Review on the interplay between NLR proteins, and RNA and DNA viruses.
Keywords: caspase, RNA virus, DNA virus, innate immunity
Abstract
NLR proteins are innate immune sensors that respond to microbial infection. Upon pathogen infection, some NLR proteins form large complexes, called inflammasomes, which activate caspase-1 and induce the production of active IL-1β and IL-18. Activation of inflammasomes can also lead to an inflammatory cell death program, named pyroptosis. In this review, we will discuss the role of various NLR proteins in sensing different viral infections, as well as the strategies used by several RNA and DNA viruses to counteract the antiviral effects of NLR-dependent inflammasomes.
INNATE IMMUNITY
The innate and adaptive arms of the host immune response work in combination to fight pathogen infection and protect the host from succumbing to the infection. Cellular receptors of the innate immune system are involved in sensing invading pathogens. The innate immune response is comprised of PRRs, which recognize PAMPs present on various microbes, including bacteria, viruses, and fungi [1]. PAMPS include pathogen components, such as lipids, nucleic acids, and proteins. PRRs can also sense endogenous DAMPs, including MSU, CPPD, and ATP, which serve as markers of tissue damage and trauma [2].
PRRs are expressed on many cell types, but the exact profile of PRRs expressed on different cell types varies. There are several classes of PRRs, including the TLRs, the RLRs, and the NLRs. The TLRs and C-type lectin receptors detect pathogens on the cell surface and in the endosome, whereas RLRs and some NLRs can detect pathogens intracellularly. PRR activation can result from direct binding of PAMPs to PRRs or indirectly, through intracellular changes, including generation of ROS or potassium ion flux or the presence of endogenous danger signals, such as uric acid. The latter is likely necessary for priming of the PRR.
Upon PAMP recognition, PRRs of the innate immune system are activated and induce a cascade of signaling events, leading to the up-regulation of IFN and inflammatory cytokines. This ultimately allows for the initiation of the adaptive immune response against the invading pathogen.
TLRs
In human cells, there are 10 known TLR proteins that are found on the cell surface or in the endosome of the cell [3]. In contrast, mice have 13 TLRs [4], which are single transmembrane proteins comprised of a LRR domain, located outside of the cell or inside of the endosome, and a TIR domain located inside of the cytoplasm or outside of the endosome [5]. PAMPs are recognized by the LRR domain, and this allows for heterotypic interactions among different TLRs. TLR3, -7, -8, and -9 detect virus-derived ssRNA or dsRNA and ssDNA or dsDNA [6] and are located within the endosome. Conversely, TLR1, -2, -4, -5, and -6 are located on the cell surface and can sense various PAMPs, including LPS, peptidoglycan, bacterial flagellum, and fungal cell wall components.
Upon binding to its cognate ligand, TLRs are activated and initiate a signal transduction cascade mediated through the TIR domain. In most cases, the TIR domain interacts with the adaptor protein MyD88, which signals through the IRAKs, including IRAK1, -2, or -4, which in turn, signal through TRAFs. The association of IRAK with TRAF activates MAPKK signaling. For example, IRAK2 and IRAK4 activate MAPKK through TRAF6, resulting in p38- and JNK-mediated phosphorylation and activation of the transcription factors ATF2/CREB and AP-1, respectively [7]. These transcription factors then translocate into the nucleus, where they can activate the promoters of proinflammatory cytokines. Additionally, IRAK1 can activate TRAF3 and TRAF6, leading to the phosphorylation of IRFs, including IRF1, -5, and -7, which bind to and activate the type I IFN promoters. TLR3 and one arm of TLR4 are Myd88-independent and interact with the TRIF instead. In a parallel pathway, TRIF-mediated TLR3 and TLR4 signal transduction leads to the interaction of TRIF with the RIP and up-regulation of NF-κB-inducible cytokines [8].
RLRs
RLRs include RIG-I and MDA-5. A third member of the RLR family, LGP2 [9], lacks a CARD and was identified originally as a negative regulator of RLR signaling by sequestering dsRNA from RIG-I and competing with MAVS for inducible IκB kinase [9–12]. However, other reports using LGP2 knockout animals suggest that LGP2 is not the primary negative regulator of type I IFN [13, 14], and yet other data show that LGP2 is required for RIG-I and MDA5-mediated antiviral responses [15]. Thus, the role of LGP2 in RLR signaling is controversial. RIG-I, MDA-5, and LGP2 are cytosolic proteins capable of sensing viral infection by binding dsRNA and 5′-triphosphate RNA. RNA-bound RIG-I becomes activated and signals through MAVS protein (IFN-β promoter stimulator protein 1), a central node in intracellular PRR transduction pathways. Whereas RIG-I can potentiate antiviral immune responses to many viruses, recent reports suggest a novel role for RIG-I in formation of an inflammasome-like structure and induction of IL-1β and IL-18. Formation of inflammasomes, as defined by recruitment of procaspase-1 and production of IL-1β and IL-18, has been extended recently to non-NLR innate immune sensors [16]. Interestingly, RIG-I associated with the inflammasome adaptor ASC to produce active caspase-1 and IL-1β independently of MAVS and NLRP3 [16]. Thus, RIG-I is able to activate NF-κB, type I IFN, and inflammatory cytokines.
NLR proteins
The NLR family of innate immune sensors has been implicated in many human diseases, including metabolic disorders, cancer, autoinflammatory conditions, and autoimmune disease [17]. The human genome contains over 22 NLR genes, many of which remain uncharacterized.
The first NLR gene that was identified was CIITA, a transcription modulator of MHCII [18]. CIITA is associated with bare lymphocyte syndrome, a disease that is characterized by defective immune responses, leading to infection-related fatality as a result of a deficiency in MHCII [19, 20].
NLRs are comprised of an N-terminal effector domain, which allows for homotypic protein–protein interactions with adaptor proteins. Alternatively, this domain can interact directly with enzymes such as procaspase-1 [21]. Typically, the NLR effector domains are a CARD or a PYD [21]. Oligomerization of NLRs is energy-dependent and requires ATP. All NLRs have a NBD, which binds to ATP. Another feature of the NLR family is the presence of a LRR domain, although the exact number of LRRs can be highly variable [21]. The LRR domain is thought to function in ligand sensing, in addition to being a negative regulator of NLR activation [17]. NLRs sense PAMPS derived from infectious agents and DAMPs derived from injured tissue or dying cells and environmental substances. Although inflammasome activation is induced by many DAMPS, PAMPS, and environmental substances, direct binding of a virus-derived component to the NLR has not yet been shown, as has been demonstrated for bacterial components [22, 23]. It is well established that inflammasomes are required for the innate immune response and for maintaining cellular homeostasis. One hypothesis is that NLRs sense intracellular differences upon viral infection [24, 25]. Another hypothesis is that NLRs bind viral PAMPs directly. A recent publication has shown that the C-terminal domain of NLRX1 is capable of binding ssRNA and dsRNA [26], suggesting that this NLR might be able to bind viral RNA directly.
Several NLR family proteins regulate innate immunity by forming large molecular complexes called inflammasomes, which lead to the up-regulation of IL-1β and IL-18. These cytokines lead to activation of NF-κB by binding their cognate receptors, IL-1R and IL-18R. Excessive production of IL-1β and IL-18 can lead to an inflammatory cell death program, called pyroptosis, which is caspase-1-dependent. Inflammasomes are comprised of homoligomerization of a NLR, procaspase-1, and ASC. Several NLR family members have been demonstrated to form inflammasomes, e.g., NLRP1, NLRP3, and NLRC4. Additionally, as alluded to above, certain non-NLR PRRs can also form inflammasomes and activate IL-1β and IL-18. These include AIM2 and RIG-I. Although caspase-1 is thought to be a key component for many inflammasomes, caspase-11 (also known as caspase-4) has been identified recently as part of a noncanonical inflammasome, where caspase-11 acts upstream of caspase-1 [27]. During noncanonical inflammasome activation, caspase-1 is necessary for IL-1β processing but is not involved in macrophage pyroptosis [27]. Most experiments published prior to this report by Dixit and colleagues [27] unknowingly used mice that were deficient in caspase-1 and caspase-11, as these two genes are too close in the genome to be segregated by recombination. Thus, in some mouse models, it is plausible that caspase-11, rather than caspase-1, is the critical mediator of inflammation [27], and previous findings suggesting the importance of caspase-1 containing inflammasomes need to be revisited.
