on biochemical parameters in control and trained rats
| Parameters | CSD | ESD | CHFD | EHFD |
|---|---|---|---|---|
| Glucose (mg/dl) | 138.14±12.08 | 125.43±10.82† | 174.06±10.31*** | 155.33±10.34*# |
| Triglyceride (mg/dl) | 57.85±3.13 | 46.98±2.92*† | 72.02±9.54** | 57.24±7.65# |
| Cholesterol (mg/dl) | 84.85±7.75 | 79.71±6.92 | 98.86 ±13.12* | 84.96±8.65# |
| HDL-C (mg/dl) | 29.02±3.59 | 30.27±4.18 | 26.28±4.79 | 28.41±3.14 |
| LDL-C (mg/dl) | 44.26±3.53 | 40.04±2.16 | 58.17±6.42*** | 45.11±4.21# |
| IL-6 (pg/ml) | 34.37±6.52 | 46.28±15.56 | 31.33±9.57 | 35.57±7.45 |
Values are expressed as mean±SD (n=7). *P<0.05, **P<0.01 and ***P<0.001 vs. CSD group; #P<0.05 vs. CHFD group; †P<0.05 vs. CHFD and EHFD groups. HDL-C: High-density-lipoprotein-cholesterol; LDL-C: low-density-lipoprotein-cholesterol; IL-6: Interlukin-6 CSD: control group with standard diet; ESD: endurance training with a standard diet; CHFD: control group with high-fat diet; EHFD: endurance training with high-fat diet
Plasma parameters Correlations
The results of the multivariate analysis of variance (MANOVA) of plasma hormonal and biochemical parameters presented in Table 2. The results showed that diet (Wilks’ λ -0.039, F=26.97, P<0.001) and exercise (Wilks’ λ -0.101, F=9.70, P <0.001) have significant effect on plasma hormonal and biochemical parameters. Furthermore, the interaction of diet and exercise (Wilks’ λ -0.211, F=4.07, P<0.01) has a synergistic effect on the plasma hormonal and biochemical parameters.
Table 2.
Results of the multivariate analysis of variance (MANOVA) of the effect of independent variables on plasma hormonal and plasma biochemical parameters
| Source | Wilks’ λ | F | Hypothesis df | Error df | P-value | ||
|---|---|---|---|---|---|---|---|
| Wilks’ Lambda | intercept | 0.001 | 750.93 | 11.00 | 12.00 | < 0.001 | |
| diet | HFD SD |
0.039 | 26.97 | 11.00 | 12.00 | < 0.001 | |
| exercise | yes no |
0.101 | 9.70 | 11.00 | 12.00 | <0.001 | |
| diet* exercise | 0.211 | 4.07 | 11.00 | 12.00 | 0.01 | ||
a. Exact statistic
b. The statistic is an upper bound on F that yields a lower bound on the significance level
c. Design: Intercept + diet + exercise + diet* exercise
Discussion
The results of this study showed that the body weight gain and food intake in the HFD groups significantly increased and exercise suppressed these effects. These findings are in agreement with the studies of Ebal et al (2), and Elj et al (3), which showed reduction in weight gain and food intake during five weeks of moderate strength exercise in rats. This significant lower body weight in trained rats may be due to the changed body composition by reducing fat mass as a result of exercise training (2, 3, 20), and negative energy balance linked with increased energy expenditure during the exercise (1). Chaolu et al, showed that the food intake of the mice in the exercise groups significantly increased as compared to the non-exercise groups (21). It is believed that exercise increases appetite and the need for energy intake.
In this study, the decreased nesfatin-1 level and increased glucose level in HFD group were observed in comparison to the standard diet group, while exercise under the High-Fat Diet antagonized these effects. These findings are in agreement with the results of Chaolu et al (21), that showed four weeks of endurance training increased plasma nesfatin-1 level. Nesfatin-1, as a neuropeptide, involved in metabolic regulation and feeding behavior (8, 22). The intraperitoneal administration of nesfatin-1, inhibits food intake and thereby reduces body weight (9). In addition, Su et al have reported that the intravenous administration of nesfatin-1 reduces the blood glucose level in hyperglycemic db/db mice (23). Furthermore, the fasting levels of nesfatin-1 are significantly lower in type 2 diabetes mellitus patients than in healthy subjects (24).
