Abstract
Lower heart rate is associated with better survival in patients with multiple organ dysfunction syndrome (MODS), a disease mostly caused by sepsis. The benefits of heart rate reduction by ivabradine during MODS are currently being investigated in the MODIfY clinical trial. Ivabradine is a selective inhibitor of the pacemaker current If and since If is impaired by lipopolysaccharide (LPS, endotoxin), a trigger of sepsis, we aimed to explore If blocking potency of ivabradine under elevated endotoxin levels in human atrial cardiomyocytes. Treatment of myocytes with S-LPS (containing the lipid A moiety, a core oligosaccharide and an O-polysaccharide chain) but not R595 (an O-chain lacking LPS-form) caused If inhibition under acute and chronic septic conditions. The specific interaction of S-LPS but not R595 to pacemaker channels HCN2 and HCN4 proves the necessity of O-chain for S-LPS–HCN interaction. The efficacy of ivabradine to block If was reduced under septic conditions, an observation that correlated with lower intracellular ivabradine concentrations in S-LPS- but not R595-treated cardiomyocytes. Computational analysis using a sinoatrial pacemaker cell model revealed that despite a reduction of If under septic conditions, ivabradine further decelerated pacemaking activity. This novel finding, i.e. If inhibition by ivabradine under elevated endotoxin levels in vitro, may provide a molecular understanding for the efficacy of this drug on heart rate reduction under septic conditions in vivo, e.g. the MODIfY clinical trial.
Keywords: Human pacemaker current, HCN channel, Ivabradine, Lipopolysaccharide, Patch clamp, Sinoatrial cell model
Highlights
-
•
S-LPS impairs If via interaction of its O-chain to HCN channels.
-
•
Efficacy of ivabradine for If blockage is reduced under elevated endotoxin levels.
-
•
S-LPS reduces intracellular ivabradine concentrations.
-
•
Ivabradine is efficient to decelerate sinoatrial pacemaking activity under septic conditions in silico.
1. Introduction
Sepsis, a microbial induced inflammatory response, encompasses a spectrum of illness that ranges from systemic infection to multiple organ dysfunction syndrome (MODS) [1]. Mortality due to septic cardiac dysfunction has been encountered in critically ill patients [2] where an increased heart rate may act as an independent risk factor [3]. Patients with lower heart rate in the early phase of MODS have better survival rates than those with higher heart rate [3].
Sepsis is induced by lipopolysaccharide (LPS, endotoxin), a major component of the outer cell wall of gram-negative bacteria. Notably, LPS from wild type bacteria usually is a mix of S-form LPS and varying proportions of so-called R-form LPS [4]. S-form LPS consists of three entities: the lipid A moiety, that harbours the endotoxic activity of the entire molecule, the core oligosaccharide and the O-polysaccharide chain, that is absent in R-form LPS [5,6]. Irrespective of their structural components, both LPS forms are capable to initiate sepsis by triggering the inflammatory response [7]. Besides initiating the inflammatory response, S-form LPS directly affects ionic channels of immune, neuronal and cardiovascular cells [8–10], the latter include channels conducting the pacemaker current If. This current is a mixed Na+/K+ inward current carried by pacemaker channels comprising four different homo- or heteromeric isoforms of hyperpolarization-activated cyclic nucleotide-gated (HCN 1–4) channels [11]. If plays an important role in the regulation of heart rate [12] by contributing to the slow diastolic depolarization phase that determines the firing rate of spontaneous action potentials of sinoatrial node cells [13]. Moreover, in response to autonomic transmitters, If contributes to the chronotropic regulation of heart rate [13]. Previously, we reported If loss-of-function in human atrial myocytes after chronic S-LPS treatment, an observation that in turn might be responsible for reduction of heart rate variability during sepsis [14,15]. Meanwhile it was shown that S-form LPS also acutely impairs If [16,17] and that the polysaccharide part (O-chain) of the LPS molecule [16] is necessary for reduction of pacemaker channel activity.
Beta-blocker administration has been shown to reduce mortality in MODS [18,19]. However, negative inotropic effects of beta-blockers restrict its use in the majority of patients. Therefore, ivabradine may be considered as an alternative therapeutic approach to reduce heart rate. This drug is a pure heart rate lowering agent that selectively inhibits If [20]. Ivabradine blocks pacemaker channels in a use-dependent way being more effective at higher heart rate while its action declines during bradycardia [21,22]. Blocking of pacemaker channels by ivabradine requires three steps: diffusion through the cell membrane, opening of respective channels and intracellular binding of the drug to the channel pore [20,23]. Heart rate reduction by ivabradine has been found to be beneficial in the treatment of cardiovascular disease, since it lowers heart rate without adversely affecting other cardiovascular functions [24].
Currently, the MODIfY trial carefully investigates potential benefits of heart rate reduction by ivabradine in sepsis and MODS [19]. Since S-LPS substantially inhibits If and ivabradine is administered in septic patients (MODIfY trial), we hypothesized that If reduction by S-LPS might interfere with ivabradine action on If. From a clinical perspective, the efficacy of If inhibition by ivabradine under septic conditions is of special therapeutic relevance.
In this study we investigated the effect of S-LPS and R595 on human atrial If under acute and chronic septic conditions as well as the interaction of both endotoxins with HCN channels. Next, we focused on the effect of endotoxins on (i) If reduction by ivabradine and (ii) intracellular ivabradine concentrations. Finally, using a computer simulation model we tried to explore the efficacy of ivabradine on deceleration of sinoatrial pacemaking activity under septic conditions.
2. Material and methods
A detailed Materials and methods section on isolation of cardiomyocytes [25–27], cell culture [28], myocyte treatments, patch-clamp measurements [14,29,30], immunoprecipitation [31], Western blot and immuno-dot-blot [32], qPCR [33], determination of ivabradine concentrations by HPLC [34] and sinoatrial pacemaker cell modeling [35] is available under the online supplemental section.
3. Results
3.1. Representative recordings of If of human atrial myocytes
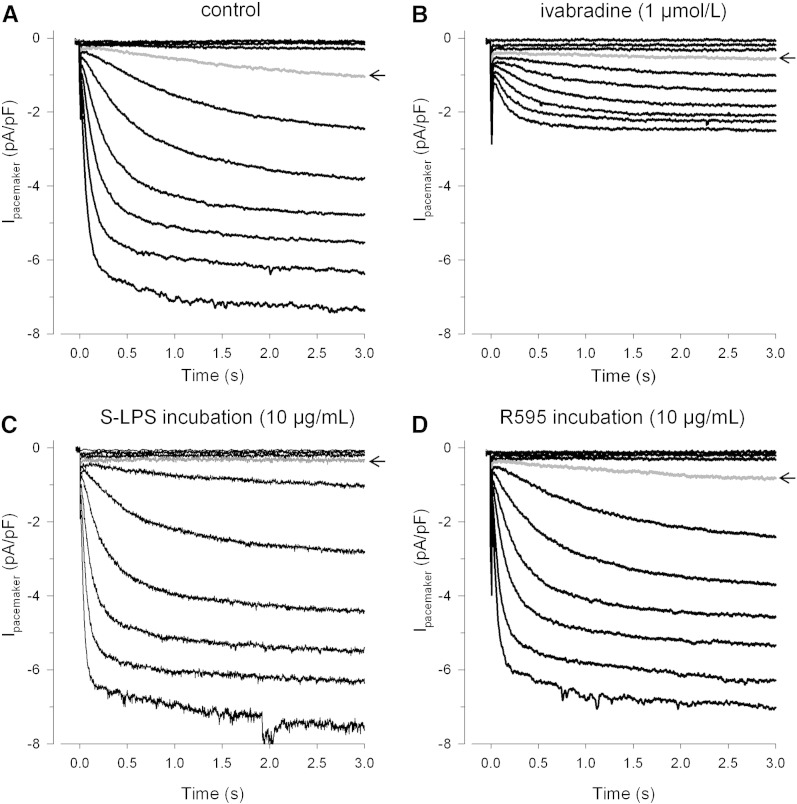
Fig. 1 shows representative traces of If under control (Fig. 1A), ivabradine (Fig. 1B) and septic conditions (mimicked in vitro by LPS incubation, Figs. 1C and D) elicited by hyperpolarizing voltage steps from − 40 to − 130 mV. If was considered to be present when current density was higher than 0.5 pA/pF at − 120 mV; this was the case in about 90% of all tested human atrial myocytes. The prominent reduction of If by ivabradine (Fig. 1B) reveals the heart rate lowering potency of this drug [36]. Under septic conditions only S-LPS (Fig. 1C) but not R595 (Fig. 1D) caused a more negative If activation threshold (control: between − 60 and − 70 mV, S-LPS: between − 70 and − 80 mV; note the arrows indicating current at − 70 mV). Irrespective of their origin, both S-LPS preparations (extracted from either Escherichia coli or Salmonella enterica serotype Minnesota) had an almost identical effect on If steady-state activation (Supplemental Fig. S1).