Similar to TLRs, the NLR proteins, NOD1 and NOD2, interact with RIP2 and induce the activation of IRF3, IRF7, and the NF-κB and MAPK signaling pathways. This leads to the up-regulation of pro-IL-1β, pro-IL-18, IL-6, TNF, and in some cases, type I IFN. Although many NLRs have been shown to activate NF-κB, some members of this family can inhibit NF-κB signaling, e.g., NLRX1 and NLRC5, which can also inhibit type I IFN signaling in response to pathogen infection [28, 29]. Thus, NLR proteins can positively and negatively regulate the inflammation and IFN pathways, and there is immense crosstalk between the IFN and inflammatory arms of the innate immune response.
NLRP1.
NLRP1 is ubiquitously expressed in all cell types, including hematopoietic and nonhematopoietic cells [30]. NLRP1 is comprised of an N-terminal PYD; a central NBD, LRR, and FIIND (function to find) domain; and a C-terminal CARD region. The LRR domain of NLRP1 has been shown to be autoinhibitory [31]. Unlike human NLRP1, mouse NLRP1 does not contain an N-terminal PYD. Additionally, human NLRP1 is encoded by a single locus in the human genome, which differs from the murine NLRP1, encoded by three loci. Hence, the current view is that human and mouse NLRP1 function very differently. Muramyl dipeptide is an activator for human and mouse NLRP1 [31, 32], whereas LT from Bacillus anthracis is an activator of only mouse NLRP1. The susceptibility of mouse strains to LT is linked to NLRP1 polymorphisms [33], suggesting that LT is a ligand for mouse NLRP1, leading to the induction of IL-1β and IL-18 [34].
NLRP1 can interact with procaspase-1 and ASC to form an inflammasome [35]. NLRP1 can also interact with caspase-2 and caspase-9 in a complex called an apoptosome, which induces cell death [36].
NLRP3.
In mice, endogenous NLRP3 expression is observed in macrophages, monocytes, and conventional DCs; splenic neutrophils, skin epithelial cells, and keratinocytes; and hepatic stellate cells [37, 38]. Similarly, in humans, NLRP3 is expressed in peripheral blood leukocytes, including monocytes and granulocytes, as well as hepatic stellate cells [38–40]. NLRP3 contains an N-terminal PYD, a central NBD region, and a C-terminal LRR domain [41]. NLRP3 interacts with ASC via its PYD to recruit procaspase-1 [42], as NLRP3 itself lacks a CARD.
NLRP3 is activated in response to DAMPs and PAMPs, including ATP [43–45], MSU and CPPD [46], cholesterol crystals [47], hyaluronic acid [48], hydroxyapatite crystals [49, 50], asbestos and silica [51–53], and amyloid β [54]. Whereas the NLRP3 inflammasome can sense alum adjuvant and is involved in IgE induction, its involvement in IgG1 induction is more controversial [55–59]. Activation of the NLRP3 inflammasome results in IL-1β and IL-18 induction. Ultraviolet radiation and skin irritants are examples of environmental substances that activate the NLRP3 inflammasome [60, 61]. NLRP3 also senses RNA and DNA viruses as described below.
NLRC5.
NLRC5 is expressed in most cell types. NLRC5 is comprised of an N-terminus CARD, a central NBD region, and a LRR domain [21]. NLRC5 has been shown to form an inflammasome with NLRP3 and respond to NLRP3 agonists, including bacterial PAMPs and crystals in cell culture systems, but does not react to bacterial pore-forming toxins [62]. NLRC5 is a positive mediator of IFN to viral infection with SeV and CMV [63, 64]. However, two other groups reported that NLRC5 is a negative regulator of the IFN, NF-kB, and AP-1 pathways [29, 65], and it attenuated the response to VSV [29]. Additionally, NLRC5 has been shown to modulate MHCI gene expression in opposing ways [65, 66]. Hence, the exact role of NLRC5 may be context-dependent. Moreover, mice with a genetic deficiency in NLRC5 were found to be competent for inflammasome activation and induced cytokines in response to RNA and DNA viruses, as well as bacterial infections [67]. Thus, it appears that there may be a species-specific, context-dependent, and cell type specific function for NLRC5.
NLRP6.
NLRP6 is expressed at high levels in intestinal tissue. NLRP6 knockout mice have an altered gut microbial ecology as a result of a reduction in the levels of IL-18, leading to expansion of a particular bacterial phyla [68]. Several groups reported that NLRP6−/− mice exhibit intestinal hyperplasia, inflammation, as well as colitis-associated tumor growth [68–70]. Moreover, a recent report showed that NLRP6 deficiency contributes to obesity and along with hyperactive TLR signaling, predisposes mice to nonalcoholic fatty liver disease [71]. Thus, the NLRP6 inflammasome plays a pivotal role in protection from carcinogenesis.
NLRP12.
The role of NLRP12 is complex. NLRP12 has been shown to act as a positive activator of inflammation in certain cases [72] and a negative regulator of inflammation in many other situations. NLRP12 was shown to up-regulate MHCI expression [73] and to down-regulate NF-κB activation and TLR signaling in certain contexts [74, 75]. NLRP12 is highly expressed in neutrophils and DCs, and mice deficient in NLRP12 had reduced inflammatory responses in two models of contact hypersensitivity and allergic dermatitis, as the NLRP12−/− DCs were hindered in their ability to migrate to draining LNs [76]. A reduced inflammatory response in these models was not a result of defective antigenic presentation or inflammasome activation [76]. Finally, similar to NLRP6 knockout mice, NLRP12−/− mice also displayed chronic inflammation and carcinogenesis in the colon [77], suggesting a role of NLRP12 as a negative regulator of inflammation. It was found that the NLRP12−/− mice were unable to down-regulate NF-κB and ERK activation in macrophages [77].
NOD2.
The NOD2 protein is a member of the NLR family and was one of the first NLRs found to modulate MAPK and NF-κB signaling [78, 79]. NOD2 contains a central NBD with a C-terminal LRR domain and two N-terminal CARDs. NOD2 interacts with RIP2 kinase via homotypic CARD–CARD interactions, thereby leading to NF-κB nuclear translocation and up-regulation of TNF-α and IL-6 [80–82]. NOD2 stimulation can also activate p38, ERK, and JNK MAPK signaling, which is critical for innate and adaptive immune responses. NOD2 was shown to interact with 2′-5′-oligoadenylate synthetase type 2, which plays a key role in the recognition and elimination of infectious RNA viruses through RNase L-mediated degradation of viral RNA [83]. NOD2 is involved in sensing viral infection as described below.
NLRX1.
NLRX1 was first described as a negative modulator of antiviral signaling to viral infection [28, 84, 85]. NLRX1 interacts with MAVS and blocks RLR-dependent NF-κB and type I IFN responses [28]. NLRX1 knockdown leads to a marked increase in antiviral responses to SeV, suggesting that NLRX1 hinders the host response to viral infection [28, 85]. Additionally, NLRX1 negatively regulates TLR-mediated NF-κB activation [86]. Upon TLR4 stimulation, NLRX1 is rapidly ubiquitinated and disassociates from TRAF6 to interact with the IKK complex to prevent NF-κB activation. Knockdown of NLRX1 activates IKK phosphorylation and NF-κB-responsive cytokines in response to TLR4 stimulation. In mice, NLRX1 loss increased the susceptibility of these animals to LPS-induced septic shock [86]. However, NLRX1 has also been shown to up-regulate ROS and NF-κB signaling and thereby, positively regulate NLR function in other systems [87, 88]. Yet another group showed no effect of NLRX1 on MAVS [89].
NON-NLR INFLAMMASOME PROTEINS
AIM2
Although not a NLR protein, AIM2 also forms inflammasomes. AIM2 is a PYHIN family member and is an IFN-inducible gene. AIM2 contains an N-terminal PYD and a C-terminal HIN200 domain [90, 91]. It can form inflammasomes by interacting with ASC and procaspase-1. AIM2 is a DNA sensor and binds DNA through its HIN200 domain, resulting in inflammasome activation and IL-1β and IL-18 secretion. It is a cytosolic protein and responds to vaccinia virus and mouse CMV but not herpes simplex virus-1 [90, 91]. AIM2 has also been shown to be activated by a number of different bacteria [90, 92, 93].
IFI16
Similar to AIM2, IFI16 is also a member of the PYHIN family. In the context of infection with KSHV, IFI16 can form a caspase-1-activating inflammasome complex [94]. In KSHV-infected endothelial cells, IFI16 bound ASC to induce caspase-1 activation and IL-1β processing [94].
RIG-I
As mentioned above, RIG-I also associates with ASC to produce active caspase-1 and IL-1β, independently of MAVS and NLRP3 [16]. The RIG-I inflammasome can activate NF-κB, type I IFN, and inflammatory cytokines.
GBP5
GBP5 can stimulate NLRP3 inflammasome assembly in response to pathogenic bacteria [95]. GBP5−/− mice displayed defects in caspase-1 activation and IL-1β and IL-18 processing and dampened NLRP3-dependent inflammatory responses in vivo. Thus, GBP5 is a modulator of the NLRP3 inflammasome [95].