Two important tonic satiety signals are insulin (released from the pancreas) and leptin (released from adipose tissue). These hormones assist in the regulation of energy balance over the long term. High concentrations of these hormones in the blood suppress appetite (4). In the present study, there were no significant changes in insulin and leptin levels in different groups. A number of studies have reported either no effect of training on leptin concentrations with short-term training (<12 weeks), or a reduction in leptin levels in long-term training (>12 weeks) (4, 25, 26). This effect could be explained by the fact that subjects were not in a fasting state and it led to the release of insulin, which is known to exert a negative feedback over leptin secretion (4). Ghrelin is present in circulation in acylated and desacyl forms, but only acylated ghrelin is thought to cross the blood-brain barrier and thus it is essential for appetite regulation. In addition, acylated ghrelin responds more rapidly to glucose infusion and exercise (12). In the present study, no significant difference was observed in acylated ghrelin level between groups. Previous studies have observed reduction (5, 27, 28), increment (12, 29, 30), or no alteration (31-34), in both total ghrelin and acylated ghrelin concentrations following exercise. However, these inconsistent findings may be due to the intensity (or energy cost) of the exercise employed and/or the sex of the exerciser (12).
Glucocorticoids were shown to inhibit ghrelin secretion (2). Exercise can cause a change in the secretion of cortisol hormone (35-37). The results of this study showed that cortisol level in HFD group increased significantly compared with the standard diet group. However, in the exercise groups, the plasma cortisol level did not differ significantly between the HFD and standard diet groups. Hazar and colleagues reported that cortisol level did not alter after doing some maximum aerobic exercises (38). Hejazi and Attarzadeh Hosseini showed that cortisol level significantly decreased during preparation phase, while it increased during pre-competition phase (36).
Exercise may increase the release of IL-6 from contracting muscles, and this release may induce multiple effects in different tissues. IL-6 possesses somewhat catabolic features, indicated by the ability to increase energy expenditure, lipolysis, and cortisol level (11, 39). In this study, no significant change in IL-6 level was observed in different groups (Table 2). Conn et al reported the rise of serum cortisol level in hamsters without any change in IL-6 level after exercise (40). Minetto et al found that the cortisol response to the exercise was not related to the amount of circulating IL-6 in elite power and endurance athletes (41). Stewart et al demonstrated that a 12-week combined aerobic and resistance training program had no effect on fasting IL-6 in healthy previously physically active and inactive subjects (42). Likewise, no change in IL-6 was observed after 2 and 6 weeks of high volume training in rowers (11, 43).
Plasma PYY concentrations are suppressed in fasting and elevated postprandial. It regulates body weight by reducing food intake and increasing energy expenditure (6, 7). Fasting circulating levels of total PYY are reported to be low in mice subjected to High-Fat Feeding (44, 45). Both forms of PYY (PYY3-36 and PYY1-36) are thought to serve as satiety signals, regulating the termination of individual meals (12). Results of this study showed that the plasma PYY3-36 level in HFD groups significantly decreased compared to the standard diet groups, while exercising under the HFD, antagonized this significant decrease (Figure 3). Therefore, HFD probably decreases PYY level and results in hyperphagia in rats and finally leads to obesity in animals. Several previous studies have found elevations in PYY following aerobic exercise (16, 27, 46). Our finding on PYY and Nesfatin-1 levels showed high differences between the CHDF and EHFD groups. However, at the 8th week, body weights were not significantly different between the CHFD and CSD groups. Thus, it seems that the hormones reflect food intake differences more clearly than body weight changes. Ueda et al, demonstrated that circulating levels of PYY3–36 rose with the increment of exercise intensity (17).
