Abstract
Mitogen-activated protein kinases (MAPKs) are involved in the regulation of cardiac hypertrophy and myocyte survival. Extracellular signal regulated protein kinase 1 and 2 (ERK1/2) are key components in the MAPK signaling pathways. Dysfunction of ERK1/2 in congenital heart diseases (Noonan syndrome and LEOPARD syndrome) leads to cardiac hypertrophy. ERK2 contributes 70% of protein content to total ERK1/2 content in myocardium; however, the specific role of ERK2 in regulating cardiac hypertrophy is yet to be further defined.
To investigate the specific role of ERK2 played in the cardiomyocytes, we generated and examined mice with cardiomyocyte-specific deletion of the erk2 gene (ERK2cko mice). Following short-term pathological hypertrophic stresses, the mutant mice showed attenuated hypertrophic remodeling characterized by a blunted increase in the cross-sectional area of individual myocytes, downregulation of hypertrophic foetal gene markers (ANP and BNP), and less interstitial fibrosis. However, increased cardiomyocyte apoptosis was observed. Upon prolonged stimulation, ERK2cko mice developed deterioration in cardiac function. However, absence of ERK2 did not affect physiological hypertrophy induced by 4 weeks of swimming exercise.
These results revealed an essential role for ERK2 in cardiomyocytes in the development of pathological hypertrophic remodeling and resistance to cell death.
Keywords: Cardiac hypertrophy, Apoptosis, Interstitial fibrosis, Signal transduction, Genetically modified mice
Highlights
-
•
ERK2 is required for pathological cardiac hypertrophy.
-
•
ERK2 is essential for cardiomyocyte survival through preventing apoptosis.
-
•
ERK2 deficiency in cardiomyocytes accelerates heart failure progress.
-
•
ERK2 is unlikely to be involved in the regulation of physiological hypertrophy.
1. Introduction
Human pathological cardiac hypertrophy is not only a risk factor for heart failure and sudden death [1], but also a common cause of mortality and morbidity in congenital heart diseases (CHDs) [2]. Noonan syndrome (NS) and LEOPARD syndrome (LS), termed as RASopathies, are two autosomal dominant disorders with congenital manifestation of heart defects. Clinical assessments showed that NS patients exhibit pulmonary valve stenosis accompanied with cardiac malformations; the dominant cardiac defect in 90% of LS cases is hypertrophic cardiomyopathy [3,4]. Genetic studies revealed that these syndromes are both, at least in part, related to the mutation of non-receptor protein tyrosine phosphatase type 11 (PTPN11) gene which encodes the SH2 containing protein tyrosine phosphatase (SHP2) [3–5]. The mutants showed dysfunction of the Ras-Raf-ERK1/2 signaling pathway. NS associated mutation potentiated Ras-ERK1/2 activation [6,7]. In addition, gain-of-function studies of SHP2 exhibited the NS-like syndrome accompanied by enhanced activation of ERK1/2 in the heart [8,9]. By contrast, LS mutants behaved as loss-of-function mutations of SHP2, impairing ERK activation [10–12].
Within the heart, ERK1/2 are activated at the plasma membrane by Raf-1, followed by activation of small G protein Ras [13]. Harris et al. showed that dominant negative Raf-1 inhibited ERK1/2 activation in transgenic mice, which were resistant to hypertrophic stress but developed significant cardiomyocyte apoptosis [14]. Although cardiac-specific Raf-1 knockout mice demonstrated heart dysfunction in the absence of cardiac hypertrophy, associated with more apoptotic cardiomyocytes through a MEK/ERK independent mechanism [15], recent studies have suggested that ERK1/2 appear to be an essential cardiac protector during stress stimulation [16–19].
ERK1/2 have been implicated as the regulators of cardiac hypertrophy in both cell culture and genetically modified mouse models. For example, in cultured cardiomyocytes, it is suggested that ERK1/2 activation promotes hypertrophic growth [20]. Transgenic mice overexpressing MEK1 had robust cardiac hypertrophy associated with activation of ERK1/2 [18]. Nevertheless, dual-specificity phosphatase 6 (DUSP6) knockout mice with spontaneously increased ERK1/2 phosphorylation induced larger hearts due to hypercellularity of myocytes compared to wild type mice [19]. However, complete inhibition of ERK1/2 activity by DUSP6 overexpression in transgenic mice or loss expression of ERK1/2 in the ERK1 null cross mated ERK2 cardiomyocyte-specific deletion mice did not reduce cardiac hypertrophy following different pathological stimuli [21]. The biological role of ERK1/2 in heart hypertrophy is conflicting; thus, the understanding of regulation by ERK1/2 in the heart remains elusive.
Global gene target studies showed that ERK1 deficient knockout mice are fertile, viable and of normal size [22]. In contrast, ERK2 knockout mice die during embryogenesis at day 11.5 due to an abnormality of placental development causing secondary effects such as a thin heart wall and growth retardation [23], which indicates that they may play different roles in vivo. ERK2 contributes 70% of protein content to total ERK1/2 content in myocardium [17]. Therefore, the unique regulation of cardiac function by ERK2 alone awaits clarification. To further determine the in vivo role of ERK2 in the heart, we generated cardiomyocyte-specific ERK2 deleted mice (ERK2cko). The deletion of ERK2 in cardiomyocytes alone did not affect heart development and was not compensated by ERK1. Thereafter, ERK2cko mice were exposed to pressure overload or β-adrenergic stimulation for various time points; the absence of ERK2 resulted in blunted hypertrophic growth and increased susceptibility to heart failure due to apoptosis. Conversely, ERK2 was not involved in the regulation of physiological hypertrophy induced by long-term swimming exercise.
2. Materials and methods
All mice used in this study were maintained in a pathogen-free facility at the University of Manchester. The animal studies performed were in accordance with the UK Home Office and institutional guidelines. An expanded Materials and methods section is available in the online supplement data.
2.1. Generation of ERK2 cardiomyocyte-specific knockout mice
Mice with modification in the erk2 gene with LoxP elements flanking exon 2 and 3 (ERK2f/f) [24] were cross mated with mice expressing Cre under a myosin light chain (MLC2v) promoter to generate cardiomyocyte-specific ERK2 knockout mice (ERK2cko).
2.2. Hypertrophic models
Cardiac hypertrophy was induced by transverse aortic constriction (TAC) or a sham operation under anaesthesia with a mixture of ketamine (100 mg/kg) and xylazine (5 mg/kg), 0.1 mg/kg buprenorphine and artificial ventilation on 8 week old male ERK2cko mice and the control littermates ERK2f/f mice, or by administration of isoproterenol (ISO, Sigma-Aldrich) at 10 mg/kg/day via osmotic mini-pumps (Alzet) implantation as previously described [25,26]. ERK2f/f and ERK2cko mice were also subjected to a 4-week swimming exercise to induce physiological cardiac hypertrophy as previously described [25].
2.3. Echocardiography
Mice were anesthetized with Avertin (200 mg/kg). To evaluate the cardiac function, transthoracic 2D M-mode echocardiographic recordings were performed using an Acuson Sequoia C256 ultrasound system (Siemens) following a protocol described previously [25,26].
2.4. Histology analyses and terminal deoxynucleotidyl transferase dUTP nick end labelling (TUNEL) assay
5 μm thick paraffin sections were cut and stained with haematoxylin & eosin to measure cardiomyocyte cross-sectional areas, and stained using the Masson's trichrome method to examine interstitial fibrosis as described [25,26]. TUNEL assay was performed to detect apoptosis using the in situ Cell Death Detection kit (Roche).
2.5. Immunoblot analyses
Protein extracts (30 μg) were subjected to Western blot analyses with the primary antibodies against ERK1 (Santa Cruz), ERK2 (Santa Cruz), ERK5 (Upstate), Phospho-ERK5 (Invitrogen), p38 (Santa Cruz), Phospho-ERK1/2, MEK1/2, Phospho-MEK1/2, Phospho-p38, JNK1/2, Phospho-JNK1/2, PKB, Phospho-PKB, active caspase 3 (Cell signaling) or α-Tubulin (Sigma-Aldrich). Immunecomplexes were detected by enhanced chemiluminescence with anti-mouse, anti-rabbit or anti-goat immunoglobulin G-coupled to horseradish peroxidase as the secondary antibody (Amersham-Pharmacia).
2.6. ERK1/2 kinase assay
Tissue samples were homogenized in triton lysis buffer (Supplementary materials and methods). Protein extracts were applied for ERK1/2 kinase assay following the manufacturer's instruction (Cell Signaling).
2.7. siRNA transfection of neonatal rat cardiomyocytes (NRCMs)
NRCMs were prepared as previously described [24–26]. NRCMs were transfected with either control siRNA (Sigma-Aldrich) or rat ERK2 siRNA (gene ID #116590; si genome SMART pool, Dharmacon) using Lipofectamine Plus reagent according to the manufacturer's instruction (Invitrogen) for 48 h prior to phenylephrine (PE, 30 mM, Sigma-Aldrich) treatments or 100 μM H2O2 treatments.
2.8. Immunocytochemistry
NRCMs on laminin-coated coverslips were transfected with control or ERK2 siRNA, followed by treatment with PE for 48 h. Thereafter, triple immunostaining was performed using anti-ANP antibody (Peninsula Laboratories) and anti-α-actinin antibody (Sigma-Aldrich) accompanied by nuclei detection by 4′,6-diamidino-2-phenylindole (DAPI, Invitrogen).