VIRUSES THAT ACTIVATE NLR-DEPENDENT INFLAMMASOMES
Although many pathogens, including fungi and bacteria, can activate NLRs, we have only focused on the interactions of viruses with the NLR protein family in this review. The NLRs that sense RNA and DNA viruses are summarized in Table 1.
Table 1. NLRs That Positively or Negatively Respond to RNA and DNA Viral Infections.
| NLR sensor | Virus | Reference |
|---|---|---|
| NLRP1 | KSHV | [111] |
| NLRP3 | Vaccinia Ankara virus, adenovirus, VZV, influenza virus, SeV, measles virus, EMCV, RSV, VSV, HCV, HIV | [96–100, 102–104, 106] |
| NLRC5 | SeV, VSV, CMV | [29, 63–65] |
| NOD2 | RSV, parainfluenza virus 3, influenza virus, VSV | [105] |
| NLRX1 | SeV, sindbis virus, influenza virus, SV5, VSV | [28, 84] |
RNA viruses
The NLRP3 inflammasome is activated by the most number of viruses to date and hence, may be a common pathway for viral detection by the host cell. Paramyxoviruses, e.g., SeV, RSV, and measles virus, as well as orthomyxoviruses, e.g., IAV, can activate the NLRP3 inflammasome [96–98]. IAV and SeV triggered caspase-1 activation in WT but not NLRP3−/− macrophages [96]. Another NLR family member, NLRC5, was also shown to sense SeV in THP-1 monocytic cells and primary human dermal fibroblasts [63]. RSV was reported to activate the NLRP3 inflammasome, resulting in IL-1β production during viral infection. Similar to other viruses, intracellular ROS and potassium efflux were shown to be important for NLRP3 inflammasome formation [97]. Yet, another paramyxovirus family member, measles virus, can induce increases in IL-1β in THP-1 monocytes in a NLRP3-dependent manner [98] and IL-1β levels were decreased upon knockdown of NLRP3 [98]. NLRP3 is also a sensor for IAV in vivo, as mice lacking NLRP3 induced lower levels of IL-1β and had higher mortality compared with WT animals [99, 100]. As a result of the fact that IAV is also known to activate TLR7/8, NLRP3 may also be a downstream responder to infection and may not necessarily sense a viral PAMP itself. Additionally, the M2 protein of IAV activates NLRP3. M2 induces a proton ion channel, suggesting that changes in intracellular ionic flux activate NLRP3 [101].
EMCV is another virus that can activate NLRP3 and MDA-5 together, suggesting that NLRs and RLRs work in synergy to inhibit EMCV replication. Thus, innate immunity against viruses may involve multiple PRRs [16, 102]. VSV [102] and HCV [103] were also shown to activate NLRP3 during viral replication. As with other viral infections, the generation of ROS appears to be important for inflammasome activation during HCV infection [103]. Finally, HIV could activate NLRP3 inflammasome and IL-1β secretion in DCs from healthy individuals but not from HIV-positive patients, suggesting that the inflammasome may play a role early in the course of HIV disease [104].
Another NLR protein, NOD2, is involved in several RNA viral infections, including VSV, RSV, parainfluenza virus 3, and IAV [105]. NOD2 expression was up-regulated in response to infection with VSV, RSV, and IAV [105]. This further resulted in IFN-β induction in human bronchial epithelial cells, macrophages, and embryonic fibroblasts. NOD2 was observed to relocalize to the mitochondria upon infection, where it interacted with MAVS. The NOD2 LRR domain and NBD were found to bind MAVS and lead to induction of type I IFN.
Unlike the other NLR members described above, NLRX1 is a negative regulator of inflammation and has been shown to attenuate the proinflammatory response to SeV, Sindbis virus, SV5, VSV, and IAV infection [28, 84].
DNA viruses
NLRP3 recognizes enveloped and nonenveloped DNA viruses, such as MVA and adenovirus [106, 107]. Depletion of NLRP3 in a monocytic cell line inhibited IL-1β secretion in response to MVA infection, and a similar effect was seen in NLRP3−/− bone marrow-derived macrophages. TLR2 and -6 also appeared to participate in this response [107]. Adenoviral DNA was demonstrated to activate IL-1β secretion in monocytic cells [106]. In NLRP3-depleted peritoneal macrophages, IL-1β activation was reduced significantly in response to adenoviral infection in vitro, as was the case in other NLRP3 knockdown cells [108]. Although NLRP3 knockout mice demonstrated reduced IL-1β induction in response to adenoviral infection, a complete reduction was not seen [106], indicating that another sensor may also recognize adenovirus. More recently, a NLRP3-independent role for sensing adenovirus was also reported through β3-integrin-mediated activation of proinflammatory responses, independent of DNA sensing [109].
Similar to MVA, herpesviruses are large, enveloped DNA viruses, eight of which are known to infect the human host. These viruses induce different NLR inflammasomes. VZV was shown to activate NLRP3 inflammasome formation in monocytic and melanoma cell lines and in skin xenografts. This resulted in the recruitment of ASC and caspase-1 [110]. Another herpesvirus, KSHV can induce the NLRP1 inflammasome upon infection of primary monocytes [111]. However, the virus encodes for a protein, named Orf63, which inhibits NLRP1, and silencing of Orf63 resulted in increased IL-1β levels following KSHV infection [111]. A third herpesvirus, HCMV, activated NLRC5 in human foreskin fibroblasts [62]. Depletion of NLRC5 resulted in inhibition of IFN-α production during HCMV infection.
Currently, it remains unclear how NLRs sense RNA and DNA viruses. As mentioned above, it is possible that a secondary event, such as ROS generation or ion flux, activates the NLR-dependent inflammasome.
VIRAL MODULATORS OF NLR FUNCTION
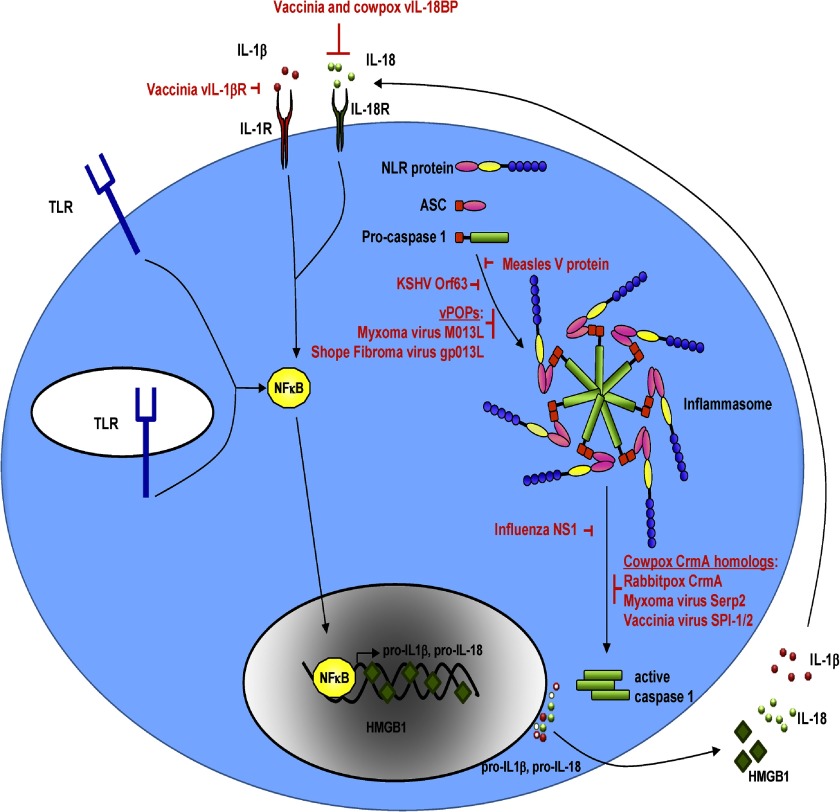
Several viruses encode inhibitors of NLR inflammasomes as depicted in Fig. 1. These include poxviruses and herpesviruses, which have large genomes and can express unique proteins or viral homologs of cellular proteins to inhibit innate immunity.
Figure 1. Viral proteins inhibit various steps of the NLR-dependent inflammasome pathway.