Fig. 1.
Representative pacemaker current recordings of human atrial myocytes.
Pacemaker currents (pA) were recorded by hyperpolarizing voltage steps ranging from − 40 to − 130 mV (10 mV increment, holding potential − 40 mV, 3 s duration) and normalized to cell capacitance (pF) for a control cell (A) and for cells treated with indicated concentrations of ivabradine (7 min) (B), S-LPS (6 h) (C) or R595 (6 h) (D). Arrows indicate current at − 70 mV.
3.2. Effect of endotoxins on If of human atrial myocytes
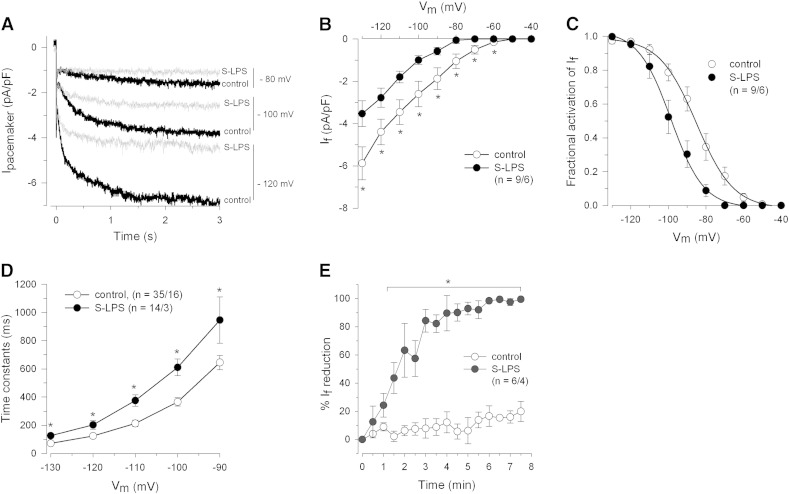
In order to investigate If under acute application of endotoxins, cells were superfused (6 min) with either S-LPS or R595. Fig. 2A shows representative pacemaker current recordings at indicated membrane potentials before and after S-LPS superfusion. S-LPS treatment resulted in a significant (i) reduction of If between − 60 and − 130 mV (Fig. 2B), (ii) shift of steady-state activation of If towards more negative membrane potentials (Fig. 2C) and (iii) slowing down of If current activation (Fig. 2D). The maximal If reduction by S-LPS occurred between 3 and 4 min (Fig. 2E) and was significantly more pronounced than the time-dependent If rundown (see also Supplemental Fig. S2). In line with a recent study where R595 had no acute effects on HCN2 current [16], we also did not observe any R595-mediated acute effects on If in human cardiomyocytes (data not shown).
Fig. 2.
Acute effects of S-LPS on pacemaker current of human atrial myocytes.
(A) Representative superimposed current recordings of a cell before and after S-LPS superfusion (10 μg/mL, 6 min). (B) I–V relationships of If before and after S-LPS superfusion. (C) If steady-state activation before and after S-LPS superfusion. Normalized conductances were fitted by a Boltzmann function. Control: V1/2 = − 85.4 ± 3.0 mV, k = 8.8 ± 0.8 mV; LPS: V1/2 = − 98.0 ± 3.1 mV, k = 6.3 ± 0.4 mV. (D) Mean time constants of If activation before and after S-LPS superfusion. Values were obtained by fitting a monoexponential function to current traces. (E) Time-dependent current reduction of If by S-LPS and under control conditions. Repetitive voltage steps were applied to − 80 mV for 3 s from a holding potential of − 40 mV (1/30 Hz, 8 min). Measurements were normalized to initial values and reduction of current densities was calculated. *p ≤ 0.05 vs. control.
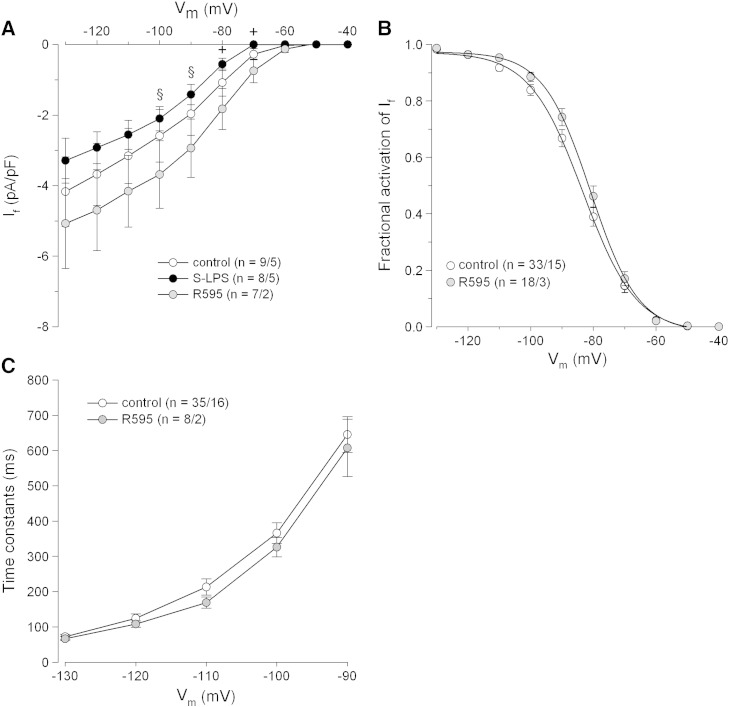
In order to investigate If under chronic conditions, myocytes were incubated with endotoxins for 6–10 h. In line with our previous findings [14] S-LPS shifted If steady-state activation towards more negative membrane potentials, slowed down its current activation (data not shown) and significantly reduced current density at − 70 and − 80 mV. In contrast to S-LPS, R595 did neither reduce If (Fig. 3A) nor alter If steady-state activation or time constants of If activation compared to controls (Figs. 3B and C).
Fig. 3.
Chronic effects of S-LPS and R595 on pacemaker current of human atrial myocytes.