2.9. Luciferase reporter assay
NRCMs transfected with either control siRNA or ERK2 siRNA were infected with virus encoding brain natriuretic peptide (BNP) Ad-BNP-Luc at 25 MOI for 24 h followed by 24 hour treatment of PE (30 μM). BNP reporter activity was analysed using luciferase assay kit (Promega).
2.10. Quantitative real-time PCR
Real time quantitative PCRs were performed using SYBR Green PCR Master Mix (Applied Biosystems) according to the manufacturer's recommendations. Primers to identify ERK2, ANP, BNP, Collα2, Col3α1, Ctgf and GAPDH were obtained from Qiagen. Thermal cycling was performed in the 7500 Real Time PCR System (Applied Biosystems), and the results were analysed using the 2-ΔΔCT method [27].
2.11. Statistical analysis
Data were expressed as means ± SEM and statistical comparisons between groups were analysed using the paired Student's t test; for multiple comparisons 1- or 2-way ANOVA followed by Bonferroni post-hoc was used where appropriate. A value of p < 0.05 was considered to be statistically significant between groups.
3. Results
3.1. Characterization of ERK2cko mice
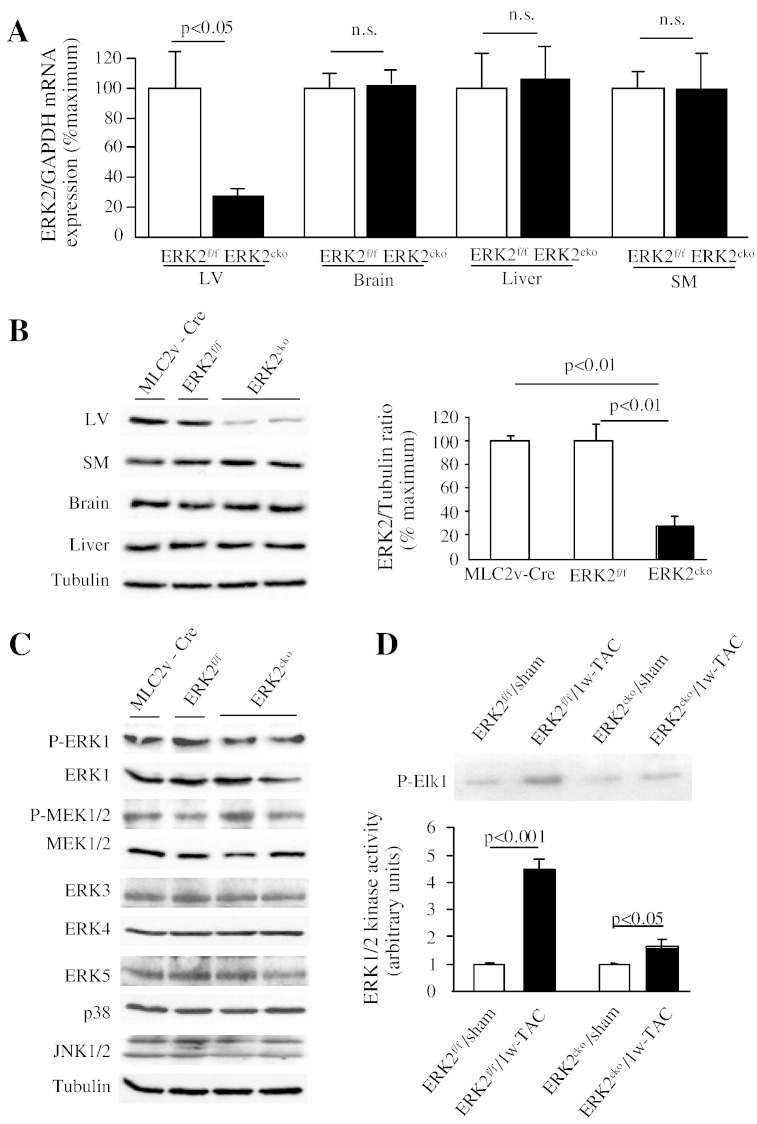
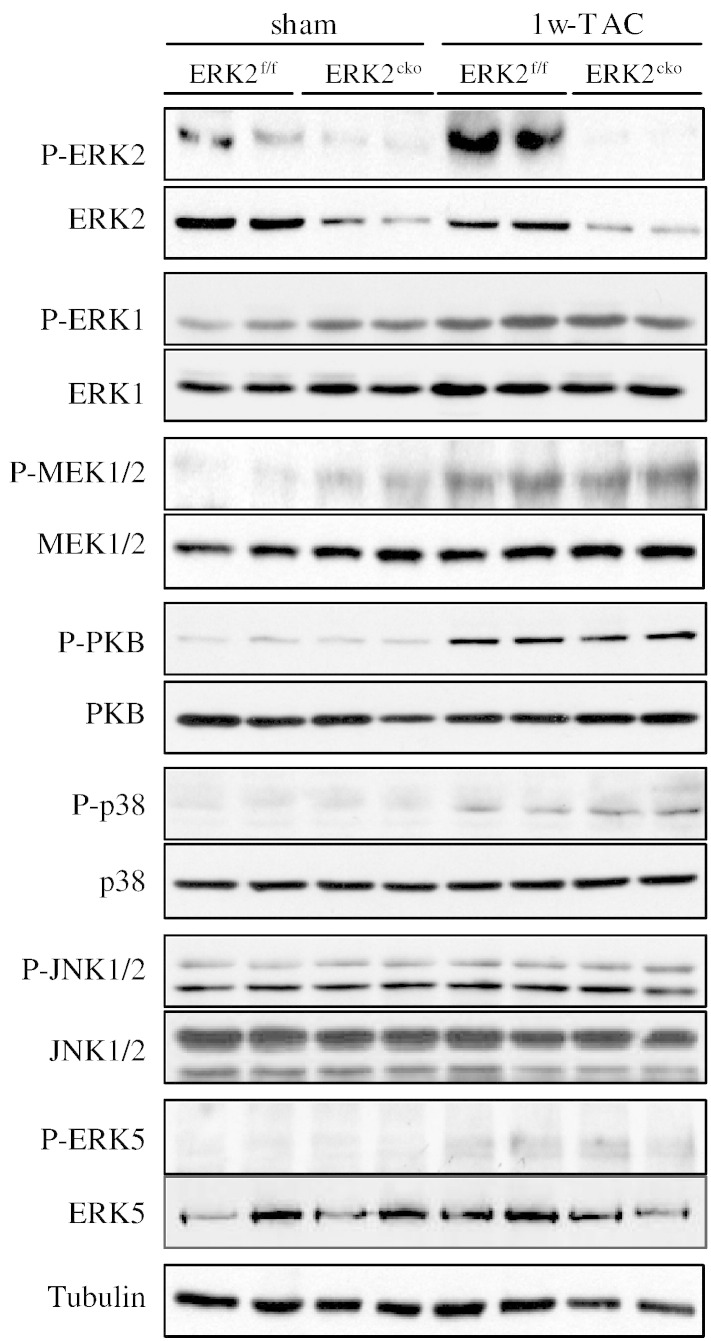
Due to the embryonic lethality caused by conventional gene targeting of the erk2 gene, we generated mice with cardiomyocyte-specific deletion of ERK2 (ERK2cko) using the Cre-LoxP system. ERK2cko mice were fertile, viable and visibly normal suggesting that the cardiomyocyte-specific deletion of ERK2 in ventricles alone did not affect heart development (data not shown). The mRNA levels of ERK2 were significantly reduced (73.34%) in ERK2cko ventricles, whereas mRNA levels in skeletal muscle (SM), brain, or liver were indistinguishable in comparison to the control group (MLC2v-Cre transgenic mice or ERK2f/f mice) (Fig. 1A). Protein level of ERK2 in the ventricle was reduced by 75.14% in ERK2cko. Comparable protein levels of ERK2 were found in brain, liver and SM in MLC2v-Cre mice, ERK2f/f or ERK2cko mice (Fig. 1B). Protein expression or phosphorylation of ERK1 or MEK1/2 was unchanged in ventricles in all mouse lines (Fig. 1C), which indicated that ERK2 expression was not affected by Cre expression or compensatory change of ERK1. In addition, the absence of ERK2 in the ventricles did not induce alterations by other pathways as the protein expression of ERK5, p38 and JNK were not different (Fig. 1C). To further determine whether ERK1 is compensating for the loss of ERK2, we examined the kinase activity of ERK1/2 in response to hypertrophic stimulation (Fig. 1D).
Fig. 1.
Characterisation of cardiomyocyte-specific deletion of ERK2. (A) Quantitative real-time PCR analysis of mRNA levels of ERK2 in the left ventricle (LV), skeletal muscle (SM), brain, and liver. A 73% decrease in mRNA level was shown only in LV of ERK2cko mice in comparison to ERK2f/f mice. Data were derived from 3 independent experiments performed in triplicate and normalized to GAPDH content (n = 3). (B) Immunoblot analysis confirmed specificity of ERK2 deletion in LV. ERK2 protein expression was comparable in various tissues. Tubulin served as protein loading control. The ratio of ERK2 expression to tubulin is shown in the bar graph. (C) Western blot analyses demonstrated unchanged activation and expression levels of ERK1 and MEK1/2. Expression of ERK3, ERK4, ERK5, p38 and JNK in ventricular extracts was the same in all genotypes. Tubulin served as protein loading control. (D) ERK1/2 kinase activity was examined on the tissue subjected to 1 week of TAC, demonstrating ERK1 activation did not compensate the loss of ERK2 in the heart (n = 5). n.s.: no significant difference. Data presented as mean ± SEM.