Endurance exercise is widely recommended in the treatment paradigm of various hyperlipoproteinemias for its putative antiatherogenic action on circulating lipids (47). The results of this study have shown that plasma TG, CS, and LDL-C levels in HFD group significantly increased as compared with the standard diet group, while these parameters decreased after endurance training (Table 1). However, several reports revealed that exercise training significantly increased HDL level (48, 49). Greene et al, demonstrated that a single exercise session on a treadmill was associated with improved blood lipids and lipoproteins in obese adults (50). In addition, Huffman et al demonstrated that, independent of diet, exercise had beneficial effects on LDL-C particle number, LDL-C size, HDL-C level, HDL-C size, and TG level (51).
Conclusion
Our findings showed that exercise in rat can counteract weight gain caused by a HFD. The endurance training may produce a reduction of food intake and weight gain via the increase in nesfatin-1 and PYY levels. The key findings of this study is that exercise training (for 8 weeks) attenuated the HFD induced increase in body weight which may be due to the increase in energy expenditure and a decrease in energy intake. Moreover, these findings may be related to an exercise mediated preservation of PYY3-36 and nesfatin-1. The greatest effects of exercise were found in the HFD-fed rats, where exercise normalized some parameters (body weight) and ameliorated others, while the effects of exercise within the standard-diet group were minimal. However, additional research is required to further clarify the present research findings and completely understanding their implications and also application of them in athletic training monitoring.
Acknowledgment
This work was supported by a grant from Exercise Physiology Research Center, Baqiyatallah University of Medical Sciences, Tehran, Iran.
Footnotes
Conflict of Interests
Authors have no conflict of interests.
References
- 1.Bi S, Scott KA, Hyun J, Ladenheim EE, Moran TH. Running wheel activity prevents hyperphagia and obesity in Otsuka long-evans Tokushima Fatty rats: role of hypothalamic signaling. Endocrinology. 2005;146:1676–1685. doi: 10.1210/en.2004-1441. [DOI] [PubMed] [Google Scholar]
- 2.Ebal E, Cavalie H, Michaux O, Lac G. Effect of a moderate exercise on the regulatory hormones of food intake in rats. Appetite. 2007;49:521–524. doi: 10.1016/j.appet.2007.03.007. [DOI] [PubMed] [Google Scholar]
- 3.Elj NE, Lac G, Tabka Z, Gharbi N, Fezaa SE. Effect of physical exercise on reducing food intake and weight gain. Procedia Soc Behav Sci. 2011;30:2027–2031. [Google Scholar]
- 4.Bilski J, Teleglow A, Zahradnik-Bilska J, Dembinski A, Warzecha Z. Effects of exercise on appetite and food intake regulation. Med Sport. 2009;13:82–94. [Google Scholar]
- 5.Broom DR, Stensel DJ, Bishop NC, Burns SF, Miyashita M. Exercise-induced suppression of acylated ghrelin in humans. J Appl Physiol. 2007;102:2165–2171. doi: 10.1152/japplphysiol.00759.2006. [DOI] [PubMed] [Google Scholar]
- 6.Stensel D. Exercise, appetite and appetite-regulating hormones: implications for food intake and weight control. Ann Nutr Metab. 2010;57:36–42. doi: 10.1159/000322702. [DOI] [PubMed] [Google Scholar]
- 7.Li J-B, Asakawa A, Li Y, Cheng K, Inui A. Effects of exercise on the levels of peptide YY and ghrelin. Exp Clin Endocrinol Diabetes. 2011;119:163–166. doi: 10.1055/s-0030-1262790. [DOI] [PubMed] [Google Scholar]
- 8.Pałasz A, Krzystanek M, Worthington J, Czajkowska B, Kostro K, Wiaderkiewicz R, et al. Nesfatin-1, a unique regulatory neuropeptide of the brain. Neuropeptides. 2012;46:105–112. doi: 10.1016/j.npep.2011.12.002. [DOI] [PubMed] [Google Scholar]