Cells were incubated with S-LPS or R595 (10 μg/mL, 6–10 h. (A) Mean I–V relationships of If in control and endotoxin-treated cells. (B) If steady-state activation for control and R595-treated cells. Normalized conductances were fitted by a Boltzmann function. Control: V1/2 = − 83.3 ± 1.2 mV, k = 7.8 ± 0.4 mV; R595: V1/2 = − 80.3 ± 1.3 mV, k = 7.0 ± 0.5 mV. (C) Mean time constants of If activation for control and R595-treated cells. Values were obtained by fitting a monoexponential function to current traces. +p ≤ 0.05 vs. control and R595; §p ≤ 0.05 vs. R595.
3.3. Interactions of endotoxins with HCN channels
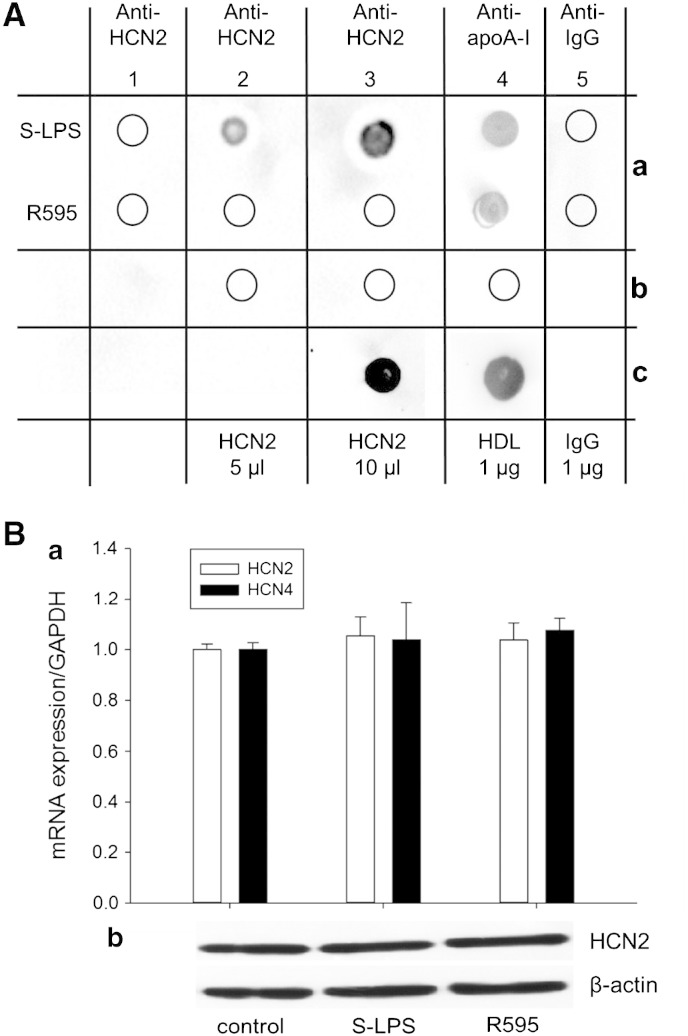
Acute S-LPS perfusion rapidly decreased If in human cardiomyocytes (Fig. 2E). Furthermore, S-LPS superfusion of HEK293 cells overexpressing HCN2 (the major isoform expressed in human atrial myocytes [37]) was found to reduce If by approx. 30% within 8 s [16]. These observations suggest a direct interaction of S-LPS with HCN channels. In order to test this hypothesis, a series of dot-blot experiments was performed. S-LPS and R595 preparations were cross-linked to activated (prewetted) PVDF membranes. After drying (inactivation) the membranes were incubated with either HCN2 or HCN4 (the major isoform in sinoatrial node cells [38]) that had been immunoprecipitated from murine heart (Supplemental Fig. S3). Using specific antibodies to follow endotoxin–HCN interactions, immunoreactive signals for HCN2 (Fig. 4A [2a, 3a]) and HCN4 (Supplemental Fig. S4) could only be detected with S-LPS (but not R595) cross-linked membranes. Observations that S-LPS and R595 interact with HDL/apoA-I (the major apolipoprotein of HDL, Fig. 4A [4a]), a known ligand for both LPS preparations [39], confirm the capacity of both endotoxin preparations to interact with candidate ligands. To further validate our findings, endotoxin-cross-linked membranes were incubated with murine total IgG, being present in immunoprecipitated HCN2; the lack of immunoreactivity (Fig. 4A [5a]) confirmed that the observed S-LPS–HCN2 interaction is specific and not due to S-LPS–IgG interaction. In addition, no immunoreactive signals were observed when inactivated membranes were incubated with either HCN2 or HDL (Fig. 4A [2b, 3b, 4b]), while incubation of HCN2 or HDL to activated membranes showed immunoreactivity (Fig. 4A [3c, 4c]). These negative and positive controls further confirm the specificity of S-LPS–HCN channel interaction.
Fig. 4.
LPS–HCN interactions.
(A) Dot-blot analysis for LPS–HCN2 binding. a: S-LPS and R595 (10 μg) were cross-linked with methanol prewetted PVDF membranes. Membranes were dried and dots were incubated with PBS (1a), indicated volumes of immunoprecipitated HCN2 protein (2a/3a), HDL (4a) or purified murine total IgG (5a). b: Prewetted PVDF membranes were dried followed by dotting with immunoprecipitated HCN2 protein (2b/3b) or HDL (4b). c: Immunoprecipitated HCN2 protein (3c) or HDL (4c) was dotted onto prewetted membranes. After all incubation steps lanes 1–5 were incubated with indicated primary antibody and immunoreactive dots were visualized; empty circles represent no immunoreactivity. One representative experiment out of 3 is shown. (B) mRNA (a, n = 8) and protein expression (b, one representative experiment out of 3 is shown) of HCN isoforms after S-LPS or R595 treatment (10 μg/mL, 6 h) in HL-1 cells using qPCR and Western blot, respectively.
As immunoprecipitated HCN2 and HCN4 were denatured by LDS sample buffer, the unspecific interaction of S-LPS with unfolded channel might have occurred. To show that also non-denatured HCN2 and HCN4 channels interact with endotoxins, cross-linked membranes were incubated with murine heart protein lysates; these experiments confirm interaction of intact HCN2 and HCN4 channels to S-LPS but not R595 (data not shown).
If is impaired in a similar manner under acute (Fig. 2B) and chronic S-LPS conditions (Fig. 3A) and S-LPS interacts with both HCN channel isoforms (Fig. 4A, Supplemental Fig. S4). To gain insights whether chronic endotoxin treatment could modulate channel expression, HL-1 cells (where If reduction after S-LPS treatment was previously reported [40]) were used. Both endotoxins did not alter mRNA (HCN2 and HCN4, up to 24 h) or protein expression (HCN2, 6 and 24 h); only the 6 h time point is shown (Fig. 4B, a/b).