Some poxviruses encode scavenger receptors (vIL-1βR) and IL-18BPs, which compete with IL-1β and IL-18 from binding their cognate receptors. Moreover, MxV and Shope fibroma virus encode vPOPs, which bind ASC to prevent inflammasome formation through caspase-1 recruitment. Measles virus encodes a nonstructural V protein that interacts with and inhibits NLRP3. Similarly, KSHV encodes the Orf63 protein, which shows similarity to cellular NLRP1 and can interact with cellular NLRP1 and NLRP3 to prevent the formation of inflammasomes. Poxvirus family members encode serpins such as CrmA, SP1/2 (SPI-1/2), and Serp2, which inhibit caspase-1 activity and prevent IL-1β and IL-18 secretion. The NS1 protein of influenza also inhibits caspase-1, IL-1β, and IL-18 activation and secretion. HMGB1, High mobility group box 1.
The family of poxviruses encodes multiple proteins that target innate immune effectors inside the cell. The first viral inhibitor described was the cowpox virus serpin protein, CrmA, which blocks caspase-1 by interacting directly with and inhibiting procaspase-1. This prevents proteolytic processing of procaspase-1 to active caspase-1 [112] and results in reduced IL-1β secretion from the infected cell [112]. Poxvirus mutant viruses deleted for CrmA display decreased virulence and lesions on chicken allantoic membranes [112] and following intratracheal and intradermal inoculation in C57BL/6 mice [113]. Moreover, MxV mutants deleted for the CrmA homolog Serp2 displayed highly attenuated viral replication [114]. However, intranasal infection of BALB/c mice with vaccinia virus lacking the CrmA homologs, SPI-1 and SPI-2 (also SP1/2), did not affect virulence, although these proteins could block IL-1β processing when expressed in THP-1 cells [115, 116].
Mammalian IL-18BP is an antagonist of IL-18, which prevents IL-18 from interacting with its receptor. Many poxviruses encode functional vIL-18BPs [117, 118]. Additionally, some poxviruses, e.g., vaccinia virus, also contain an IL-1βR homolog (vIL-103R or vIL-1βR), which can bind and sequester IL-1β [119].
MxV, a poxvirus, was the first virus identified to encode a viral protein that inhibited NLRs. The M013L protein of MxV and the gp013L protein of Shope fibroma virus display homology to cellular POPs, which are known to negatively regulate inflammasome formation by hindering the association of ASC with NLRs [120]. The M013 protein of MxV can bind ASC to inhibit IL-1β activation [121]. A M013 mutant virus was attenuated, and its replicative capacity was impaired in lymphocytes and monocytes. Moreover, increased caspase-1 activity and secreted IL-1β were observed with the mutant virus. Similarly, Shope fibroma virus expresses a vPOP homolog, called gp013L (or S013L), which interacts with ASC [122]. gp013L was demonstrated to inhibit the NLRP3 inflammasome and hinder IL-1β secretion [122].
The herpesvirus KSHV encodes an Orf63 protein, which could inhibit NLRP1- and NLRP3-dependent caspase-1 activation and processing of IL-1β and IL-18. Silencing of Orf63 expression led to an increase in IL-1β during primary infection and reactivation of KSHV [111].
Other viruses also thwart innate immune effectors. IAV is known to activate the inflammasome as described above. However, this virus also has a mechanism to dampen inflammatory responses, whereby the NS1 IAV protein from the H1N1 strain contains a RNA-binding domain, which suppresses the activation of IL-1β and IL-18 [123]. Additionally, the nonstructural V protein of Measles virus can interact with NLRP3 through its carboxyl-terminal domain, and cells expressing the V protein suppress NLRP3-dependent IL-1β production [98]. Furthermore, a mutant virus lacking the V protein induced more IL-1β production during infection [98].
SUMMARY
Inflammasome activation is an important means by which the host can induce inflammation through the activation and secretion of IL-1β and IL-18. Excessive IL-1β and IL-18 are detrimental to viral infection and can lead to pyroptosis, thereby eliminating the virus-infected cell from the host. The discovery of pathogen-encoded inhibitors of NLRs highlights the importance of this arm of the host immune response in combating infection to multiple viruses. A wide range of viruses, including but not limited to paramyxoviruses, orthomyxoviruses, herpesviruses, poxviruses, and retroviruses, can activate NLR-dependent inflammasomes. Some of these viruses have evolved to counteract the inflammasome response to viral infection through a diverse array of strategies that inhibit inflammasome activation. Future goals include determining how exactly NLR proteins sense viruses and how the NLR proteins interface with other PRRs to mount a successful response to viral infection.
ACKNOWLEDGMENTS
S.R.J. was supported by the Pathogenesis training grant (T32- AI007151). B.D. is supported by grant DE018281. Because of space limitations, we apologize for many important publications that were not cited in this review.
Footnotes
- −/−
- deficient
- AIM2
- absent in melanoma 2
- ASC
- apoptotic-associated speck-like protein containing a caspase recruitment domain
- CIITA
- class II transactivator
- CARD
- caspase recruitment domain
- CPPD
- calcium pyrophosphate dihydrate
- CrmA
- cytokine response modifier A
- DAMP
- danger-associated molecular pattern
- EMCV
- encephalomyocarditis virus
- GBP5
- guanylate-binding protein 5
- HCMV
- human CMV
- HCV
- hepatitis C virus
- HIN200
- hematopoietic IFN-inducible nuclear antigens with 200 aa repeats
- IAV
- influenza A virus
- IFI16
- IFN-inducible protein 16
- IL-18BP
- IL-18 binding protein
- IRAK
- IL-1R-associated kinase
- IRF
- IFN regulatory factor
- KSHV
- Kaposi's sarcoma-associated herpesvirus
- LGP2
- laboratory of genetics and physiology 2
- LRR
- leucine-rich repeat
- LT
- lethal toxin
- MAVS
- mitochondrial antiviral signaling
- MDA
- melanoma differentiation-associated gene
- MSU
- monosodium urate
- MVA
- modified vaccinia Ankara
- MxV
- myxoma virus
- NBD
- nucleotide-binding domain
- NLR
- nucleotide-binding domain, leucine-rich repeat
- NOD
- nucleotide-binding oligomerization
- POP
- pyrin domain-only protein
- PYD
- pyrin domain
- PYHIN
- pyrin and hematopoietic IFN-inducible nuclear antigens with 200 aa repeats
- RIG-I
- retinoic acid-inducible gene I
- RIP
- receptor-interacting protein
- RLR
- retinoic acid-like receptor
- RSV
- respiratory syncytial virus
- Serp2
- serpin 2
- SeV
- Sendai virus
- SPI-1/2
- vaccinia virus serpin proteins
- TIR
- Toll-IL-1R
- TRIF
- Toll-IL-1R-domain-containing adaptor protein, inducing IFN-β
- vIL
- viral interleukin
- vPOP
- viral pyrin domain-only protein
- VZV
- Varicella-zoster virus
REFERENCES
- 1. Kawai T., Akira S. (2008) Toll-like receptor and RIG-I-like receptor signaling. Ann. N. Y. Acad. Sci. 1143, 1–20 [DOI] [PubMed] [Google Scholar]
- 2. Bianchi M. E. (2007) DAMPs, PAMPs and alarmins: all we need to know about danger. J. Leukoc. Biol. 81, 1–5 [DOI] [PubMed] [Google Scholar]
- 3. Medzhitov R., Preston-Hurlburt P., Janeway C. A., Jr., (1997) A human homologue of the Drosophila Toll protein signals activation of adaptive immunity. Nature 388, 394–397 [DOI] [PubMed] [Google Scholar]
- 4. Leulier F., Lemaitre B. (2008) Toll-like receptors—taking an evolutionary approach. Nat. Rev. Genet. 9, 165–178 [DOI] [PubMed] [Google Scholar]
- 5. Kawai T., Akira S. (2007) TLR signaling. Sem. Immunol. 19, 24–32 [DOI] [PubMed] [Google Scholar]
- 6. Kawai T., Akira S. (2010) The role of pattern-recognition receptors in innate immunity: update on Toll-like receptors. Nature 11, 373–384 [DOI] [PubMed] [Google Scholar]
- 7. Oda K., Kitano H. (2006) A comprehensive map of the Toll-like receptor signaling network. Mol. Syst. Biol. 2, 0015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. O'Neill L. A., Bowie A. G. (2007) The family of five: TIR-domain-containing adaptors in Toll-like receptor signalling. Nat. Rev. Immunol. 7, 353–364 [DOI] [PubMed] [Google Scholar]