- 9.Shimizu H, Oh S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, et al. Peripheral administration of nesfatin-1 reduces food intake in mice: the leptin-independent mechanism. Endocrinology. 2009;150:662–671. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 10.Ghanbari-Niaki A, Kraemer RR, Soltani R. Plasma nesfatin-1 and glucoregulatory hormone responses to two different anaerobic exercise sessions. Eur J Appl Physiol. 2010;110:863–868. doi: 10.1007/s00421-010-1531-6. [DOI] [PubMed] [Google Scholar]
- 11.Jurimae J, Maestu J, Jurimae T, Mangus B, von Duvillard SP. Peripheral signals of energy homeostasis as possible markers of training stress in athletes: a review. Metabolism. 2011;60:335–350. doi: 10.1016/j.metabol.2010.02.009. [DOI] [PubMed] [Google Scholar]
- 12.Larson-Meyer DE, Palm S, Bansal A, Austin KJ, Hart AM, Alexander BM. Influence of running and walking on hormonal regulators of appetite in women. J Obes. 2012;2012:730409. doi: 10.1155/2012/730409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Leidy HJ, Dougherty KA, Frye BR, Duke KM, Williams NI. Twenty-four-hour ghrelin is elevated after calorie restriction and exercise training innon-obese women. Obesity (Silver Spring) 2007;15:446–455. doi: 10.1038/oby.2007.542. [DOI] [PubMed] [Google Scholar]
- 14.Mackelvie KJ, Meneilly GS, Elahi D, Wong AC, Barr SI, Chanoine J-P. Regulation of appetite in lean and obese adolescents after exercise: role of acylated and desacyl ghrelin. J Clin Endocrinol Metab. 2007;92:648–654. doi: 10.1210/jc.2006-1028. [DOI] [PubMed] [Google Scholar]
- 15.Chelikani PK, Haver AC, Reidelberger RD. Comparison of the inhibitory effects of PYY (3-36) and PYY (1-36) on gastric emptying in rats. Am J Physiol Regul Integr Comp Physiol. 2004;287:R1064–R1070. doi: 10.1152/ajpregu.00376.2004. [DOI] [PubMed] [Google Scholar]
- 16.Ueda S-y, Yoshikawa T, Katsura Y, Usui T, Nakao H, Fujimoto S. Changes in gut hormone levels and negative energy balance during aerobic exercise in obese young males. J Endocrinol. 2009;201:151–159. doi: 10.1677/JOE-08-0500. [DOI] [PubMed] [Google Scholar]
- 17.Ueda S-y, Yoshikawa T, Katsura Y, Usui T, Fujimoto S. Comparable effects of moderate intensity exercise on changes in anorectic gut hormone levels and energy intake to high intensity exercise. J Endocrinol. 2009;203:357–364. doi: 10.1677/JOE-09-0190. [DOI] [PubMed] [Google Scholar]
- 18.Hariri N, Thibault L. High-fat diet-induced obesity in animal models. Nutr Res Rev. 2010;23:270–299. doi: 10.1017/S0954422410000168. [DOI] [PubMed] [Google Scholar]
- 19.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 20.MacLean PS, Higgins JA, Wyatt HR, Melanson EL, Johnson GC, Jackman MR, et al. Regular exercise attenuates the metabolic drive to regain weight after long-term weight loss. Am J Physiol Regul Integr Comp Physiol. 2009;297:R793–R802. doi: 10.1152/ajpregu.00192.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chaolu H, Asakawa A, Ushikai M, Li Y-X, Cheng K-C, Li J-B, et al. Effect of exercise and high-fat diet on plasma adiponectin and nesfatin levels in mice. Exp Ther Med. 2011;2:369–373. doi: 10.3892/etm.2011.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, et al. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–1301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- 23.Su Y, Zhang J, Tang Y, Bi F, Liu J-N. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun. 2010;391:1039–1042. doi: 10.1016/j.bbrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 24.Li Q-C, Wang H-Y, Chen X, Guan H-Z, Jiang Z-Y. Fasting plasma levels of nesfatin-1 in patients with type 1 and type 2 diabetes mellitus and the nutrient-related fluctuation of nesfatin-1 level in normal humans. Regul Pept. 2010;159:72–77. doi: 10.1016/j.regpep.2009.11.003. [DOI] [PubMed] [Google Scholar]