3.4. Effect of ivabradine on If of human atrial myocytes
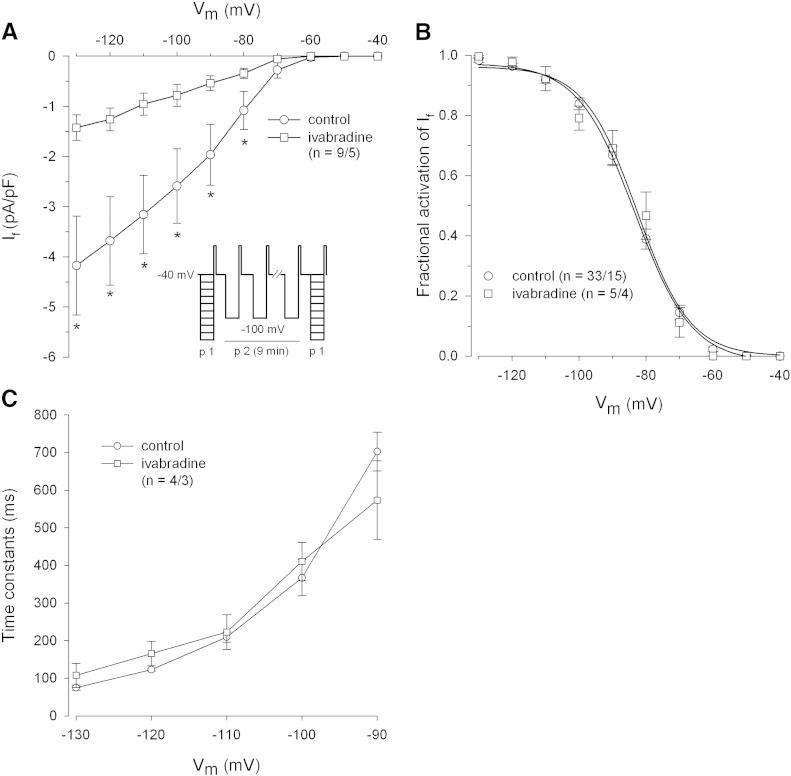
Fig. 5A shows the effect of ivabradine on If elicited by protocol 1 (p1, see inset) before and after ivabradine superfusion of cardiomyocytes. A use-dependent If block by ivabradine was established by protocol 2 (p2, see inset). Ivabradine significantly reduced If (from − 80 to − 130 mV) but did not change steady-state (Fig. 5B) or time constants of If activation (Fig. 5C). A current rundown was observed after stimulation of control cardiomyocytes using protocol p2. Ivabradine-induced If reduction (dark grey bars) was significantly higher compared to current rundown in controls (white bars) at all given membrane potentials (Fig. 6D).
Fig. 5.
Effect of ivabradine on pacemaker current of human atrial myocytes.
(A) Mean I–V relationships of If before and after ivabradine superfusion (1 μmol/L, 7 min). Inset shows the voltage clamp protocol. (B) If steady-state activation in control and ivabradine superfused cells. Normalized conductances were fitted by a Boltzmann function. Control: V1/2 = − 83.3 ± 1.2 mV, k = 7.8 ± 0.4 mV; ivabradine: V1/2 = − 84.1 ± 4.0 mV, k = 7.9 ± 2.5 mV. (C) Mean time constants of If activation before and after ivabradine superfusion. Values were obtained by fitting a monoexponential function to current traces. *p ≤ 0.05 vs. control.
Fig. 6.
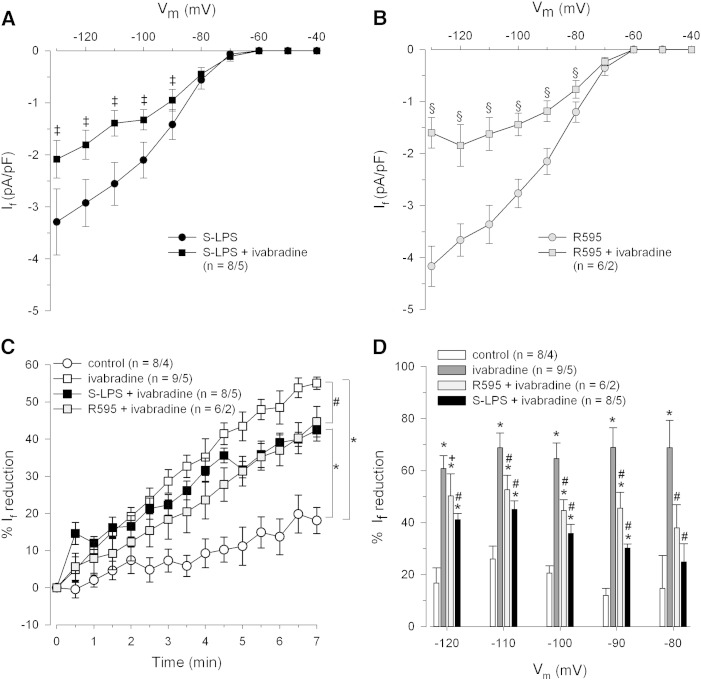
Effect of ivabradine on If characteristics in human atrial myocytes under septic conditions.
Cells were incubated with endotoxins (10 μg/mL, 6–10 h) and superfused with ivabradine (1 μmol/L, 7 min). Mean I–V relationships of If in S-LPS (A) or R595 incubated cells (B) before and after ivabradine superfusion. (C) Use-dependent development of ivabradine blockage of If. Currents were elicited by hyperpolarizing voltage steps to − 100 mV (3 s duration) at 1/6 Hz. (D). Percentage of If reduction by ivabradine was calculated from the underlying I–V relationships before and after ivabradine perfusion. ‡p ≤ 0.05 vs. S-LPS; §p ≤ 0.05 vs. R595; *p ≤ 0.05 vs. control; #p ≤ 0.05 vs. ivabradine.
3.5. Effect of ivabradine on If of human atrial myocytes under septic conditions
Ivabradine treatment significantly reduced If at membrane potentials from − 90 to − 130 mV in both S-LPS- and R595-incubated cardiomyocytes (Figs. 6A and B). The significantly reduced If current density at a membrane potential of − 80 mV in R595 incubated cells (Fig. 6B) did not differ from current rundown (Fig. 6D).
Fig. 6C shows the time-dependent development of ivabradine induced If blockage in control and S-LPS/R595 incubated cells at a membrane potential of − 100 mV (protocol p2, see inset Fig. 5A). In order to calculate If reduction five consecutive measurements of current densities during a 30 s period were averaged and normalized to initial values. If blockage by ivabradine was significantly higher in controls than in endotoxin incubated cells but did not differ between the two LPS forms. Furthermore, current rundown in control cardiomyocytes (beginning 2.5 min after protocol p2 had been started) was significantly smaller than If reduction in all ivabradine-treated cardiomyocytes. Fig. 6D shows that the efficacy of ivabradine to block If is significantly reduced in endotoxin incubated myocytes over a wide membrane potential range. If reduction was calculated from the underlying I–V relationships before and after ivabradine superfusion.
If steady-state activation (which is shifted towards more negative membrane potentials by S-LPS but not R595) was not further affected by ivabradine in endotoxin-treated myocytes (data not shown).
3.6. Effect of endotoxins on intracellular ivabradine concentrations in guinea pig ventricular myocytes
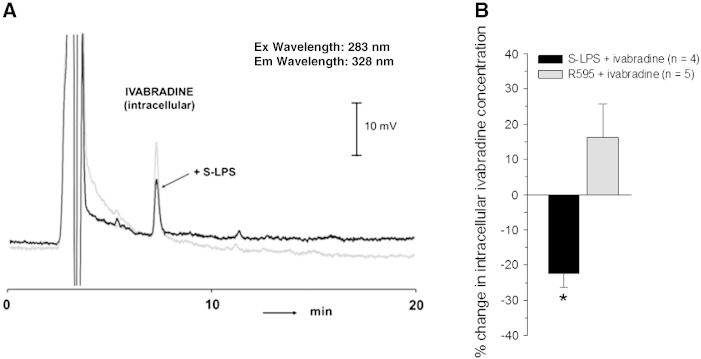
Under septic conditions If inhibition by ivabradine is reduced compared to controls (Figs. 6C and D). Since ivabradine diffuses across the cell membrane [23] and S-LPS is known to change cell membrane integrity [41], a reduction in intracellular ivabradine concentrations in endotoxin incubated myocytes might be the consequence. To test this hypothesis, intracellular ivabradine concentrations were measured in guinea pig cardiomyocytes due to the limited yield of human myocytes. Representative HPLC-chromatograms are shown in Fig. 7A. Intracellular ivabradine concentrations ranged between 1.55 and 3.31 pmoL/mg total cell protein in different control myocyte preparations. From Fig. 7B, it is evident that incubation of cells with S-LPS (but not R595) significantly reduced intracellular ivabradine concentrations compared to respective controls.