- 9. Yoneyama M., Kikuchi M., Matsumoto K., Imaizumi T., Miyagishi M., Taira K., Foy E., Loo Y. M., Gale M., Jr., Akira S., Yonehara S., Kato A., Fujita T. (2005) Shared and unique functions of the DExD/H-box helicases RIG-I, MDA5, and LGP2 in antiviral innate immunity. J. Immunol. 175, 2851–2858 [DOI] [PubMed] [Google Scholar]
- 10. Saito T., Hirai R., Loo Y. M., Owen D., Johnson C. L., Sinha S. C., Akira S., Fujita T., Gale M., Jr. (2007) Regulation of innate antiviral defenses through a shared repressor domain in RIG-I and LGP2. Proc. Natl. Acad. Sci. USA 104, 582–587 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Komuro A., Horvath C. M. (2006) RNA- and virus-independent inhibition of antiviral signaling by RNA helicase LGP2. J. Virol. 80, 12332–12342 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Rothenfusser S., Goutagny N., DiPerna G., Gong M., Monks B. G., Schoenemeyer A., Yamamoto M., Akira S., Fitzgerald K. A. (2005) The RNA helicase Lgp2 inhibits TLR-independent sensing of viral replication by retinoic acid-inducible gene-I. J. Immunol. 175, 5260–5268 [DOI] [PubMed] [Google Scholar]
- 13. Venkataraman T., Valdes M., Elsby R., Kakuta S., Caceres G., Saijo S., Iwakura Y., Barber G. N. (2007) Loss of DExD/H box RNA helicase LGP2 manifests disparate antiviral responses. J. Immunol. 178, 6444–6455 [DOI] [PubMed] [Google Scholar]
- 14. Pollpeter D., Komuro A., Barber G. N., Horvath C. M. (2011) Impaired cellular responses to cytosolic DNA or infection with Listeria monocytogenes and vaccinia virus in the absence of the murine LGP2 protein. PloS One 6, e18842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Satoh T., Kato H., Kumagai Y., Yoneyama M., Sato S., Matsushita K., Tsujimura T., Fujita T., Akira S., Takeuchi O. (2010) LGP2 is a positive regulator of RIG-I- and MDA5-mediated antiviral responses. Proc. Natl. Acad. Sci. USA 107, 1512–1517 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Poeck H., Bscheider M., Gross O., Finger K., Roth S., Rebsamen M., Hannesschlager N., Schlee M., Rothenfusser S., Barchet W., Kato H., Akira S., Inoue S., Endres S., Peschel C., Hartmann G., Hornung V., Ruland J. (2010) Recognition of RNA virus by RIG-I results in activation of CARD9 and inflammasome signaling for interleukin 1 β production. Nat. Immunol. 11, 63–69 [DOI] [PubMed] [Google Scholar]
- 17. Davis B. K., Wen H., Ting J. P. (2011) The inflammasome NLRs in immunity, inflammation, and associated diseases. Annu. Rev. Immunol. 29, 707–735 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Steimle V., Otten L. A., Zufferey M., Mach B. (1993) Complementation cloning of an MHC class II transactivator mutated in hereditary MHC class II deficiency (or bare lymphocyte syndrome). Cell 75, 135–146 [PubMed] [Google Scholar]
- 19. Steimle V., Siegrist C. A., Mottet A., Lisowska-Grospierre B., Mach B. (1994) Regulation of MHC class II expression by interferon-γ mediated by the transactivator gene CIITA. Science 265, 106–109 [DOI] [PubMed] [Google Scholar]
- 20. Cressman D. E., Chin K. C., Taxman D. J., Ting J. P. (1999) A defect in the nuclear translocation of CIITA causes a form of type II bare lymphocyte syndrome. Immunity 10, 163–171 [DOI] [PubMed] [Google Scholar]
- 21. Ting J. P., Lovering R. C., Alnemri E. S., Bertin J., Boss J. M., Davis B. K., Flavell R. A., Girardin S. E., Godzik A., Harton J. A., Hoffman H. M., Hugot J. P., Inohara N., Mackenzie A., Maltais L. J., Nunez G., Ogura Y., Otten L. A., Philpott D., Reed J. C., Reith W., Schreiber S., Steimle V., Ward P. A. (2008) The NLR gene family: a standard nomenclature. Immunity 28, 285–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhao Y., Yang J., Shi J., Gong Y. N., Lu Q., Xu H., Liu L., Shao F. (2011) The NLRC4 inflammasome receptors for bacterial flagellin and type III secretion apparatus. Nature 477, 596–600 [DOI] [PubMed] [Google Scholar]
- 23. Kofoed E. M., Vance R. E. (2011) Innate immune recognition of bacterial ligands by NAIPs determines inflammasome specificity. Nature 477, 592–595 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Zhou R., Tardivel A., Thorens B., Choi I., Tschopp J. (2009) Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat. Immunol. 11, 136–140 [DOI] [PubMed] [Google Scholar]
- 25. Petrilli V., Papin S., Dostert C., Mayor A., Martinon F., Tschopp J. (2007) Activation of the NALP3 inflammasome is triggered by low intracellular potassium concentration. Cell Death Differ. 14, 1583–1589 [DOI] [PubMed] [Google Scholar]
- 26. Hong M., Yoon S. I., Wilson I. A. (2012) Structure and functional characterization of the RNA-binding element of the NLRX1 innate immune modulator. Immunity 36, 337–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Kayagaki N., Warming S., Lamkanfi M., Vande Walle L., Louie S., Dong J., Newton K., Qu Y., Liu J., Heldens S., Zhang J., Lee W. P., Roose-Girma M., Dixit V. M. (2011) Non-canonical inflammasome activation targets caspase-11. Nature 479, 117–121 [DOI] [PubMed] [Google Scholar]
- 28. Moore C. B., Bergstralh D. T., Duncan J. A., Lei Y., Morrison T. E., Zimmermann A. G., Accavitti-Loper M. A., Madden V. J., Sun L., Ye Z., Lich J. D., Heise M. T., Chen Z., Ting J. P. (2008) NLRX1 is a regulator of mitochondrial antiviral immunity. Nature 451, 573–577 [DOI] [PubMed] [Google Scholar]
- 29. Cui J., Zhu L., Xia X., Wang H. Y., Legras X., Hong J., Ji J., Shen P., Zheng S., Chen Z. J., Wang R. F. (2010) NLRC5 negatively regulates the NF-κB and type I interferon signaling pathways. Cell 141, 483–496 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Kummer J. A., Broekhuizen R., Everett H., Agostini L., Kuijk L., Martinon F., van Bruggen R., Tschopp J. (2007) Inflammasome components NALP 1 and 3 show distinct but separate expression profiles in human tissues suggesting a site-specific role in the inflammatory response. J. Histochem. Cytochem. 55, 443–452 [DOI] [PubMed] [Google Scholar]
- 31. Faustin B., Lartigue L., Bruey J. M., Luciano F., Sergienko E., Bailly-Maitre B., Volkmann N., Hanein D., Rouiller I., Reed J. C. (2007) Reconstituted NALP1 inflammasome reveals two-step mechanism of caspase-1 activation. Mol. Cell 25, 713–724 [DOI] [PubMed] [Google Scholar]
- 32. Hsu L. C., Ali S. R., McGillivray S., Tseng P. H., Mariathasan S., Humke E. W., Eckmann L., Powell J. J., Nizet V., Dixit V. M., Karin M. (2008) A NOD2-NALP1 complex mediates caspase-1-dependent IL-1β secretion in response to Bacillus anthracis infection and muramyl dipeptide. Proc. Natl. Acad. Sci. USA 105, 7803–7808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Boyden E. D., Dietrich W. F. (2006) Nalp1b controls mouse macrophage susceptibility to anthrax lethal toxin. Nat. Genet. 38, 240–244 [DOI] [PubMed] [Google Scholar]
- 34. Nour A. M., Yeung Y. G., Santambrogio L., Boyden E. D., Stanley E. R., Brojatsch J. (2009) Anthrax lethal toxin triggers the formation of a membrane-associated inflammasome complex in murine macrophages. Infect. Immun. 77, 1262–1271 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Martinon F., Burns K., Tschopp J. (2002) The inflammasome: a molecular platform triggering activation of inflammatory caspases and processing of proIL-β. Mol. Cell 10, 417–426 [DOI] [PubMed] [Google Scholar]
- 36. Chu Z. L., Pio F., Xie Z., Welsh K., Krajewska M., Krajewski S., Godzik A., Reed J. C. (2001) A novel enhancer of the Apaf1 apoptosome involved in cytochrome c-dependent caspase activation and apoptosis. J. Biol. Chem. 276, 9239–9245 [DOI] [PubMed] [Google Scholar]
- 37. Guarda G., Zenger M., Yazdi A. S., Schroder K., Ferrero I., Menu P., Tardivel A., Mattmann C., Tschopp J. (2011) Differential expression of NLRP3 among hematopoietic cells. J. Immunol. 186, 2529–2534 [DOI] [PubMed] [Google Scholar]