- 25.Martins C, Morgan L, Truby H. A review of the effects of exercise on appetite regulation: an obesity perspective. Int J Obes (Lond) 2008;32:1337–1347. doi: 10.1038/ijo.2008.98. [DOI] [PubMed] [Google Scholar]
- 26.Ramezankhany A, Nazar AP, Hedayati M. Comparing effects of aerobics, pilates exercises and low calorie diet on leptin levels and lipid profiles in sedentary women. Iran J Basic Med Sci. 2011;14:256–263. [Google Scholar]
- 27.Broom DR, Batterham RL, King JA, Stensel DJ. Influence of resistance and aerobic exercise on hunger, circulating levels of acylated ghrelin, and peptide YY in healthy males. Am J Physiol Regul Integr Comp Physiol. 2009;296:R29–R35. doi: 10.1152/ajpregu.90706.2008. [DOI] [PubMed] [Google Scholar]
- 28.King JA, Wasse LK, Stensel DJ. The acute effects of swimming on appetite, food intake, and plasma acylated ghrelin. J Obes. 2010;2011:351628. doi: 10.1155/2011/351628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Christ ER, Zehnder M, Boesch C, Trepp R, Mullis PE, Diem P, et al. The effect of increased lipid intake on hormonal responses during aerobic exercise in endurance-trained men. Eur J Endocrinol. 2006;154:397–403. doi: 10.1530/eje.1.02106. [DOI] [PubMed] [Google Scholar]
- 30.Russell M, Misra M. Influence of ghrelin and adipocytokines on bone mineral density in adolescent female athletes with amenorrhea and eumenorrheic athletes. Med Sport Sci. 2010;55:103–113. doi: 10.1159/000321975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Erdmann J, Tahbaz R, Lippl F, Wagenpfeil S, Schusdziarra V. Plasma ghrelin levels during exercise effects of intensity and duration. Regul Pept. 2007;143:127–135. doi: 10.1016/j.regpep.2007.05.002. [DOI] [PubMed] [Google Scholar]
- 32.Ghahraman M, Rohani H. AG. The acute effects of aerobic and resistance exercise on plasma acylated ghrelin and hungerin overweight men. World Appl Sci J. 2013;21:888–893. [Google Scholar]
- 33.King JA, Wasse LK, Broom DR, Stensel DJ. Influence of brisk walking on appetite, energy intake, and plasma acylated ghrelin. Med Sci Sports Exerc. 2010;42:485–492. doi: 10.1249/MSS.0b013e3181ba10c4. [DOI] [PubMed] [Google Scholar]
- 34.Ebrahimi M, Rahmani-Nia F, Damirchi A, Mirzaie B, Pur SA. Effect of short-term exercise on appetite, energy intake and energy-regulating hormones. Iran J Basic Med Sci. 2013;16:829–834. [PMC free article] [PubMed] [Google Scholar]
- 35.Dimitriou L, Sharp N, Doherty M. Circadian effects on the acute responses of salivary cortisol and IgA in well trained swimmers. Br J Sports Med. 2002;36:260–264. doi: 10.1136/bjsm.36.4.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hejazi K, Hosseini S-RA. Influence of selected exercise on serum immunoglobulin, testosterone and cortisol in semi-endurance elite runners. Asian J Sports Med. 2012;3:185–192. [PMC free article] [PubMed] [Google Scholar]
- 37.McGuigan MR, Egan AD, Foster C. Salivary cortisol responses and perceived exertion during high intensity and low intensity bouts of resistance exercise. J Sports Sci Med. 2004;3:8–15. [PMC free article] [PubMed] [Google Scholar]
- 38.Hazar S, Hazar M, Korkmaz S, Bayil S, Gurkan A. The effect of graded maximal aerobic exercise on some metabolic hormones, muscle damage and some metabolic end products in sportsmen. Sci Res Essays. 2011;6:1337–1343. [Google Scholar]