3.2. ERK2cko mice had an attenuated hypertrophic response to pressure overload
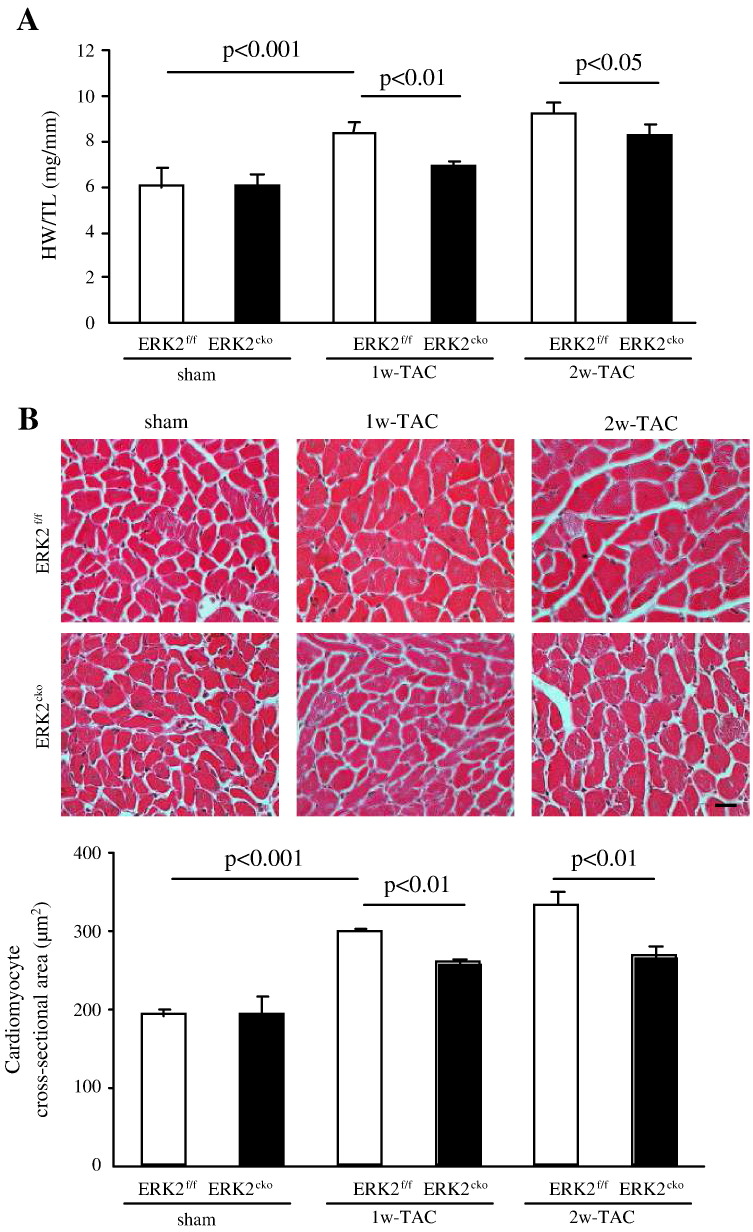
To determine whether ERK2 is essential for the development of pressure overload-induced cardiac hypertrophy, 1 or 2 weeks of TAC was performed on ERK2f/f and ERK2cko mice. Under the similar pressure gradients (35–40 mm Hg), whilst ERK2f/f mice showed a 30% and 53% increase in heart weight/tibia length (HW/TL) ratio following 1w- or 2w-TAC, respectively, the increase in ERK2cko mice was significantly lower (only 11% or 33% respectively) (Fig. 2A). Consistent with these results, the cardiomyocyte cross-sectional area was smaller in ERK2cko 1w-TAC cardiomyocytes (258.91 ± 3.27 μm2) in comparison to ERK2f/f cardiomyocytess (298.80 ± 2.33 μm2) (Fig. 2B); furthermore; ERK2cko cardiomyocyte (267.31 ± 13.87 μm2) also showed smaller cross-sectional area than ERK2f/f cardiomyocyte (332.81 ± 17.02 μm2) after 2w TAC, suggesting a blunted enlargement of cardiomyocyte hypertrophy in ERK2cko mice subjected short-term TAC.
Fig. 2.
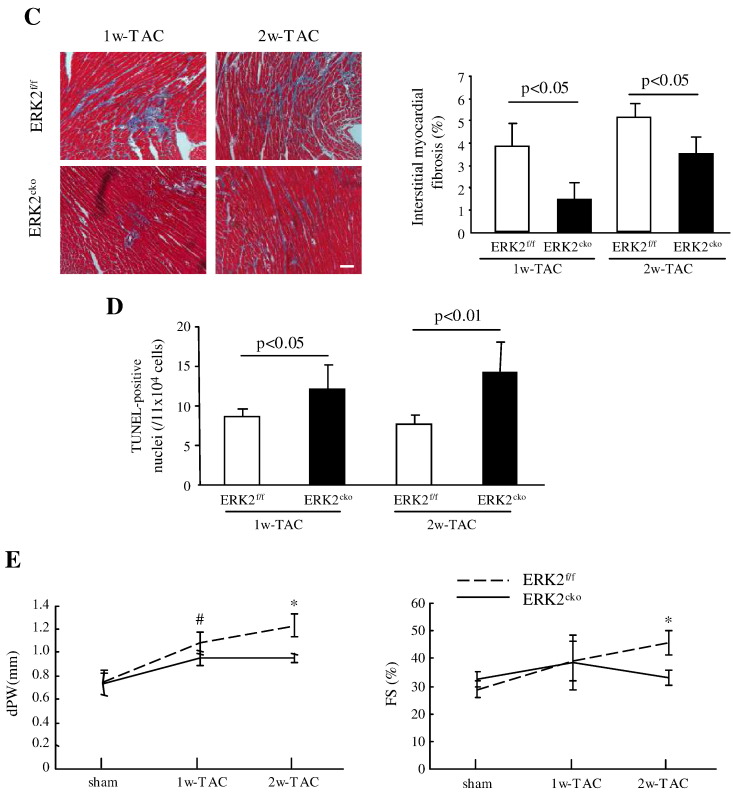
Attenuated hypertrophic response in ERK2cko mice following short-term TAC. (A) HW/TL ratios of ERK2f/f and ERK2cko mice were calculated following sham or TAC treatment. (B) ERK2cko mice developed less hypertrophy after 1 or 2 weeks of TAC. Haematoxylin/eosin staining of ventricular cross-sections (top panel, scale = 20 μm). The measurement of mean cross-sectional area showed less enlarged ERK2cko cardiomyocytes (lower panel). (C) Masson's trichrome staining of the hearts demonstrated less interstitial ventricular fibrosis in ERK2cko-TAC hearts (scale = 50 μm). Quantification of the relative area of fibrosis is expressed as a percentage of the fibrosis area in the microscope view. (D) The bar graph summarizes the number of apoptotic nuclei in ERK2cko ventricles compared to that in ERK2f/f ventricles after 1w- or 2w-TAC. (E) Parameters of end-diastolic left ventricular posterior wall thickness (dPW) and fractional shortening (FS%) were shown to demonstrate cardiac function. n = 7. # or *, P < 0.05 ERK2cko versus ERK2f/f. Data presented as mean ± SEM.
Besides hypertrophic growth, pressure overload also induces accumulation of interstitial fibrosis. However, Masson's trichrome staining showed 62% less ventricular fibrosis in ERK2cko 1w-TAC mice compared to the ERK2f/f 1w-TAC group, while ERK2cko had 33% less fibrosis than ERK2f/f after 2w-TAC (Fig. 2C). We also investigated apoptosis post pressure overload using TUNEL staining. After 1 or 2 weeks of TAC, 38% or 50% pronounced TUNEL-positive nuclei in ERK2cko ventricles were detected compared to the control-TAC group (Fig. 2D), indicating that ERK2 was required for cardiomyocyte survival in response to TAC. Consistent with cardiomyocyte growth by H&E staining, echocardiography substantiated reduced end-diastolic left ventricular posterior wall thickness (dPW) in ERK2cko compared to ERK2f/f heart subjected to 1w- or 2w-TAC. However, ERK2cko showed decreased FS (33.1 ± 2.5% versus 45.7 ± 4.2% [ERK2f/f]) following 2 weeks of TAC (Fig. 2E).