- 38. Watanabe A., Sohail M. A., Gomes D. A., Hashmi A., Nagata J., Sutterwala F. S., Mahmood S., Jhandier M. N., Shi Y., Flavell R. A., Mehal W. Z. (2009) Inflammasome-mediated regulation of hepatic stellate cells. Am. J. Physiol. Gastrointest. Liver Physiol. 296, G1248–G1257 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Anderson J. P., Mueller J. L., Rosengren S., Boyle D. L., Schaner P., Cannon S. B., Goodyear C. S., Hoffman H. M. (2004) Structural, expression, and evolutionary analysis of mouse CIAS1. Gene 338, 25–34 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Manji G. A., Wang L., Geddes B. J., Brown M., Merriam S., Al-Garawi A., Mak S., Lora J. M., Briskin M., Jurman M., Cao J., DiStefano P. S., Bertin J. (2002) PYPAF1, a PYRIN-containing Apaf1-like protein that assembles with ASC and regulates activation of NF-κ B. J. Biol. Chem. 277, 11570–11575 [DOI] [PubMed] [Google Scholar]
- 41. Hoffman H. M., Mueller J. L., Broide D. H., Wanderer A. A., Kolodner R. D. (2001) Mutation of a new gene encoding a putative pyrin-like protein causes familial cold autoinflammatory syndrome and Muckle-Wells syndrome. Nat. Genet. 29, 301–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Agostini L., Martinon F., Burns K., McDermott M. F., Hawkins P. N., Tschopp J. (2004) NALP3 forms an IL-1β-processing inflammasome with increased activity in Muckle-Wells autoinflammatory disorder. Immunity 20, 319–325 [DOI] [PubMed] [Google Scholar]
- 43. Rock K. L., Lai J. J., Kono H. (2011) Innate and adaptive immune responses to cell death. Immunol. Rev. 243, 191–205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Iyer S. S., Pulskens W. P., Sadler J. J., Butter L. M., Teske G. J., Ulland T. K., Eisenbarth S. C., Florquin S., Flavell R. A., Leemans J. C., Sutterwala F. S. (2009) Necrotic cells trigger a sterile inflammatory response through the Nlrp3 inflammasome. Proc. Natl. Acad. Sci. USA 106, 20388–20393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Mariathasan S., Weiss D. S., Newton K., McBride J., O'Rourke K., Roose-Girma M., Lee W. P., Weinrauch Y., Monack D. M., Dixit V. M. (2006) Cryopyrin activates the inflammasome in response to toxins and ATP. Nature 440, 228–232 [DOI] [PubMed] [Google Scholar]
- 46. Martinon F., Petrilli V., Mayor A., Tardivel A., Tschopp J. (2006) Gout-associated uric acid crystals activate the NALP3 inflammasome. Nature 440, 237–241 [DOI] [PubMed] [Google Scholar]
- 47. Duewell P., Kono H., Rayner K. J., Sirois C. M., Vladimer G., Bauernfeind F. G., Abela G. S., Franchi L., Nunez G., Schnurr M., Espevik T., Lien E., Fitzgerald K. A., Rock K. L., Moore K. J., Wright S. D., Hornung V., Latz E. (2010) NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 464, 1357–1361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Yamasaki K., Muto J., Taylor K. R., Cogen A. L., Audish D., Bertin J., Grant E. P., Coyle A. J., Misaghi A., Hoffman H. M., Gallo R. L. (2009) NLRP3/cryopyrin is necessary for interleukin-1β (IL-1β) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J. Biol. Chem. 284, 12762–12771 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Jin C., Frayssinet P., Pelker R., Cwirka D., Hu B., Vignery A., Eisenbarth S. C., Flavell R. A. (2011) NLRP3 inflammasome plays a critical role in the pathogenesis of hydroxyapatite-associated arthropathy. Proc. Natl. Acad. Sci. USA 108, 14867–14872 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Pazar B., Ea H. K., Narayan S., Kolly L., Bagnoud N., Chobaz V., Roger T., Liote F., So A., Busso N. (2011) Basic calcium phosphate crystals induce monocyte/macrophage IL-1β secretion through the NLRP3 inflammasome in vitro. J. Immunol. 186, 2495–2502 [DOI] [PubMed] [Google Scholar]
- 51. Dostert C., Petrilli V., Van Bruggen R., Steele C., Mossman B. T., Tschopp J. (2008) Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 320, 674–677 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Cassel S. L., Eisenbarth S. C., Iyer S. S., Sadler J. J., Colegio O. R., Tephly L. A., Carter A. B., Rothman P. B., Flavell R. A., Sutterwala F. S. (2008) The Nalp3 inflammasome is essential for the development of silicosis. Proc. Natl. Acad. Sci. USA 105, 9035–9040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Hornung V., Bauernfeind F., Halle A., Samstad E. O., Kono H., Rock K. L., Fitzgerald K. A., Latz E. (2008) Silica crystals and aluminum salts activate the NALP3 inflammasome through phagosomal destabilization. Nat. Immunol. 9, 847–856 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Halle A., Hornung V., Petzold G. C., Stewart C. R., Monks B. G., Reinheckel T., Fitzgerald K. A., Latz E., Moore K. J., Golenbock D. T. (2008) The NALP3 inflammasome is involved in the innate immune response to amyloid-β. Nat. Immunol. 9, 857–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Franchi L., Nunez G. (2008) The Nlrp3 inflammasome is critical for aluminium hydroxide-mediated IL-1β secretion but dispensable for adjuvant activity. Eur. J. Immunol. 38, 2085–2089 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Eisenbarth S. C., Colegio O. R., O'Connor W., Sutterwala F. S., Flavell R. A. (2008) Crucial role for the Nalp3 inflammasome in the immunostimulatory properties of aluminium adjuvants. Nature 453, 1122–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Kool M., Petrilli V., De Smedt T., Rolaz A., Hammad H., van Nimwegen M., Bergen I. M., Castillo R., Lambrecht B. N., Tschopp J. (2008) Cutting edge: alum adjuvant stimulates inflammatory dendritic cells through activation of the NALP3 inflammasome. J. Immunol. 181, 3755–3759 [DOI] [PubMed] [Google Scholar]
- 58. McKee A. S., Munks M. W., MacLeod M. K., Fleenor C. J., Van Rooijen N., Kappler J. W., Marrack P. (2009) Alum induces innate immune responses through macrophage and mast cell sensors, but these sensors are not required for alum to act as an adjuvant for specific immunity. J. Immunol. 183, 4403–4414 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59. Li H., Willingham S. B., Ting J. P., Re F. (2008) Cutting edge: inflammasome activation by alum and alum's adjuvant effect are mediated by NLRP3. J. Immunol. 181, 17–21 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Feldmeyer L., Keller M., Niklaus G., Hohl D., Werner S., Beer H. D. (2007) The inflammasome mediates UVB-induced activation and secretion of interleukin-1β by keratinocytes. Curr. Biol. 17, 1140–1145 [DOI] [PubMed] [Google Scholar]
- 61. Watanabe H., Gaide O., Petrilli V., Martinon F., Contassot E., Roques S., Kummer J. A., Tschopp J., French L. E. (2007) Activation of the IL-1β-processing inflammasome is involved in contact hypersensitivity. J. Invest. Dermatol. 127, 1956–1963 [DOI] [PubMed] [Google Scholar]
- 62. Davis B. K., Roberts R. A., Huang M. T., Willingham S. B., Conti B. J., Brickey W. J., Barker B. R., Kwan M., Taxman D. J., Accavitti-Loper M. A., Duncan J. A., Ting J. P. (2011) Cutting edge: NLRC5-dependent activation of the inflammasome. J. Immunol. 186, 1333–1337 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Kuenzel S., Till A., Winkler M., Hasler R., Lipinski S., Jung S., Grotzinger J., Fickenscher H., Schreiber S., Rosenstiel P. (2010) The nucleotide-binding oligomerization domain-like receptor NLRC5 is involved in IFN-dependent antiviral immune responses. J. Immunol. 184, 1990–2000 [DOI] [PubMed] [Google Scholar]
- 64. Neerincx A., Lautz K., Menning M., Kremmer E., Zigrino P., Hosel M., Buning H., Schwarzenbacher R., Kufer T. A. (2010) A role for the human nucleotide-binding domain, leucine-rich repeat-containing family member NLRC5 in antiviral responses. J. Biol. Chem. 285, 26223–26232 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Benko S., Magalhaes J. G., Philpott D. J., Girardin S. E. (2010) NLRC5 limits the activation of inflammatory pathways. J. Immunol. 185, 1681–1691 [DOI] [PubMed] [Google Scholar]