- 39.Fischer CP. Interleukin-6 in acute exercise and training: what is the biological relevance. Exerc Immunol Rev. 2006;12:6–33. [PubMed] [Google Scholar]
- 40.Conn CA, Kozak WE, Tooten P, Niewold TA, Borer KT, Kluger MJ. Effect of exercise and food restriction on selected markers of the acute phase response in hamsters. J Appl Physiol. 1995;78:458–465. doi: 10.1152/jappl.1995.78.2.458. [DOI] [PubMed] [Google Scholar]
- 41.Minetto M, Rainoldi A, Gazzoni M, Ganzit G, Saba L, Paccotti P. Interleukin-6 response to isokinetic exercise in elite athletes: relationships to adrenocortical function and to mechanical and myoelectric fatigue. Eur J Appl Physiol. 2006;98:373–382. doi: 10.1007/s00421-006-0285-7. [DOI] [PubMed] [Google Scholar]
- 42.Stewart LK, Flynn MG, Campbell WW, Craig BA, Robinson JP, Timmerman KL, et al. The influence of exercise training on inflammatory cytokines and C-reactive protein. Med Sci Sports Exerc. 2007;39:1714–1719. doi: 10.1249/mss.0b013e31811ece1c. [DOI] [PubMed] [Google Scholar]
- 43.Ramson R, Jurimae J, Jurimae T, Maestu J. The influence of increased training volume on cytokines and ghrelin concentration in college level male rowers. Eur J Appl Physiol. 2008;104:839–846. doi: 10.1007/s00421-008-0839-y. [DOI] [PubMed] [Google Scholar]
- 44.Yang N, Wang C, Xu M, Mao L, Liu L, Sun X. Interaction of dietary composition and PYY gene expression in diet-induced obesity in rats. J Huazhong Univ Sci Technolog Med Sci. 2005;25:243–246. doi: 10.1007/BF02828131. [DOI] [PubMed] [Google Scholar]
- 45.Sloth B, Holst JJ, Flint A, Gregersen NT, Astrup A. Effects of PYY1–36 and PYY3–36 on appetite, energy intake, energy expenditure, glucose and fat metabolism in obese and lean subjects. Am J Physiol Endocrinol Metab. 2007;292:E1062–E8. doi: 10.1152/ajpendo.00450.2006. [DOI] [PubMed] [Google Scholar]
- 46.Cooper J, Watras A, Paton C, Wegner F, Adams A, Schoeller D. Impact of exercise and dietary fatty acid composition from a high-fat diet on markers of hunger and satiety. Appetite. 2011;56:171–178. doi: 10.1016/j.appet.2010.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Crouse SF, O’Brien BC, Grandjean PW, Lowe RC, Rohack JJ, Green JS. Effects of training and a single session of exercise on lipids and apolipoproteins in hypercholesterolemic men. J Appl Physiol. 1997;83:2019–2028. doi: 10.1152/jappl.1997.83.6.2019. [DOI] [PubMed] [Google Scholar]
- 48.Lee M-G, Park K-S, Kim D-U, Choi S-M, Kim H-J. Effects of high-intensity exercise training on body composition, abdominal fat loss, and cardiorespiratory fitness in middle-aged Korean females. Appl Physiol Nutr Metab. 2012;37:1019–1027. doi: 10.1139/h2012-084. [DOI] [PubMed] [Google Scholar]
- 49.Nounou HA, Deif MM, Shalaby MA. Effect of flaxseed supplementation and exercise training on lipid profile, oxidative stress and inflammation in rats with myocardial ischemia. Lipids Health Dis. 2012;11:129. doi: 10.1186/1476-511X-11-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Greene NP, Fluckey JD, Lambert BS, Greene ES, Riechman SE, Crouse SF. Regulators of blood lipids and lipoproteins? PPARδ and AMPK, induced by exercise, are correlated with lipids and lipoproteins in overweight/obese men and women. Am J Physiol Endocrinol Metab. 2012;303:E1212–E1221. doi: 10.1152/ajpendo.00309.2012. [DOI] [PubMed] [Google Scholar]
- 51.Huffman KM, Hawk VH, Henes ST, Ocampo CI, Orenduff MC, Slentz CA, et al. Exercise effects on lipids in persons with varying dietary patterns-does diet matter if they exercise? Responses in Studies of a Targeted Risk Reduction Intervention through Defined Exercise I. Am Heart J. 2012;164:117–124. doi: 10.1016/j.ahj.2012.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]