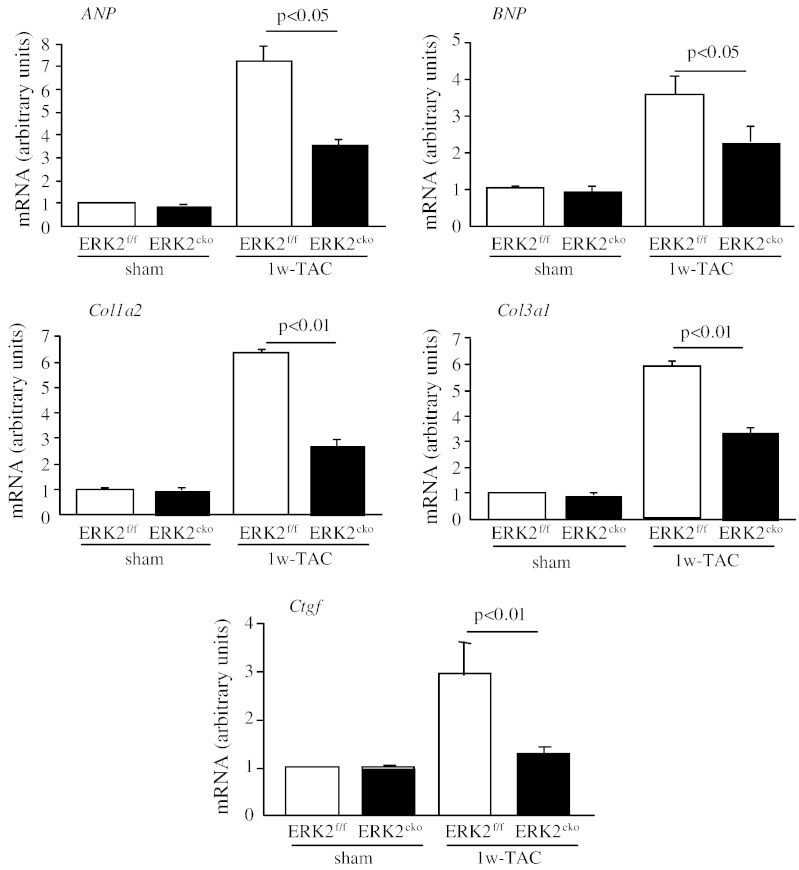
In concordance with the above, upregulation of the transcripts of hypertrophic gene markers, atrial natriuretic peptide (ANP) and brain natriuretic peptide (BNP), was remarkably blunted in ERK2cko TAC mice (Fig. 3). We also found that the fibrosis marker genes procollagen type 1α2 (Col1a2), procollagen type 3α3 (Col3a3) and connective tissue growth factor (Ctgf) were significantly less upregulated in ERK2cko ventricles compared to the controls (Fig. 3), suggesting that ERK2cko mice developed less interstitial fibrosis.
Fig. 3.
Quantitative real-time PCR analyses of hypertrophic gene markers and fibrosis gene markers. ANP or BNP and Col1α2, Col3α1 or Ctgf. Data derived from 3 independent experiments performed in triplicate and normalized to GAPDH content (n = 3). Data presented as mean ± SEM.
To further investigate the mechanisms involved in the attenuated hypertrophic response, we examined the activation and expression of both anti- and pro-hypertrophic signaling pathways including ERK2, ERK1, MEK1/2, PKB, p38, JNK1/2 and ERK5 following 1 week of TAC stimulation. Notably, normal heart subjected to TAC showed activation of ERK2. ERK1 was not able to induce compensatory changes in ERK2cko-TAC ventricles (Fig. 4). In addition, the activation of the other hypertrophic regulators was also comparable between the two genotypes. Taken together, these results suggested that the blunted hypertrophic response to pressure overload was due to the absence of ERK2 from the ventricles.
Fig. 4.
Analysis of hypertrophic regulators in ERK2f/f and ERK2cko ventricles. Protein extracts from ERK2f/f and ERK2cko ventricles after 1 week of sham or TAC operation were subjected to immunoblot analyses for total ERK2, ERK1, MEK1/2, PKB, p38, JNK and ERK5 expression as well as their phosphorylation levels using specific antibodies.
3.3. ERK2cko mice were susceptible to heart failure in response to prolonged TAC
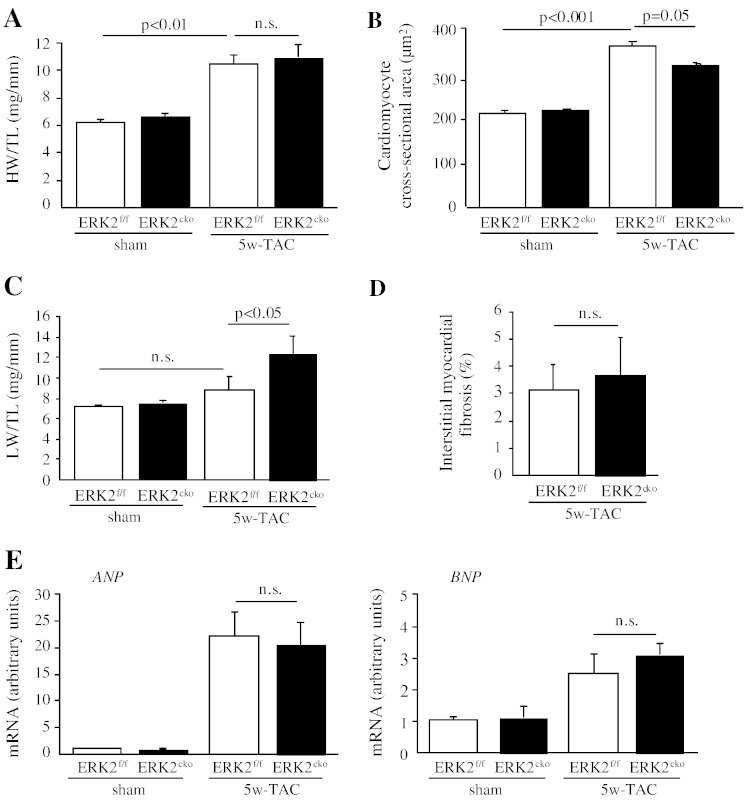
To investigate whether loss of ERK2 in cardiomyocytes predisposes mice to heart failure, the duration of pressure overload in both genotypes was extended. After 5 weeks of TAC treatment, ERK2cko and ERK2f/f mice showed comparably elevated HW/TL ratios (Fig. 5A). Nevertheless, cardiomyocyte cross-sectional area showed a decrease in ERK2cko compared to ERK2f/f (Fig. 5B). The greater lung weight/tibia length (LW/TL) ratio observed in ERK2cko 5w-TAC mice indicated pulmonary edema, likely due to contractile insufficiently (Fig. 5C). Under prolonged hypertrophic stress, both ERK2f/f and the ERK2cko groups had an increase in fibrosis (Fig. 5D). Expression levels of ANP and BNP were remarkably increased following 5 weeks of TAC in both ERK2f/f and ERK2cko mice (Fig. 5E).
Fig. 5.
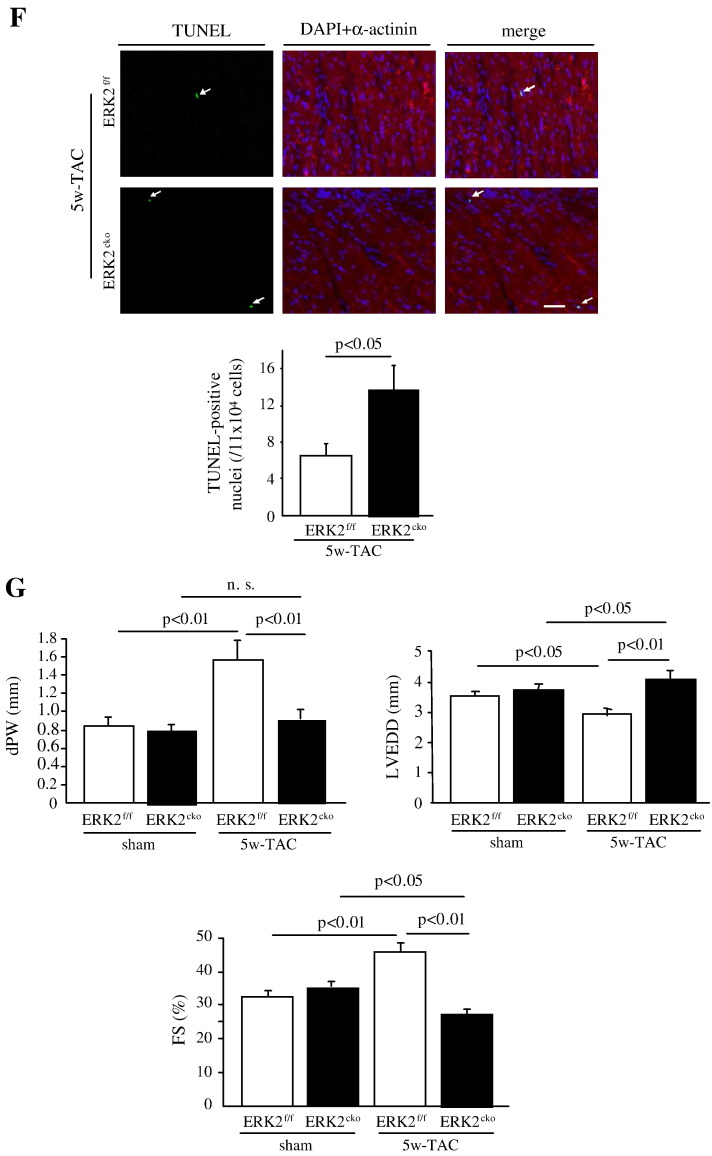
ERK2cko mice were prone to heart failure after prolonged pressure overload. (A) After 5 weeks of TAC, ERK2f/f and ERK2cko mice both showed increased HW/TL ratio compared to sham operated groups. (B) The measurement of mean cross-sectional area showed blunted ERK2cko cardiomyocytes. (C) ERK2cko mice showed significantly increased lung weight/tibia length (LW/TL) ratio. (D) Quantification of interstitial ventricular fibrosis in ERK2f/f and ERK2cko mice. (E) Quantitative real-time PCR analyses of ANP and BNP. Data derived from 3 independent experiments performed in triplicate and normalized to GAPDH content. (F) Augmented apoptosis in ERK2cko ventricular cardiomyocytes was detected by TUNEL assay (arrows indicate TUNEL positive nuclei, scale = 100 μm). Triple staining was performed: TUNEL (green), DAPI (blue), α-actinin (red). (G) M-mode echocardiography of ERK2f/f and ERK2cko mice following sham or TAC revealed detrimental cardiac dysfunction in ERK2cko 5w-TAC group. dPW, left ventricular end-diastolic dimension (LVEDD), and FS% are shown. n = 9 to 10. n.s.: no significant difference. Data presented as means ± SEM.