Fig. 7.
Intracellular ivabradine concentrations in guinea pig ventricular myocytes under septic conditions.
Untreated (controls) and endotoxin-treated (S-LPS or R595, 10 μg/mL, 6 h) cells were incubated with ivabradine (1 μmol/L, 15 min). (A) Representative HPLC-chromatograms for intracellular ivabradine content in control and S-LPS treated cells. (B) Percentage change of intracellular ivabradine concentrations in S-LPS and R595 incubated cells compared to respective controls. *p ≤ 0.05 vs. control.
3.7. Effect of ivabradine on sinoatrial pacemaking activity in the presence of S-LPS: a computer simulation model
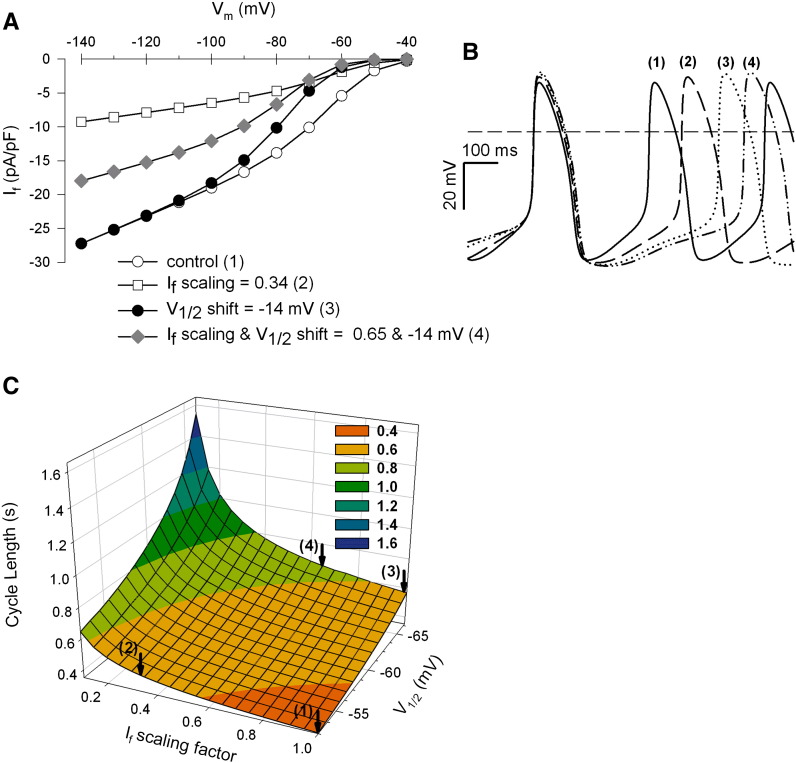
To gain first indications whether an impaired If (by S-LPS) is still a suitable target for ivabradine to reduce sinoatrial beating rate, experimental data were implemented in a sinoatrial computer cell model [35]. Based on our present and previously reported [14] experimental data using human myocytes, the effect of S-LPS was implemented in the model as an If V1/2 shift of − 14 mV (which represents a shift of If V1/2 in the sinoatrial cell model from − 52.5 to − 66.5 mV). The action of ivabradine was simulated by reduction of If conductance (66% and 35% in control and S-LPS incubated cells, respectively; see Figs. 5A and 6A).
In this simulation model, ivabradine (open squares) reduced If at all membrane potentials, while S-LPS (filled circles) had a reducing effect on If between − 50 and − 100 mV (Fig. 8A). At membrane potentials positive to approximately − 65 mV, If reduction by S-LPS was more pronounced compared to ivabradine. Combining effects of both compounds, the most prominent decrease of If between − 40 and − 70 mV became apparent (grey diamonds). The gradual decrease of If at physiologically relevant membrane potentials (Fig. 8A) resulted in a gradual increase in cycle length (CL) from 355 ms (controls (1)) to 455 ms (ivabradine (2)), 569 ms (S-LPS (3)) and 604 ms (S-LPS and ivabradine (4), Fig. 8B). A systematic variation of the two If model parameters (Fig. 8C) predicts that CL is more strongly influenced by an If V1/2 shift (to more negative membrane potentials) than by reduction of If conductance. For example, CL alteration by an If V1/2 shift of only − 2 mV is equivalent to a 20% decrease in If conductance. Moreover, the simulation model clearly shows that at all given V1/2 values (shifted by S-LPS), a reduction of If conductance due to ivabradine treatment leads to an additional decrease in CL. In conclusion, ivabradine is efficient to decelerate sinoatrial pacemaking activity in silico in the presence of S-LPS.
Fig. 8.
Effects of S-LPS and ivabradine on pacemaker activity of a sinoatrial cell model.
A rabbit sinoatrial pacemaker cell model was used to investigate S-LPS-induced V1/2 shift of − 14 mV and ivabradine-induced If reduction (66% and 35% in control and S-LPS-treated cells, respectively). (A) I–V relationships of If using the computational model: If was voltage clamped from − 40 to − 140 mV in 10 mV steps of 3 s duration for indicated conditions (1) control, (2) ivabradine, (3) S-LPS, (4) S-LPS and ivabradine. (B) Time course of spontaneous action potentials computed for indicated conditions (1) to (4). (C) Changes in cycle length (CL) are represented as a function of (i) reduction in If conductance (If scaling factor) and (ii) If–V1/2 shift to more negative membrane potentials. The arrows indicate the specific parameter setting (1) to (4).
4. Discussion
Sepsis and sepsis induced MODS are considered as highly complex diseases where heart pacemaking function is expected to be modulated by direct and indirect endotoxin effects [15,42]. In the present study, we exclusively focused on the direct effects of endotoxin on human atrial pacemaker current (If). We here report that S-LPS exerts besides a chronic also an acute inhibitory effect on human If. Our data provide an explanation for S-LPS-induced impairment of If, namely the interaction of the O-chain of S-LPS to the HCN channel. Furthermore, we explored the effect of ivabradine on If under septic conditions. To our knowledge, we are the first to show that the inhibitory action of ivabradine on If is reduced under elevated endotoxin concentrations. However, our in silico results show that the efficacy of ivabradine to decelerate pacemaking activity is preserved under septic conditions.
Previously we have reported If impairment in human myocytes under chronic S-LPS conditions [14]. We now report reduction of If by S-LPS (but not R595) under acute conditions. The data are in line with findings obtained in other cellular models (HEK293 cells overexpressing HCN2 or HL-1 cells [16,17]). In addition, our data reveal direct interactions between S-LPS (but not R595) and cardiac HCN channels, namely HCN2 (the predominant isoform in human atrial myocytes [37]) and HCN4 (the prevalent isoform in sinoatrial node cells [38]). However, the exact binding site(s) of S-LPS to the respective HCN channels remain to be explored.
HCN4 is considered as the main isoform contributing to the sinoatrial pacemaking process [13]. Thus, binding of S-LPS to HCN4 most likely accounts for If impairment in murine sinoatrial node cells [16] and may contribute to reduction of beating rate in spontaneously active murine HL-1 cells [17].