- 66. Meissner T. B., Li A., Biswas A., Lee K. H., Liu Y. J., Bayir E., Iliopoulos D., van den Elsen P. J., Kobayashi K. S. (2010) NLR family member NLRC5 is a transcriptional regulator of MHC class I genes. Proc. Natl. Acad. Sci. USA 107, 13794–13799 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kumar H., Pandey S., Zou J., Kumagai Y., Takahashi K., Akira S., Kawai T. (2011) NLRC5 deficiency does not influence cytokine induction by virus and bacteria infections. J. Immunol. 186, 994–1000 [DOI] [PubMed] [Google Scholar]
- 68. Elinav E., Strowig T., Kau A. L., Henao-Mejia J., Thaiss C. A., Booth C. J., Peaper D. R., Bertin J., Eisenbarth S. C., Gordon J. I., Flavell R. A. (2011) NLRP6 inflammasome regulates colonic microbial ecology and risk for colitis. Cell 145, 745–757 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Normand S., Delanoye-Crespin A., Bressenot A., Huot L., Grandjean T., Peyrin-Biroulet L., Lemoine Y., Hot D., Chamaillard M. (2011) Nod-like receptor pyrin domain-containing protein 6 (NLRP6) controls epithelial self-renewal and colorectal carcinogenesis upon injury. Proc. Natl. Acad. Sci. USA 108, 9601–9606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Chen G. Y., Liu M., Wang F., Bertin J., Nunez G. (2011) A functional role for Nlrp6 in intestinal inflammation and tumorigenesis. J. Immunol. 186, 7187–7194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71. Henao-Mejia J., Elinav E., Jin C., Hao L., Mehal W. Z., Strowig T., Thaiss C. A., Kau A. L., Eisenbarth S. C., Jurczak M. J., Camporez J. P., Shulman G. I., Gordon J. I., Hoffman H. M., Flavell R. A. (2012) Inflammasome-mediated dysbiosis regulates progression of NAFLD and obesity. Nature 482, 179–185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72. Wang L., Manji G. A., Grenier J. M., Al-Garawi A., Merriam S., Lora J. M., Geddes B. J., Briskin M., DiStefano P. S., Bertin J. (2002) PYPAF7, a novel PYRIN-containing Apaf1-like protein that regulates activation of NF-κ B and caspase-1-dependent cytokine processing. J. Biol. Chem. 277, 29874–29880 [DOI] [PubMed] [Google Scholar]
- 73. Williams K. L., Taxman D. J., Linhoff M. W., Reed W., Ting J. P. (2003) Cutting edge: monarch-1: a pyrin/nucleotide-binding domain/leucine-rich repeat protein that controls classical and nonclassical MHC class I genes. J. Immunol. 170, 5354–5358 [DOI] [PubMed] [Google Scholar]
- 74. Williams K. L., Lich J. D., Duncan J. A., Reed W., Rallabhandi P., Moore C., Kurtz S., Coffield V. M., Accavitti-Loper M. A., Su L., Vogel S. N., Braunstein M., Ting J. P. (2005) The CATERPILLER protein monarch-1 is an antagonist of Toll-like receptor-, tumor necrosis factor α-, and Mycobacterium tuberculosis-induced pro-inflammatory signals. J. Biol. Chem. 280, 39914–39924 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Lich J. D., Williams K. L., Moore C. B., Arthur J. C., Davis B. K., Taxman D. J., Ting J. P. (2007) Monarch-1 suppresses non-canonical NF-κB activation and p52-dependent chemokine expression in monocytes. J. Immunol. 178, 1256–1260 [DOI] [PubMed] [Google Scholar]
- 76. Arthur J. C., Lich J. D., Ye Z., Allen I. C., Gris D., Wilson J. E., Schneider M., Roney K. E., O'Connor B. P., Moore C. B., Morrison A., Sutterwala F. S., Bertin J., Koller B. H., Liu Z., Ting J. P. (2010) Cutting edge: NLRP12 controls dendritic and myeloid cell migration to affect contact hypersensitivity. J. Immunol. 185, 4515–4519 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Zaki M. H., Vogel P., Malireddi R. K., Body-Malapel M., Anand P. K., Bertin J., Green D. R., Lamkanfi M., Kanneganti T. D. (2011) The NOD-like receptor NLRP12 attenuates colon inflammation and tumorigenesis. Cancer Cell 20, 649–660 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Ogura Y., Inohara N., Benito A., Chen F. F., Yamaoka S., Nunez G. (2001) Nod2, a Nod1/Apaf-1 family member that is restricted to monocytes and activates NF-κB. J. Biol. Chem. 276, 4812–4818 [DOI] [PubMed] [Google Scholar]
- 79. Kobayashi K., Inohara N., Hernandez L. D., Galan J. E., Nunez G., Janeway C. A., Medzhitov R., Flavell R. A. (2002) RICK/Rip2/CARDIAK mediates signalling for receptors of the innate and adaptive immune systems. Nature 416, 194–199 [DOI] [PubMed] [Google Scholar]
- 80. Park J. H., Kim Y. G., McDonald C., Kanneganti T. D., Hasegawa M., Body-Malapel M., Inohara N., Nunez G. (2007) RICK/RIP2 mediates innate immune responses induced through Nod1 and Nod2 but not TLRs. J. Immunol. 178, 2380–2386 [DOI] [PubMed] [Google Scholar]
- 81. Hasegawa M., Fujimoto Y., Lucas P. C., Nakano H., Fukase K., Nunez G., Inohara N. (2008) A critical role of RICK/RIP2 polyubiquitination in Nod-induced NF-κB activation. EMBO J. 27, 373–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82. Yang Y., Yin C., Pandey A., Abbott D., Sassetti C., Kelliher M. A. (2007) NOD2 pathway activation by MDP or Mycobacterium tuberculosis infection involves the stable polyubiquitination of Rip2. J. Biol. Chem. 282, 36223–36229 [DOI] [PubMed] [Google Scholar]
- 83. Dugan J. W., Albor A., David L., Fowlkes J., Blackledge M. T., Martin T. M., Planck S. R., Rosenzweig H. L., Rosenbaum J. T., Davey M. P. (2009) Nucleotide oligomerization domain-2 interacts with 2′-5′-oligoadenylate synthetase type 2 and enhances RNase-L function in THP-1 cells. Mol. Immunol. 47, 560–566 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Allen I. C., Moore C. B., Schneider M., Lei Y., Davis B. K., Scull M. A., Gris D., Roney K. E., Zimmermann A. G., Bowzard J. B., Ranjan P., Monroe K. M., Pickles R. J., Sambhara S., Ting J. P. (2011) NLRX1 protein attenuates inflammatory responses to infection by interfering with the RIG-I-MAVS and TRAF6-NF-κB signaling pathways. Immunity 34, 854–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. Jia Y., Song T., Wei C., Ni C., Zheng Z., Xu Q., Ma H., Li L., Zhang Y., He X., Xu Y., Shi W., Zhong H. (2009) Negative regulation of MAVS-mediated innate immune response by PSMA7. J. Immunol. 183, 4241–4248 [DOI] [PubMed] [Google Scholar]
- 86. Xia X., Cui J., Wang H. Y., Zhu L., Matsueda S., Wang Q., Yang X., Hong J., Songyang Z., Chen Z. J., Wang R. F. (2011) NLRX1 negatively regulates TLR-induced NF-κB signaling by targeting TRAF6 and IKK. Immunity 34, 843–853 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87. Arnoult D., Soares F., Tattoli I., Castanier C., Philpott D. J., Girardin S. E. (2009) An N-terminal addressing sequence targets NLRX1 to the mitochondrial matrix. J. Cell Sci. 122, 3161–3168 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Abdul-Sater A. A., Said-Sadier N., Lam V. M., Singh B., Pettengill M. A., Soares F., Tattoli I., Lipinski S., Girardin S. E., Rosenstiel P., Ojcius D. M. (2010) Enhancement of reactive oxygen species production and chlamydial infection by the mitochondrial Nod-like family member NLRX1. J. Biol. Chem. 285, 41637–41645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89. Rebsamen M., Vazquez J., Tardivel A., Guarda G., Curran J., Tschopp J. (2011) NLRX1/NOD5 deficiency does not affect MAVS signalling. Cell Death Differ. 18, 1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Fernandes-Alnemri T., Yu J. W., Juliana C., Solorzano L., Kang S., Wu J., Datta P., McCormick M., Huang L., McDermott E., Eisenlohr L., Landel C. P., Alnemri E. S. (2010) The AIM2 inflammasome is critical for innate immunity to Francisella tularensis. Nat. Immunol. 11, 385–393 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Rathinam V. A., Jiang Z., Waggoner S. N., Sharma S., Cole L. E., Waggoner L., Vanaja S. K., Monks B. G., Ganesan S., Latz E., Hornung V., Vogel S. N., Szomolanyi-Tsuda E., Fitzgerald K. A. (2010) The AIM2 inflammasome is essential for host defense against cytosolic bacteria and DNA viruses. Nat. Immunol. 11, 395–402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Sauer J. D., Witte C. E., Zemansky J., Hanson B., Lauer P., Portnoy D. A. (2010) Listeria monocytogenes triggers AIM2-mediated pyroptosis upon infrequent bacteriolysis in the macrophage cytosol. Cell Host Microbe 7, 412–419 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jones J. W., Kayagaki N., Broz P., Henry T., Newton K., O'Rourke K., Chan S., Dong J., Qu Y., Roose-Girma M., Dixit V. M., Monack D. M. (2010) Absent in melanoma 2 is required for innate immune recognition of Francisella tularensis. Proc. Natl. Acad. Sci. USA 107, 9771–9776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Kerur N., Veettil M. V., Sharma-Walia N., Bottero V., Sadagopan S., Otageri P., Chandran B. (2011) IFI16 acts as a nuclear pathogen sensor to induce the inflammasome in response to Kaposi sarcoma-associated herpesvirus infection. Cell Host Microbe 9, 363–375 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Shenoy A. R., Wellington D. A., Kumar P., Kassa H., Booth C. J., Cresswell P., Macmicking J. D. (2012) GBP5 promotes NLRP3 inflammasome assembly and immunity in mammals. Science 336, 481–485 [DOI] [PubMed] [Google Scholar]