Whilst, same as displayed in 1w-TAC heart, the long-term pressure overload also led to enhanced apoptosis in ERK2cko cardiomyocytes (14/1 × 104 cells) in comparison to the control group (6.5/1 × 104 cells) (Fig. 5F), which indicated that the increased ventricular wall stress due to the lack of appropriate hypertrophy in ERK2cko induced more cell death. Echocardiography revealed that the end-diastolic left ventricular posterior wall thickness (dPW) was reduced in the ERK2cko heart after 5 weeks of TAC (0.9 ± 0.12 mm versus 1.57 ± 0.20 mm in ERK2f/f mice), while left ventricular end-diastolic dimension (LVEDD) was increased (Fig. 5G). This structural change was accompanied by cardiac dysfunction in ERK2cko mice, in which a substantially reduced FS was observed (28.62 ± 1.99%) versus ERK2f/f controls (45.68 ± 2.8%) (Fig. 5G). Collectively, these data demonstrated that, following 5 weeks of TAC, lack of ERK2 led to increased interstitial fibrosis and apoptosis. ERK2cko mice were more vulnerable to long-term pressure overload-induced stress and showed early signs of heart failure.
3.4. Blunted cardiac hypertrophic response was shown in ERK2cko mice subjected to chronic β-adrenergic stimulation
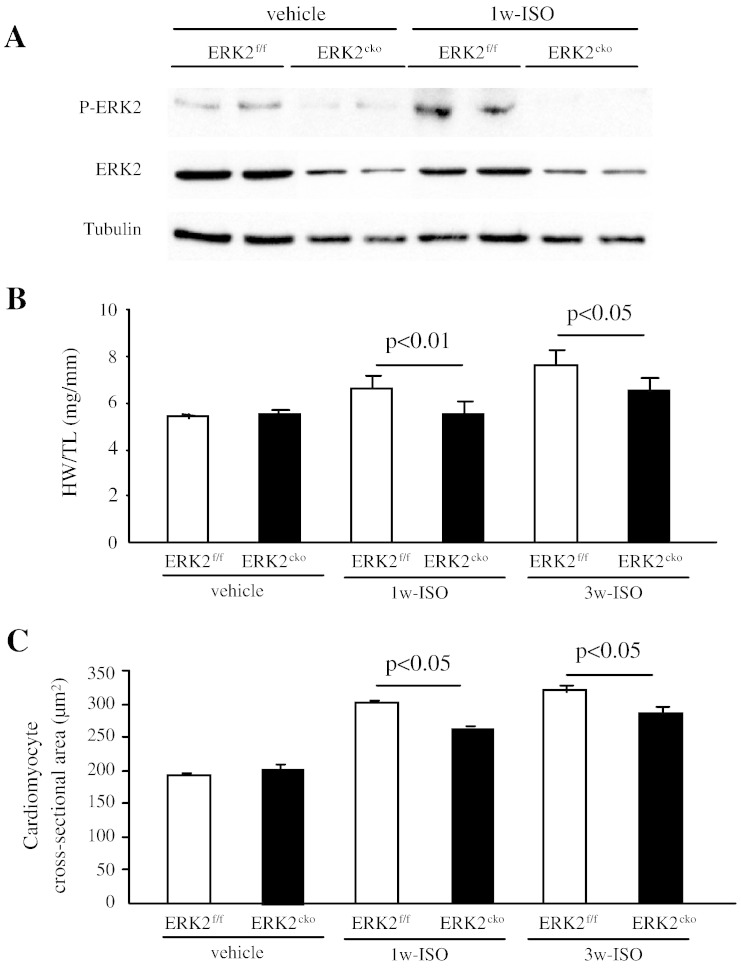
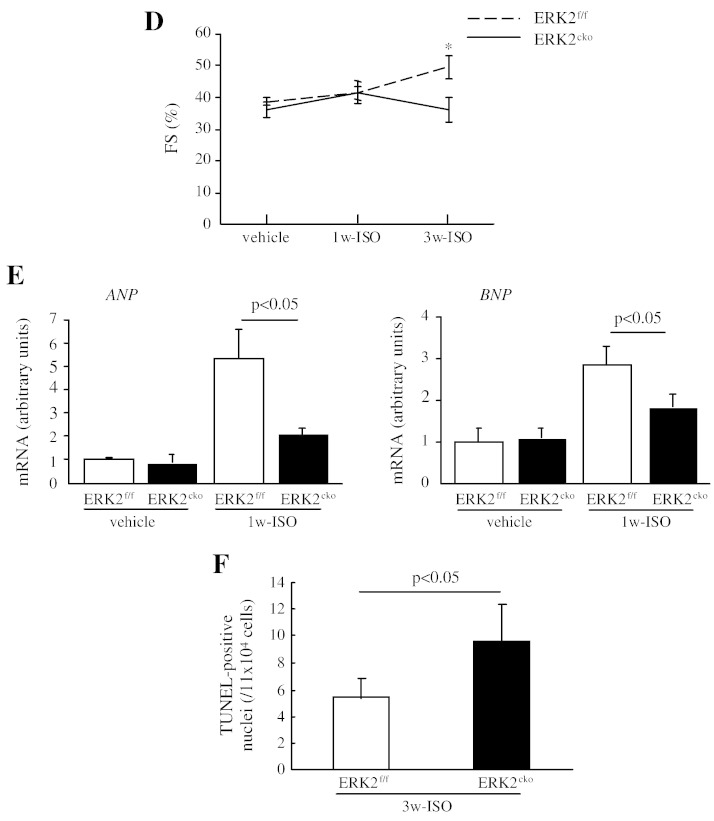
We next explored whether ERK2 is required for the hypertrophic cardiac response to chronic β-adrenergic stimulation. After 1 week of ISO administration (10 mg/kg/day), ERK2 was activated in the normal heart (Fig. 6A). The heart tissues subjected 1w- or 3w-ISO stimulus were analysed by morphological and biochemical studies. After 1 or 3 weeks of ISO, HW/TL ratio was 17% or 15% less in ERK2cko than that in ERK2f/f respectively (Fig. 6B). Histology performed to investigate hypertrophic growth at the cellular level indicated that the cardiomyocyte cross-sectional area in ERK2cko 1w-ISO treated mice (264.75 ± 2.80 μm2) was less than the ERK2f/f 1w-ISO group (300.71 ± 4.92 μm2) (Fig. 6C). In addition, 3 weeks of ISO also induced a blunted cross-sectional area in ERK2cko (287.12 ± 10.8 versus 322.12 ± 8.1 in ERK2f/f) (Fig. 6C); nevertheless, FS was decreased in ERK2cko followed by 3 week of ISO stimulation (Fig. 6D). In line with the above results, the transcript levels of the hypertrophic markers ANP and BNP were lower in ERK2cko 1w-ISO treated mice than in ERK2f/f 1w-ISO ventricles (Fig. 6E). After long-term stimulation of ISO, ERK2cko displayed augmented apoptosis (Fig. 6F). To summarize, these results indicated that ERK2 is required for hypertrophic remodeling also caused by chronic β-adrenergic stimulation; furthermore, loss of ERK2 led to increased susceptibility to heart failure by long-term ISO stimulation.
Fig. 6.
ERK2 mediated cardiac hypertrophy induced by isoproterenol. (A) Following 1 week of ISO stimulation, immunoblot analysis revealed that the activation of ERK2 was increased in normal heart. (B) ERK2cko mice showed less enlargement of the HW/TL ratio compared to control 1w- or 3w-ISO treated group. (C) The quantification of haematoxylin/eosin staining of ventricular cross-sections also displayed less cardiac hypertrophic growth in ERK2cko hearts. (D) FS% indicated the reduced cardiac function in ERK2cko following 3 weeks of ISO. n = 6. *, P < 0.05 ERK2cko versus ERK2f/f. (E) Real-time PCR analyses indicating lower level of ANP and BNP mRNA expression in ERK2cko 1w-ISO ventricles. Data were derived from 3 independent experiments performed in triplicate and normalized to GAPDH content (n = 3). (F) Increased apoptosis in ERK2cko cardiomyocytes after 3 weeks of ISO stimulation by TUNEL assay. n = 5. Data presented as means ± SEM.