Pacemaker channels may also be indirectly affected by inflammatory cytokines (e.g. TNFα) released in response to bacterial and viral infections. Cytokines have been reported to play a pivotal role in gram-negative sepsis, particularly in myocardial dysfunction [43,44], probably by causing alterations in calcium homeostasis and production of nitric oxide (NO). The latter has been shown to modulate a number of cardiac ionic currents [45,46] including the sinoatrial pacemaker current [47] which can be stimulated by NO. This stimulation may partly contribute to sinus tachycardia in septic shock. Similar cascades are evoked by gram-positive sepsis which also may modulate pacemaker channel activity and hence heart rate [48,49]. Furthermore, inflammatory cytokines (interferons) released in response to viral brain infections have been reported to inhibit neuronal pacemaker channel activity [50]. Taken together, cytokine-dependent modulations of pacemaker channels provide a further cellular mechanism to alter sinoatrial pacemaking as well as neuronal network oscillations.
Ivabradine blocks If in cardiomyocytes including both human atrial [37] and rabbit sinoatrial node cells [20] as well as HCN4 overexpressing CHO cells [51]. We also observed If reduction (approx. 66%) by 1 μmol/L ivabradine (at membrane potentials from − 80 to − 130 mV), a concentration which has no effects on other membrane currents [20,52].
Under septic conditions, If block by ivabradine is significantly attenuated. If blocking capacity in the presence of S-LPS is paralleled by reduced intracellular ivabradine concentrations. As a consequence, less ivabradine is likely to be available for binding to the inner channel pore. Moreover, an altered access and/or binding behaviour of ivabradine due to S-LPS–HCN interaction might further influence ivabradine's blocking potency. None of these mechanisms are likely to occur for R595 treatment since this LPS-form did not alter intracellular ivabradine concentration or interact with the HCN channels. The exact mechanism how R595 modulates action of ivabradine on If remains to be further investigated. It is worth mentioning that the susceptibility of gram-negative bacteria towards hydrophobic agents also depends on the LPS-form on their surface [53]. For example, in case of deep-rough Re-LPS mutant strains, hydrophobic antibiotics such as macrolides readily access the cell surface and permeate into the cell interior through the lipid bilayer, while no accumulation was observed in strains having S-LPS [54]. Therefore, one may speculate that incorporation of S-LPS in human myocytes hinders the accumulation and transfer of the hydrophobic ivabradine.
Our data show that in the presence of S-LPS, If current densities are reduced at physiologically relevant membrane potentials. Therefore, the question arises whether a decreased If as found under septic conditions is still a suitable target for ivabradine in order to reduce sinoatrial beating rate. It is known that during the diastolic depolarization phase of the sinoatrial action potential, very small net inward currents are sufficient to depolarize the cell membrane towards the action potential threshold [55]. Since If of mammalian sinoatrial [16] and human atrial myocytes is impaired by S-LPS in a similar manner and the inhibitory effect of ivabradine on human atrial If is comparable to that on sinoatrial mammalian If [37], we implemented our experimental data into a sinoatrial cell model. This in silico approach suggests that ivabradine should still be capable of decreasing sinoatrial beating rate under septic conditions through If inhibition. Nevertheless, it has to be mentioned that sinoatrial pacemaking in vivo is a very complex process due to the close interaction of the membrane clock (cyclic opening and closing of membrane ion channels) and the calcium clock (rhythmic spontaneous Ca2 + release from the sarcoplasmatic reticulum) [56]. Furthermore, the data presented in this study are gained from quiescent atrial myocytes, which exhibit differences in ion channel expression and hence electrophysiological characteristics. These differences might influence experimental outcome. Therefore further investigations using spontaneously active cardiomyocytes are required to confirm our conclusions and to get a deeper insight in the effect of ivabradine on heart rate under septic conditions in vivo. Such studies are of special interest since currently the clinical MODIfY trial studies the benefits of heart rate reduction by ivabradine in patients suffering from MODS [19], where heart rate is usually elevated due to increased oxygen demand and cytokine levels [57] and has been proved to predict survival [3,58]. Pure heart rate reduction by ivabradine could therefore improve the outcome of patients in shock by lowering myocardial oxygen demand, improving diastolic coronary perfusion and acting on the negative force-frequency relationship of the failing heart [57].
In summary, our data demonstrate for the first time that O-chain-dependent interaction of endotoxin to HCN channels mediates If reduction under septic conditions. Even though If blocking capacity of ivabradine is reduced under elevated endotoxin levels in vitro, this drug is effective to decelerate sinoatrial beating rate in the presence of S-LPS in silico. Thus, the medical application of ivabradine as a heart rate reducing agent in critically ill patients (during MODS and sepsis) might favor a better therapeutic outcome.
Sources of funding
This work was supported by grants of the Austrian Science Fund (FWF): P21159-B19, F3007 and W1226-B18 within the “Doctoral College Metabolic and Cardiovascular Disease” at the Medical University of Graz.
Disclosures
None.
Conflict of interest
None of the authors has any conflict of interest to disclose.
Acknowledgments
We are thankful to Servier Laboratories (France) for providing ivabradine, to Dr. Klaus Brandenburg (Germany) for providing R595 and to Dr. Peter Schaffer (Austria) for helpful comments.
Contributor Information
Klaus Zorn-Pauly, Email: klaus.zornpauly@medunigraz.at.
Brigitte Pelzmann, Email: brigitte.pelzmann@medunigraz.at.
Appendix A. Supplementary data
Supplementary material.
References
- 1.Cohen J. The immunopathogenesis of sepsis. Nature. 2002;420:885–891. doi: 10.1038/nature01326. [DOI] [PubMed] [Google Scholar]
- 2.Fernandes C.J., Jr., de Assuncao M.S. Myocardial dysfunction in sepsis: a large, unsolved puzzle. Crit Care Res Pract. 2012;2012:896430. doi: 10.1155/2012/896430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hoke R.S., Müller-Werdan U., Lautenschlager C., Werdan K., Ebelt H. Heart rate as an independent risk factor in patients with multiple organ dysfunction: a prospective, observational study. Clin Res Cardiol. 2012;101:139–147. doi: 10.1007/s00392-011-0375-3. [DOI] [PubMed] [Google Scholar]
- 4.Huber M., Kalis C., Keck S., Jiang Z., Georgel P., Du X. R-form LPS, the master key to the activation of TLR4/MD-2-positive cells. Eur J Immunol. 2006;36:701–711. doi: 10.1002/eji.200535593. [DOI] [PubMed] [Google Scholar]
- 5.Seydel U., Hawkins L., Schromm A.B.., Heine H., Scheel O., Koch M.H. The generalized endotoxic principle. Eur J Immunol. 2003;33:1586–1592. doi: 10.1002/eji.200323649. [DOI] [PubMed] [Google Scholar]
- 6.Brandenburg K., Wiese A. Endotoxins: relationships between structure, function, and activity. Curr Top Med Chem. 2004;4:1127–1146. doi: 10.2174/1568026043388213. [DOI] [PubMed] [Google Scholar]
- 7.Nielsen J.S., Larsson A., Ledet T., Turina M., Tonnesen E., Krog J. Rough-form-lipopolysaccharide increase apoptosis in human CD4(+) and CD8(+) T-lymphocytes. Scand J Immunol. 2012;75:193–202. doi: 10.1111/j.1365-3083.2011.02613.x. [DOI] [PubMed] [Google Scholar]
- 8.Blunck R., Scheel O., Muller M., Brandenburg K., Seitzer U., Seydel U. New insights into endotoxin-induced activation of macrophages: involvement of a K+ channel in transmembrane signaling. J Immunol. 2001;166:1009–1015. doi: 10.4049/jimmunol.166.2.1009. [DOI] [PubMed] [Google Scholar]
- 9.Hoang L.M., Chen C., Mathers D.A. Lipopolysaccharide rapidly activates K+ channels at the intracellular membrane face of rat cerebral artery smooth muscle cells. Neurosci Lett. 1997;231:25–28. doi: 10.1016/s0304-3940(97)00519-3. [DOI] [PubMed] [Google Scholar]
- 10.Wilkinson M.F., Earle M.L., Triggle C.R., Barnes S. Interleukin-1beta, tumor necrosis factor-alpha, and LPS enhance calcium channel current in isolated vascular smooth muscle cells of rat tail artery. FASEB J. 1996;10:785–791. doi: 10.1096/fasebj.10.7.8635696. [DOI] [PubMed] [Google Scholar]
- 11.Biel M., Wahl-Schott C., Michalakis S., Zong X. Hyperpolarization-activated cation channels: from genes to function. Physiol Rev. 2009;89:847–885. doi: 10.1152/physrev.00029.2008. [DOI] [PubMed] [Google Scholar]
- 12.Bucchi A., Barbuti A., DiFrancesco D., Baruscotti M. Funny current and cardiac rhythm: insights from HCN knockout and transgenic mouse models. Front Physiol. 2012;3:240. doi: 10.3389/fphys.2012.00240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.DiFrancesco D. The role of the funny current in pacemaker activity. Circ Res. 2010;106:434–446. doi: 10.1161/CIRCRESAHA.109.208041. [DOI] [PubMed] [Google Scholar]
- 14.Zorn-Pauly K., Pelzmann B., Lang P., Machler H., Schmidt H., Ebelt H. Endotoxin impairs the human pacemaker current If. Shock. 2007;28:655–661. [PubMed] [Google Scholar]
- 15.Papaioannou V.E., Verkerk A.O., Amin A.S., de Bakker J.M. Intracardiac origin of heart rate variability, pacemaker funny current and their possible association with critical illness. Curr Cardiol Rev. 2013;9:82–96. doi: 10.2174/157340313805076359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Klöckner U., Rueckschloss U., Grossmann C., Ebelt H., Müller-Werdan U., Loppnow H. Differential reduction of HCN channel activity by various types of lipopolysaccharide. J Mol Cell Cardiol. 2011;51:226–235. doi: 10.1016/j.yjmcc.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 17.Wondergem R., Graves B.M., Ozment-Skelton T.R., Li C., Williams D.L. Lipopolysaccharides directly decrease Ca2 + oscillations and the hyperpolarization-activated nonselective cation current If in immortalized HL-1 cardiomyocytes. Am J Physiol Cell Physiol. 2010;299:C665–C671. doi: 10.1152/ajpcell.00129.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmidt H., Hoyer D., Hennen R., Heinroth K., Rauchhaus M., Prondzinsky R. Autonomic dysfunction predicts both 1- and 2-month mortality in middle-aged patients with multiple organ dysfunction syndrome. Crit Care Med. 2008;36:967–970. doi: 10.1097/CCM.0B013E3181653263. [DOI] [PubMed] [Google Scholar]
- 19.Nuding S., Ebelt H., Hoke R.S., Krummenerl A., Wienke A., Müller-Werdan U. Reducing elevated heart rate in patients with multiple organ dysfunction syndrome by the If (funny channel current) inhibitor ivabradine: MODI(f)Y trial. Clin Res Cardiol. 2011;100:915–923. doi: 10.1007/s00392-011-0323-2. [DOI] [PubMed] [Google Scholar]
- 20.Bois P., Bescond J., Renaudon B., Lenfant J. Mode of action of bradycardic agent, S 16257, on ionic currents of rabbit sinoatrial node cells. Br J Pharmacol. 1996;118:1051–1057. doi: 10.1111/j.1476-5381.1996.tb15505.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ferrari R., Ceconi C. Selective and specific If inhibition with ivabradine: new perspectives for the treatment of cardiovascular disease. Expert Rev Cardiovasc Ther. 2011;9:959–973. doi: 10.1586/erc.11.99. [DOI] [PubMed] [Google Scholar]
- 22.Sulfi S., Timmis A.D. Ivabradine—the first selective sinus node If channel inhibitor in the treatment of stable angina. Int J Clin Pract. 2006;60:222–228. doi: 10.1111/j.1742-1241.2006.00817.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bucchi A., Tognati A., Milanesi R., Baruscotti M., DiFrancesco D. Properties of ivabradine-induced block of HCN1 and HCN4 pacemaker channels. J Physiol. 2006;572:335–346. doi: 10.1113/jphysiol.2005.100776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Speranza L., Franceschelli S., Riccioni G. The biological effects of ivabradine in cardiovascular disease. Molecules. 2012;17:4924–4935. doi: 10.3390/molecules17054924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.World Medical Association General Assembly World Medical Association Declaration of Helsinki: ethical principles for medical research involving human subjects. J Int Bioethique. 2004;15:124–129. [PubMed] [Google Scholar]
- 26.Pelzmann B., Schaffer P., Mächler H., Rigler B., Koidl B. Adenosine inhibits the L-type calcium current in human atrial myocytes. Naunyn Schmiedebergs Arch Pharmacol. 1995;351:293–297. doi: 10.1007/BF00233249. [DOI] [PubMed] [Google Scholar]
- 27.Piper H.M., Probst I., Schwartz P., Hutter F.J., Spieckermann P.G. Culturing of calcium stable adult cardiac myocytes. J Mol Cell Cardiol. 1982;14:397–412. doi: 10.1016/0022-2828(82)90171-7. [DOI] [PubMed] [Google Scholar]
- 28.Claycomb W.C., Lanson N.A., Jr., Stallworth B.S., Egeland D.B., Delcarpio J.B., Bahinski A. HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A. 1998;95:2979–2984. doi: 10.1073/pnas.95.6.2979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porciatti F., Pelzmann B., Cerbai E., Schaffer P., Pino R., Bernhart E. The pacemaker current If in single human atrial myocytes and the effect of beta-adrenoceptor and A1-adenosine receptor stimulation. Br J Pharmacol. 1997;122:963–969. doi: 10.1038/sj.bjp.0701473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Barry P.H. JPCalc, a software package for calculating liquid junction potential corrections in patch-clamp, intracellular, epithelial and bilayer measurements and for correcting junction potential measurements. J Neurosci Methods. 1994;51:107–116. doi: 10.1016/0165-0270(94)90031-0. [DOI] [PubMed] [Google Scholar]