3.5. ERK2 regulates hypertrophy by nuclear induction of ANP expression and antagonizes apoptosis
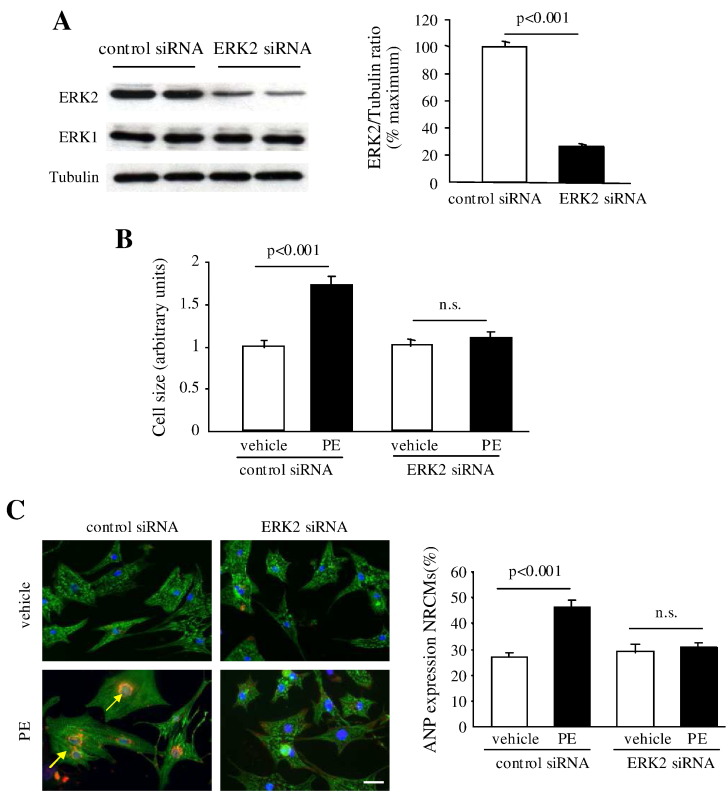
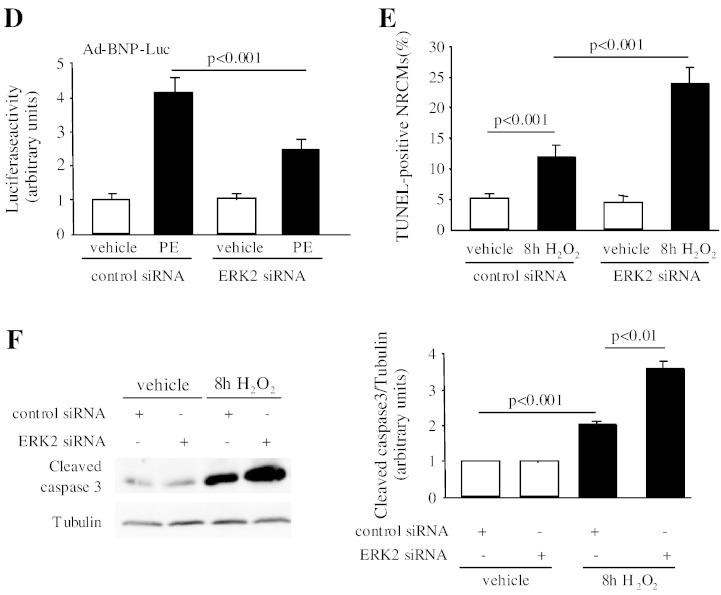
Next, we investigated the molecular mechanism by which ERK2 regulates cardiac hypertrophy by performing in vitro studies in NRCMs. To diminish expression of ERK2, NRCMs were transfected with rat ERK2 siRNA. Western blot result showed that expression of ERK2 was 78% decreased by ERK2 siRNA, while ERK1 expression was not changed (Fig. 7A). NRCMs transfected with either control siRNA or ERK2 siRNA were subsequently treated with PE (30 mM) for 48 h. ERK2 siRNA treated NRCMs did not undergo a hypertrophic growth, whereas NRCMs transfected with control siRNA showed a remarkable increase in cell size (Fig. 7B). The role of ERK2 in reactivation of ANP and BNP was further assessed. Firstly, loss of ERK2 abrogated the hypertrophic effect of PE, showing reduced ANP expression in NRCMs (Fig. 7C). Next, a luciferase reporter assay was used to detect the BNP reporter activity. In response to PE stress, adenoviral infection of the BNP-luciferase reporter (Ad-BNP-Luc) in control NRCMs led to enhanced BNP reporter activity. However, knockdown of ERK2 showed less increase in BNP activity (Fig. 7D). The results strongly suggested an important role for ERK2 as an activator of ANP and BNP expression in hypertrophic growth.
Fig. 7.
Knockdown of ERK2 diminished hypertrophic growth and promoted apoptosis in neonatal rat cardiomyocytes (NRCMs). (A) Western blot analysis showed that expression of ERK2 was decreased by transfection of rat ERK2 siRNA. (B) Cardiomyocyte cell size was not increased in NRCMs transfected with ERK2 siRNA following PE administration for 48 h. (C) Representative images of triple staining of NRCMs (red staining for ANP [arrows], green for α-actinin, and blue for DAPI, scale bar = 20 μm). Quantification of ANP expression in NRCMs is presented by the graph. (D) ERK2 siRNA treated NRCMs displayed blunted BNP reporter activity compared to the significantly increased activity in control NRCMs after PE treatment. (E) Enhanced apoptosis levels were detected in ERK2 siRNA transfected NRCMs following 100 μM H2O2 treatment for 8 h by TUNEL assay. The bar graphs represent the number of apoptotic nuclei. (F) Immunoblot analysis of protein levels of active caspase 3. Tubulin expression is the protein loading control. The ratio of active caspase 3 to tubulin was dramatically increased in the NRCMs with loss of ERK2 after 8 h treatment of H2O2. n = 5. n.s.: no significant difference. Data presented as mean ± SEM.
In addition, we examined the role of ERK2 in apoptosis with a pro-apoptotic agent, hydrogen peroxide (H2O2, 100 μM). Following 8 h of H2O2 treatment, NRCMs transfected with ERK2 siRNA showed more obvious apoptosis (the number of TUNEL-positive nuclei was 2 times of that in control NRCMs) (Fig. 7E), along with an augmented level of the active caspase 3 (Fig. 7F). Taken together, ERK2 was required for cardiac hypertrophic growth in NRCMs in response to PE stimulation, and loss of ERK2 led to a dramatic increase in apoptotic cardiomyocytes.
3.6. ERK2f/f and ERK2cko mice showed similar physiological cardiac hypertrophy in response to swimming exercise
Finally, the role of ERK2 in physiological hypertrophy was determined. To induce physiological hypertrophy, both genotypes were subjected to swimming exercise for 4 weeks. Histological analysis demonstrated comparable hypertrophic growth with increased myocardial cross-sectional areas in both swimming groups (Supplement Fig. IA, B). In addition, increased expression levels of pathological hypertrophic markers (ANP, BNP) were not observed (Supplement Fig. IC). Echocardiographic assessment demonstrated a similarly preserved cardiac function in both genotypes (Supplement Table I). To estimate whether ERK2 is involved in the regulation of physiological hypertrophy, we examined the activation of ERK2 and found that ERK2 was not activated in normal heart after long-term swimming (Supplement Fig. ID). Taken together, swimming induced hypertrophic growth; however, ERK2 was unlikely to be involved in the regulation of physiological hypertrophy.
4. Discussion
In response to pathological stress, the heart undergoes compensatory hypertrophic enlargement characterized by the increased cross sectional area of individual cardiomyocytes. With prolonged hypertrophy, loss of myocytes by apoptosis and interstitial fibrosis lead to heart failure. In the current study, the specific biological role of ERK2 in the heart was investigated by using the ERK2 cardiomyocyte-specific knockout mouse model. In the absence of ERK2, the heart exhibited attenuated cardiac hypertrophic remodeling with short-term pressure overload or chronic β-adrenergic stimulation. However, when exposed to prolonged pathological stimulation, the mice with cardiomyocyte specific deletion of ERK2 were susceptible to heart failure.
4.1. ERK2 is required for pathological hypertrophic growth in response to overload stress
The majority of the previous studies to discover the role of ERK1/2 in myocytes have been performed on several genetically modified mouse models. Transgenic mice overexpressing MEK1 or dual-specificity phosphatase 6 (DUSP6) knockouts increased the basal phosphorylation of ERK1/2, indicating a crucial role of ERK1/2 in developing cardiac hypertrophy [18,19]. However, reduction of ERK1 (ERK1−/−) or ERK2 (ERK2+/−) expression or complete inhibition of ERK1/2 by overexpression of DUSP6 revealed no reduction in pathologic stimulus (TAC)-induced heart hypertrophy [21], indicating that ERK1/2 may not be necessary for cardiac hypertrophy. A recent study also revealed that the ERK1−/− ERK2cko mice, which contained a deletion of both ERK1 and ERK2, led to generation of spontaneous cardiac hypertrophy without external stimuli and augmented eccentric hypertrophy after 2 weeks of TAC [28].
On the contrary, Sbroggio et al. showed that the absence of IQ motif-containing GTPase-activating protein 1 (IQGAP1), as a scaffold for the ERK1/2 cascade, resulted in impaired cardiomyocyte hypertrophy accompanied by the blunted reactivation of the gene programme through diminishing the activity of ERK1/2 [29]. Further evidence indicated that transgenic mice with enhanced ERK2 Thr188 autophosphorylation displayed increased hypertrophic growth whereas mice with suppressed ERK2 Thr188 phosphorylation showed resistance to pressure overload-induced hypertrophy [30]. Therefore, the role of ERK1 or ERK2 in the heart needed to be specified. We established the mouse line with specific absence of ERK2 but normal expression of ERK1 in cardiomyocytes which did not show abnormal cardiac development. ERK2cko mice demonstrated blunted hypertrophic growth characterized by smaller cross-sectional areas associated with an attenuated increase in transcript levels of hypertrophic biomarkers. ERK2cko mice also showed identical expression levels of MEK1/2, ERK1, ERK3, ERK4, ERK5, p38 and JNK. Therefore, this study specifically portrayed the contribution of ERK2 in cardiac hypertrophic remodeling. In line with these observations, we also found unvaried reactivation levels of ANP and BNP in ERK2 siRNA transfected NRCMs following hypertrophic stimulation. Together, it is plausible to propose that ERK2 activation driven by autophosphorylation may play a more direct role in regulating cardiac hypertrophy.
Exercise-induced cardiac hypertrophy is a prototype of physiological heart growth, characterized by normal cardiac structure and preserved or improved cardiac function. Several lines of evidence have demonstrated that IGF-PI3K-AKT signaling pathway plays a central role in the development of physiological in response to exercise [31,32]. In addition, a recent study discovered that transcriptional factor C/EBPβ was downregulated with exercise stimulation to derepress cardiomyocyte growth and proliferation [33,34]. The potential ability of cardiomyocyte proliferation is known to serve as a protective basis for pathological cardiac remodeling [35]. The negative regulation of C/EBPβ by AKT [33] and cross-talk of AKT-mTOR-p70S6K with Raf-MEK-ERK in cells [36] led us to investigate whether ERK2 has function in mediating physiological hypertrophy. Surprisingly, in the current study, EKR2cko mice displayed comparable heart growth by morphological studies after 4 weeks of swimming exercise. Although ERK1/2 was reported to modulate proliferation via ribosomal s6 kinase (RSK) or transcriptional factors such as Elk1, c-Fos, and Sp1 in other cells [37], further studies are required to clarify the molecular mechanism of how ERK2 is involved in regulation of cardiomyocyte proliferation.
4.2. EKR2 is an anti-apoptotic factor in the cardiomyocytes
We also examined the role of ERK2 in regulating cardiomyocyte survival. The present study demonstrated the cardiomyocyte protective function of ERK2. A previous study reported that pro-apoptotic genes, such as Bad and p53, were upregulated in the absence of ERK1/2 [30], suggesting that ERK1/2 suppressed apoptotic response within the heart. Consistently, the current study detected enhanced caspase 3 activity in ERK2 knocked down NRCMs following stimulation by H2O2. Caspase 3 is known to interact with caspase 9 which were demonstrated to be inhibited by activation of the ERK pathway [38]. Several convincing evidence indicated that ERK1/2 has anti-apoptotic function in cells due to inactivation of pro-apoptotic factors, e.g. Bim and BAD, to inhibit the release of caspase members; in the meantime, they can activate pro-survival factors, e.g. Bcl-Xl and CREB [39]. However, ERK1 and ERK2 have distinct target factors of cell death and survival, for instance, ERK2 was shown not only to dominantly inactivate the pro-apoptotic gene Noxa and p53 upregulated modulator of apoptosis (PUMA) but also efficiently repress caspase 8 and cleaved poly ADP ribose polymerase (PARP) in melanoma cells [40]. Although further studies are required to uncover specific downstream targets of ERK2 in protecting cardiomyocytes following stress, ERK2 seems to be necessary for maintaining cardiac function during hypertrophic remodeling and for protecting cardiomyocytes against pathologic stress-induced apoptosis.
4.3. The role of ERK2 in regulating the formation of interstitial fibrosis awaits determination
Accumulation of interstitial fibrosis is also a key mediator for heart function. The present study showed less fibrosis in ERK2cko mice following short-term TAC, determined by histological staining and decreased expression of the fibrotic gene markers. CTGF, as a key mediator of cardiac fibrosis, can be expressed and secreted from cardiomyocytes to induce fibrosis in response to stimulation [41]. Previous studies determined the regulation of CTGF expression by transcriptional factor MEF2 or STAT3 in fibroblasts [42,43]. Thus, less interstitial fibrosis and Ctgf mRNA in ERK2cko 1w- or 2w-TAC hearts are likely due to downregulation of MEF2 or STAT3.
However, following prolonged pathological stress-induced cardiac dysfunction, comparable fibrosis formation was detected in ERK2cko 5w-TAC hearts compared with the ERK2f/f group. It is plausible that ERK2 knockout cardiomyocytes are sensitized to stress-induced cell death and sequentially beget heart dysfunction, in which fibroblasts are activated to generate more fibrosis. In addition, Thum et al. demonstrated that, in cardiac fibroblasts, micro RNA-21 promoted cardiac fibrosis by promoting ERK1/2 signaling following long-term pressure overload [44]. Nevertheless, future studies are required to specifically focus on the role of ERK2 in cardiac fibrosis formation during pathological hypertrophy by using mice with cardiac fibroblast-specific deletion of ERK2.
5. Conclusion
In conclusion, this study provides convincing evidence that clarifies the in vivo role of ERK2 in the heart. ERK2 is required for adaptive hypertrophic remodeling and protection of cardiomyocytes in response to pathological. However, ERK2 is not involved in physiologic cardiac growth, which contributes the invaluable information for the development of new therapeutic strategies treating heart diseases.
Disclosures
None.
Acknowledgment
This work was supported by the British Heart Foundation (PG/09/052/27833, PG/12/76/29852) and the Medical Research Council (G1002082).
Appendix A. Supplementary data
Supplementary material.
References
- 1.Hill J.A., Olson E.N. Cardiac plasticity. N Engl J Med. 2008;358:1370–1380. doi: 10.1056/NEJMra072139. [DOI] [PubMed] [Google Scholar]
- 2.Maron B.J. Hypertrophic cardiomyopathy in childhood. Pediatr Clin North Am. 2004;51:1305–1346. doi: 10.1016/j.pcl.2004.04.017. [DOI] [PubMed] [Google Scholar]
- 3.Ogata T., Yoshida R. PTPN11 mutations and genotype-phenotype correlations in Noonan and LEOPARD syndromes. Pediatr Endocrinol Rev. 2005;2:669–674. [PubMed] [Google Scholar]
- 4.Burch M., Sharland M., Shinebourne E., Smith G., Patton M., McKenna W. Cardiologic abnormalities in Noonan syndrome: phenotypic diagnosis and echocardiographic assessment of 118 patients. J Am Coll Cardiol. 1993;22:1189–1192. doi: 10.1016/0735-1097(93)90436-5. [DOI] [PubMed] [Google Scholar]
- 5.Tartaglia M., Mehler E.L., Goldberg R., Zampino G., Brunner H.G., Kremer H. Mutations in PTPN11, encoding the protein tyrosine phosphatase SHP-2, cause Noonan syndrome. Nat Genet. 2001;29:465–468. doi: 10.1038/ng772. [DOI] [PubMed] [Google Scholar]
- 6.Araki T., Mohi M.G., Ismat F.A., Bronson R.T., Williams I.R., Kutok J.L. Mouse model of Noonan syndrome reveals cell type- and gene dosage-dependent effects of Ptpn11 mutation. Nat Med. 2004;10:849–857. doi: 10.1038/nm1084. [DOI] [PubMed] [Google Scholar]
- 7.Fragale A., Tartaglia M., Wu J., Gelb B.D. Noonan syndrome-associated SHP2/PTPN11 mutants cause EGF-dependent prolonged GAB1 binding and sustained ERK2/MAPK1 activation. Hum Mutat. 2004;23:267–277. doi: 10.1002/humu.20005. [DOI] [PubMed] [Google Scholar]
- 8.Nakamura T., Colbert M., Krenz M., Molkentin J.D., Hahn H.S., Dorn G.W., II Mediating ERK 1/2 signaling rescues congenital heart defects in a mouse model of Noonan syndrome. J Clin Invest. 2007;117:2123–2132. doi: 10.1172/JCI30756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Krenz M., Gulick J., Osinska H.E., Colbert M.C., Molkentin J.D., Robbins J. Role of ERK1/2 signaling in congenital valve malformations in Noonan syndrome. Proc Natl Acad Sci U S A. 2008;105:18930–18935. doi: 10.1073/pnas.0806556105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kontaridis M.I., Swanson K.D., David F.S., Barford D., Neel B.G. PTPN11 (Shp2) mutations in LEOPARD syndrome have dominant negative, not activating, effects. J Biol Chem. 2006;281:6785–6792. doi: 10.1074/jbc.M513068200. [DOI] [PubMed] [Google Scholar]