- 31.Kitz K., Windischhofer W., Leis H.J., Huber E., Kollroser M., Malle E. 15-Deoxy-Δ12,14-prostaglandin J2 induces Cox-2 expression in human osteosarcoma cells through MAPK and EGFR activation involving reactive oxygen species. Free Radic Biol Med. 2011;50:854–865. doi: 10.1016/j.freeradbiomed.2010.12.039. [DOI] [PubMed] [Google Scholar]
- 32.Rossmann C., Rauh A., Hammer A., Windischhofer W., Zirkl S., Sattler W. Hypochlorite-modified high-density lipoprotein promotes induction of HO-1 in endothelial cells via activation of p42/44 MAPK and zinc finger transcription factor Egr-1. Arch Biochem Biophys. 2011;509:16–25. doi: 10.1016/j.abb.2011.02.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Semlitsch M., Shackelford R.E., Zirkl S., Sattler W., Malle E. ATM protects against oxidative stress induced by oxidized low-density lipoprotein. DNA Repair (Amst) 2011;10:848–860. doi: 10.1016/j.dnarep.2011.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Klippert P., Jeanniot J.P., Polve S., Lefevre C., Merdjan H. Determination of ivabradine and its N-demethylated metabolite in human plasma and urine, and in rat and dog plasma by a validated high-performance liquid chromatographic method with fluorescence detection. J Chromatogr B Biomed Sci Appl. 1998;719:125–133. doi: 10.1016/s0378-4347(98)00406-x. [DOI] [PubMed] [Google Scholar]
- 35.Severi S., Fantini M., Charawi L.A., DiFrancesco D. An updated computational model of rabbit sinoatrial action potential to investigate the mechanisms of heart rate modulation. J Physiol. 2012;590:4483–4499. doi: 10.1113/jphysiol.2012.229435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Thollon C., Vilaine J.P. If inhibition in cardiovascular diseases. Adv Pharmacol. 2010;59:53–92. doi: 10.1016/S1054-3589(10)59003-3. [DOI] [PubMed] [Google Scholar]
- 37.El Chemaly A., Magaud C., Patri S., Jayle C., Guinamard R., Bois P. The heart rate-lowering agent ivabradine inhibits the pacemaker current If in human atrial myocytes. J Cardiovasc Electrophysiol. 2007;18:1190–1196. doi: 10.1111/j.1540-8167.2007.00955.x. [DOI] [PubMed] [Google Scholar]
- 38.Postea O., Biel M. Exploring HCN channels as novel drug targets. Nat Rev Drug Discov. 2011;10:903–914. doi: 10.1038/nrd3576. [DOI] [PubMed] [Google Scholar]
- 39.Ulevitch R.J., Johnston A.R., Weinstein D.B. New function for high density lipoproteins. Isolation and characterization of a bacterial lipopolysaccharide-high density lipoprotein complex formed in rabbit plasma. J Clin Invest. 1981;67:827–837. doi: 10.1172/JCI110100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wondergem R., Graves B.M., Li C., Williams D.L. Lipopolysaccharide prolongs action potential duration in HL-1 mouse cardiomyocytes. Am J Physiol Cell Physiol. 2012;303:C825–C833. doi: 10.1152/ajpcell.00173.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Cardoso F.L., Kittel A., Veszelka S., Palmela I., Toth A., Brites D. Exposure to lipopolysaccharide and/or unconjugated bilirubin impair the integrity and function of brain microvascular endothelial cells. PLoS One. 2012;7:e35919. doi: 10.1371/journal.pone.0035919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Werdan K., Schmidt H., Ebelt H., Zorn-Pauly K., Koidl B., Hoke R.S. Impaired regulation of cardiac function in sepsis, SIRS, and MODS. Can J Physiol Pharmacol. 2009;87:266–274. doi: 10.1139/Y09-012. [DOI] [PubMed] [Google Scholar]
- 43.Kumar A., Krieger A., Symeoneides S., Kumar A., Parrillo J.E. Myocardial dysfunction in septic shock: Part II. Role of cytokines and nitric oxide. J Cardiothorac Vasc Anesth. 2001;15:485–511. doi: 10.1053/jcan.2001.25003. [DOI] [PubMed] [Google Scholar]
- 44.Hunter J.D., Doddi M. Sepsis and the heart. Br J Anaesth. 2010;104:3–11. doi: 10.1093/bja/aep339. [DOI] [PubMed] [Google Scholar]
- 45.Fischmeister R., Castro L., Abi-Gerges A., Rochais F., Vandecasteele G. Species-and tissue-dependent effects of NO and cyclic GMP on cardiac ion channels. Comp Biochem Physiol A Mol Integr Physiol. 2005;142:136–143. doi: 10.1016/j.cbpb.2005.04.012. [DOI] [PubMed] [Google Scholar]
- 46.Aoki Y., Hatakeyama N., Yamamoto S., Kinoshita H., Matsuda N., Hattori Y. Role of ion channels in sepsis-induced atrial tachyarrhythmias in guinea pigs. Br J Pharmacol. 2012;166:390–400. doi: 10.1111/j.1476-5381.2011.01769.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Musialek P., Lei M., Brown H.F., Paterson D.J., Casadei B. Nitric oxide can increase heart rate by stimulating the hyperpolarization-activated inward current, I(f) Circ Res. 1997;81:60–68. doi: 10.1161/01.res.81.1.60. [DOI] [PubMed] [Google Scholar]
- 48.Knuefermann P., Sakata Y., Baker J.S., Huang C.H., Sekiguchi K., Hardarson H.S. Toll-like receptor 2 mediates Staphylococcus aureus-induced myocardial dysfunction and cytokine production in the heart. Circulation. 2004;110:3693–3698. doi: 10.1161/01.CIR.0000143081.13042.04. [DOI] [PubMed] [Google Scholar]
- 49.Boehm O., Knuefermann P., Plueck J., Schwederski M., Ehrentraut H., Kebir S. TLR2 stimulation induces cardiac inflammation but not cardiac depression in vivo. J Inflamm (Lond) 2013;10:33. doi: 10.1186/1476-9255-10-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Stadler K., Bierwirth C., Stoenica L., Battefeld A., Reetz O., Mix E. Elevation in type I interferons inhibits HCN1 and slows cortical neuronal oscillations. Cereb Cortex. 2014;24:199–210. doi: 10.1093/cercor/bhs305. [DOI] [PubMed] [Google Scholar]
- 51.Thollon C., Bedut S., Villeneuve N., Coge F., Piffard L., Guillaumin J.P. Use-dependent inhibition of hHCN4 by ivabradine and relationship with reduction in pacemaker activity. Br J Pharmacol. 2007;150:37–46. doi: 10.1038/sj.bjp.0706940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Koncz I., Szel T., Bitay M., Cerbai E., Jaeger K., Fulop F. Electrophysiological effects of ivabradine in dog and human cardiac preparations: potential antiarrhythmic actions. Eur J Pharmacol. 2011;668:419–426. doi: 10.1016/j.ejphar.2011.07.025. [DOI] [PubMed] [Google Scholar]
- 53.Delcour A.H. Outer membrane permeability and antibiotic resistance. Biochim Biophys Acta. 2009;1794:808–816. doi: 10.1016/j.bbapap.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Tsujimoto H., Gotoh N., Nishino T. Diffusion of macrolide antibiotics through the outer membrane of Moraxella catarrhalis. J Infect Chemother. 1999;5:196–200. doi: 10.1007/s101560050034. [DOI] [PubMed] [Google Scholar]
- 55.Verkerk A.O., Wilders R. Hyperpolarization-activated current, If, in mathematical models of rabbit sinoatrial node pacemaker cells. Biomed Res Int. 2013;2013:872454. doi: 10.1155/2013/872454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Monfredi O., Maltsev V.A., Lakatta E.G. Modern concepts concerning the origin of the heartbeat. Physiology (Bethesda) 2013;28:74–92. doi: 10.1152/physiol.00054.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.De Santis V., Vitale D., Santoro A., Magliocca A., Porto A.G., Nencini C. Ivabradine: potential clinical applications in critically ill patients. Clin Res Cardiol. 2013;102:171–178. doi: 10.1007/s00392-012-0516-3. [DOI] [PubMed] [Google Scholar]
- 58.Parker M.M., Shelhamer J.H., Natanson C., Alling D.W., Parrillo J.E. Serial cardiovascular variables in survivors and nonsurvivors of human septic shock: heart rate as an early predictor of prognosis. Crit Care Med. 1987;15:923–929. doi: 10.1097/00003246-198710000-00006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.