- 11.Hanna N., Montagner A., Lee W.H., Miteva M., Vidal M., Vidaud M. Reduced phosphatase activity of SHP-2 in LEOPARD syndrome: consequences for PI3K binding on Gab1. FEBS Lett. 2006;580:2477–2482. doi: 10.1016/j.febslet.2006.03.088. [DOI] [PubMed] [Google Scholar]
- 12.Marin T.M., Keith K., Davies B., Conner D.A., Guha P., Kalaitzidis D. Rapamycin reverses hypertrophic cardiomyopathy in a mouse model of LEOPARD syndrome-associated PTPN11 mutation. J Clin Invest. 2011;121:1026–1043. doi: 10.1172/JCI44972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wellbrock C., Karasarides M., Marais R. The RAF proteins take centre stage. Nat Rev Mol Cell Biol. 2004;5:875–885. doi: 10.1038/nrm1498. [DOI] [PubMed] [Google Scholar]
- 14.Harris I.S., Zhang S., Treskov I., Kovacs A., Weinheimer C., Muslin A.J. Raf-1 kinase is required for cardiac hypertrophy and cardiomyocyte survival in response to pressure overload. Circulation. 2004;110:718–723. doi: 10.1161/01.CIR.0000138190.50127.6A. [DOI] [PubMed] [Google Scholar]
- 15.Yamaguchi O., Watanabe T., Nishida K., Kashiwase K., Higuchi Y., Takeda T. Cardiac-specific disruption of the c-raf-1 gene induces cardiac dysfunction and apoptosis. J Clin Invest. 2004;114:937–943. doi: 10.1172/JCI20317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kehat I., Molkentin J.D. Extracellular signal-regulated kinase 1/2 (ERK1/2) signaling in cardiac hypertrophy. Ann N Y Acad Sci. 2010;1188:96–102. doi: 10.1111/j.1749-6632.2009.05088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Lips D.J., Bueno O.F., Wilkins B.J., Purcell N.H., Kaiser R.A., Lorenz J.N. MEK1-ERK2 signaling pathway protects myocardium from ischemic injury in vivo. Circulation. 2004;109:1938–1941. doi: 10.1161/01.CIR.0000127126.73759.23. [DOI] [PubMed] [Google Scholar]
- 18.Bueno O.F., De Windt L.J., Tymitz K.M., Witt S.A., Kimball T.R., Klevitsky R. The MEK1-ERK1/2 signaling pathway promotes compensated cardiac hypertrophy in transgenic mice. EMBO J. 2000;19:6341–6350. doi: 10.1093/emboj/19.23.6341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Maillet M., Purcell N.H., Sargent M.A., York A.J., Bueno O.F., Molkentin J.D. DUSP6 (MKP3) null mice show enhanced ERK1/2 phosphorylation at baseline and increased myocyte proliferation in the heart affecting disease susceptibility. J Biol Chem. 2008;283:31246–31255. doi: 10.1074/jbc.M806085200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bueno O.F., Molkentin J.D. Involvement of extracellular signal-regulated kinases 1/2 in cardiac hypertrophy and cell death. Circ Res. 2002;91:776–781. doi: 10.1161/01.res.0000038488.38975.1a. [DOI] [PubMed] [Google Scholar]
- 21.Purcell N.H., Wilkins B.J., York A., Saba-El-Leil M.K., Meloche S., Robbins J. Genetic inhibition of cardiac ERK1/2 promotes stress-induced apoptosis and heart failure but has no effect on hypertrophy in vivo. Proc Natl Acad Sci U S A. 2007;104:14074–14079. doi: 10.1073/pnas.0610906104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pages G., Guerin S., Grall D., Bonino F., Smith A., Anjuere F. Defective thymocyte maturation in p44 MAP kinase (Erk 1) knockout mice. Science. 1999;286:1374–1377. doi: 10.1126/science.286.5443.1374. [DOI] [PubMed] [Google Scholar]
- 23.Hatano N., Mori Y., Oh-hora M., Kosugi A., Fujikawa T., Nakai N. Essential role for ERK2 mitogen-activated protein kinase in placental development. Genes Cells. 2003;8:847–856. doi: 10.1046/j.1365-2443.2003.00680.x. [DOI] [PubMed] [Google Scholar]
- 24.Satoh Y., Endo S., Nakata T., Kobayashi Y., Yamada K., Ikeda T. ERK2 contributes to the control of social behaviors in mice. J Neurosci. 2011;31:11953–11967. doi: 10.1523/JNEUROSCI.2349-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu W., Zi M., Jin J., Prehar S., Oceandy D., Kimura T.E. Cardiac-specific deletion of Mkk4 reveals its role in pathological hypertrophic remodeling, but not in physiological cardiac growth. Circ Res. 2009;104:905–914. doi: 10.1161/CIRCRESAHA.108.188292. [DOI] [PubMed] [Google Scholar]
- 26.Liu W., Zi M., Naumann R., Ulm S., Jin J., Taglieri D.M. Pak1 as a novel therapeutic target for antihypertrophic treatment in the heart. Circulation. 2011;124:2702–2715. doi: 10.1161/CIRCULATIONAHA.111.048785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Livak K.L., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2-ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 28.Kehat I., Davis J., Tiburcy M., Accornero F., Saba-El-Leil M.K., Maillet M. Extracellular signal-regulated kinases 1 and 2 regulate the balance between eccentric and concentric cardiac growth. Circ Res. 2011;108:176–183. doi: 10.1161/CIRCRESAHA.110.231514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Sbroggio M., Carnevale D., Bertero A., Cifelli G., De Blasio E., Mascio G. IQGAP1 regulates ERK1/2 and AKT signalling in the heart and sustains functional remodelling upon pressure overload. Cardiovasc Res. 2011;91:456–464. doi: 10.1093/cvr/cvr103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Lorenz K., Schmitt J.P., Schmitteckert E.M., Lohse M.J. A new type of ERK1/2 autophosphorylation causes cardiac hypertrophy. Nat Med. 2009;15:75–83. doi: 10.1038/nm.1893. [DOI] [PubMed] [Google Scholar]
- 31.Walsh K. Akt signaling and growth of the heart. Circulation. 2006;113:2032–2034. doi: 10.1161/CIRCULATIONAHA.106.615138. [DOI] [PubMed] [Google Scholar]
- 32.Ellison G.M., Waring C.D., Vicinanza C., Torella D. Physiological cardiac remodelling in response to endurance exercise training: cellular and molecular mechanisms. Heart. 2012;98:5–10. doi: 10.1136/heartjnl-2011-300639. [DOI] [PubMed] [Google Scholar]
- 33.Bostrom P., Mann N., Wu J., Quintero P.A., Plovie E.R., Panakova D. C/EBPβ controls exercise-induced cardiac growth and protects against pathological cardiac remodeling. Cell. 2010;143:1072–1083. doi: 10.1016/j.cell.2010.11.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Molkentin J.D. The transcription factor C/EBPbeta serves as a master regulator of physiologic cardiac hypertrophy. Circ Res. 2011;108:277–278. doi: 10.1161/RES.0b013e31820ff484. [DOI] [PubMed] [Google Scholar]
- 35.Bersell K., Arab S., Haring B., Kuhn B. Neuregulin1/ErbB4 signaling induces cardiomyocyte proliferation and repair of heart injury. Cell. 2009;138:257–270. doi: 10.1016/j.cell.2009.04.060. [DOI] [PubMed] [Google Scholar]
- 36.Moelling K., Schad K., Bosse M., Zimmermann S., Schweneker M. Regulation of Raf-Akt cross-talk. J Biol Chem. 2002;277:31099–31106. doi: 10.1074/jbc.M111974200. [DOI] [PubMed] [Google Scholar]
- 37.Roskoski R., Jr. ERK1/2 MAP kinases: structure, function, and regulation. Pharmacol Res. 2012;66:105–143. doi: 10.1016/j.phrs.2012.04.005. [DOI] [PubMed] [Google Scholar]
- 38.Allan L.A., Morrice N., Brady S., Magee G., Pathak S., Clarke P.R. Inhibition of caspase-9 through phosphorylation at Thr 125 by ERK MAPK. Nat Cell Biol. 2003;5:647–654. doi: 10.1038/ncb1005. [DOI] [PubMed] [Google Scholar]
- 39.Balmanno K., Cook S.J. Tumour cell survival signalling by the ERK1/2 pathway. Cell Death Differ. 2009;16:368–377. doi: 10.1038/cdd.2008.148. [DOI] [PubMed] [Google Scholar]
- 40.Qin J., Xin H., Nickoloff B.J. Specifically targeting ERK1 or ERK2 kills melanoma cells. J Transl Med. 2012;10:15. doi: 10.1186/1479-5876-10-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Chen M.M., Lam A., Abraham J.A., Schreiner G.F., Joly A.H. CTGF expression is induced by TGF-beta in cardiac fibroblasts and cardiac myocytes: a potential role in heart fibrosis. J Mol Cell Cardiol. 2000;32:1805–1819. doi: 10.1006/jmcc.2000.1215. [DOI] [PubMed] [Google Scholar]
- 42.Van Oort R.J., van Rooij E., Bourajjaj M., Schimmel J., Jansen M.A., van der Nagel R. MEF2 activates a genetic program promoting chamber dilation and contractile dysfunction in calcineurin-induced heart failure. Circulation. 2006;114:298–308. doi: 10.1161/CIRCULATIONAHA.105.608968. [DOI] [PubMed] [Google Scholar]
- 43.Radhakrishnan S.S., Blalock T.D., Robinson P.M., Secker G., Daniels J., Grotendorst G.R. Effect of connective tissue growth factor on protein kinase expression and activity in human corneal fibroblasts. Invest Ophthalmol Vis Sci. 2012;53:8076–8085. doi: 10.1167/iovs.12-10790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Thum T., Gross C., Fiedler J., Fischer T., Kissler S., Bussen M. MicroRNA-21 contributes to myocardial disease by stimulating MAP kinase signalling in fibroblasts. Nature. 2008;456:980–984. doi: 10.1038/nature07511. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material.