- 96. Kanneganti T. D., Body-Malapel M., Amer A., Park J. H., Whitfield J., Franchi L., Taraporewala Z. F., Miller D., Patton J. T., Inohara N., Nunez G. (2006) Critical role for cryopyrin/Nalp3 in activation of caspase-1 in response to viral infection and double-stranded RNA. J. Biol. Chem. 281, 36560–36568 [DOI] [PubMed] [Google Scholar]
- 97. Segovia J., Sabbah A., Mgbemena V., Tsai S. Y., Chang T. H., Berton M. T., Morris I. R., Allen I. C., Ting J. P., Bose S. (2012) TLR2/MyD88/NF-κB pathway, reactive oxygen species, potassium efflux activates NLRP3/ASC inflammasome during respiratory syncytial virus infection. PloS One 7, e29695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Komune N., Ichinohe T., Ito M., Yanagi Y. (2011) Measles virus V protein inhibits NLRP3 inflammasome-mediated interleukin-1β secretion. J. Virol. 85, 13019–13026 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Allen I. C., Scull M. A., Moore C. B., Holl E. K., McElvania-TeKippe E., Taxman D. J., Guthrie E. H., Pickles R. J., Ting J. P. (2009) The NLRP3 inflammasome mediates in vivo innate immunity to influenza A virus through recognition of viral RNA. Immunity 30, 556–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Ichinohe T., Lee H. K., Ogura Y., Flavell R., Iwasaki A. (2009) Inflammasome recognition of influenza virus is essential for adaptive immune responses. J. Exp. Med. 206, 79–87 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Ichinohe T., Pang I. K., Iwasaki A. (2010) Influenza virus activates inflammasomes via its intracellular M2 ion channel. Nat. Immunol. 11, 404–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Rajan J. V., Rodriguez D., Miao E. A., Aderem A. (2011) The NLRP3 inflammasome detects encephalomyocarditis virus and vesicular stomatitis virus infection. J. Virol. 85, 4167–4172 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103. Burdette D., Haskett A., Presser L., McRae S., Iqbal J., Waris G. (2012) Hepatitis C virus activates interleukin-1β via caspase-1-inflammasome complex. J. Gen. Virol. 93, 235–246 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 104. Pontillo A., Silva L. T., Oshiro T. M., Finazzo C., Crovella S., Duarte A. J. (2012) HIV-1 induces NALP3-inflammasome expression and interleukin-1β secretion in dendritic cells from healthy individuals but not from HIV-positive patients. AIDS 26, 11–18 [DOI] [PubMed] [Google Scholar]
- 105. Sabbah A., Chang T. H., Harnack R., Frohlich V., Tominaga K., Dube P. H., Xiang Y., Bose S. (2009) Activation of innate immune antiviral responses by Nod2. Nat. Immunol. 10, 1073–1080 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Muruve D. A., Petrilli V., Zaiss A. K., White L. R., Clark S. A., Ross P. J., Parks R. J., Tschopp J. (2008) The inflammasome recognizes cytosolic microbial and host DNA and triggers an innate immune response. Nature 452, 103–107 [DOI] [PubMed] [Google Scholar]
- 107. Delaloye J., Roger T., Steiner-Tardivel Q. G., Le Roy D., Knaup Reymond M., Akira S., Petrilli V., Gomez C. E., Perdiguero B., Tschopp J., Pantaleo G., Esteban M., Calandra T. (2009) Innate immune sensing of modified vaccinia virus Ankara (MVA) is mediated by TLR2-TLR6, MDA-5 and the NALP3 inflammasome. PLoS Pathog. 5, e1000480. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 108. Barlan A. U., Griffin T. M., McGuire K. A., Wiethoff C. M. (2011) Adenovirus membrane penetration activates the NLRP3 inflammasome. J. Virol. 85, 146–155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Di Paolo N. C., Miao E. A., Iwakura Y., Murali-Krishna K., Aderem A., Flavell R. A., Papayannopoulou T., Shayakhmetov D. M. (2009) Virus binding to a plasma membrane receptor triggers interleukin-1 α-mediated proinflammatory macrophage response in vivo. Immunity 31, 110–121 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Nour A. M., Reichelt M., Ku C. C., Ho M. Y., Heineman T. C., Arvin A. M. (2011) Varicella-zoster virus infection triggers formation of an interleukin-1β (IL-1β)-processing inflammasome complex. J. Biol. Chem. 286, 17921–17933 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Gregory S. M., Davis B. K., West J. A., Taxman D. J., Matsuzawa S., Reed J. C., Ting J. P., Damania B. (2011) Discovery of a viral NLR homolog that inhibits the inflammasome. Science 331, 330–334 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112. Ray C. A., Black R. A., Kronheim S. R., Greenstreet T. A., Sleath P. R., Salvesen G. S., Pickup D. J. (1992) Viral inhibition of inflammation: cowpox virus encodes an inhibitor of the interleukin-1 β converting enzyme. Cell 69, 597–604 [DOI] [PubMed] [Google Scholar]
- 113. MacNeill A. L., Moldawer L. L., Moyer R. W. (2009) The role of the cowpox virus crmA gene during intratracheal and intradermal infection of C57BL/6 mice. Virology 384, 151–160 [DOI] [PubMed] [Google Scholar]
- 114. Messud-Petit F., Gelfi J., Delverdier M., Amardeilh M. F., Py R., Sutter G., Bertagnoli S. (1998) Serp2, an inhibitor of the interleukin-1β-converting enzyme, is critical in the pathobiology of myxoma virus. J. Virol. 72, 7830–7839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Kettle S., Blake N. W., Law K. M., Smith G. L. (1995) Vaccinia virus serpins B13R (SPI-2) and B22R (SPI-1) encode M(r) 38.5 and 40K, intracellular polypeptides that do not affect virus virulence in a murine intranasal model. Virology 206, 136–147 [DOI] [PubMed] [Google Scholar]
- 116. Kettle S., Alcami A., Khanna A., Ehret R., Jassoy C., Smith G. L. (1997) Vaccinia virus serpin B13R (SPI-2) inhibits interleukin-1β-converting enzyme and protects virus-infected cells from TNF- and Fas-mediated apoptosis, but does not prevent IL-1β-induced fever. J. Gen. Virol. 78, 677–685 [DOI] [PubMed] [Google Scholar]
- 117. Smith V. P., Bryant N. A., Alcami A. (2000) Ectromelia, vaccinia and cowpox viruses encode secreted interleukin-18-binding proteins. J. Gen. Virol. 81, 1223–1230 [DOI] [PubMed] [Google Scholar]
- 118. Xiang Y., Moss B. (1999) IL-18 binding and inhibition of interferon γ induction by human poxvirus-encoded proteins. Proc. Natl. Acad. Sci. USA 96, 11537–11542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119. Alcami A., Smith G. L. (1996) A mechanism for the inhibition of fever by a virus. Proc. Natl. Acad. Sci. USA 93, 11029–11034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Stehlik C., Krajewska M., Welsh K., Krajewski S., Godzik A., Reed J. C. (2003) The PAAD/PYRIN-only protein POP1/ASC2 is a modulator of ASC-mediated nuclear-factor-κ B and pro-caspase-1 regulation. Biochem. J. 373, 101–113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121. Johnston J. B., Barrett J. W., Nazarian S. H., Goodwin M., Ricciuto D., Wang G., McFadden G. (2005) A poxvirus-encoded pyrin domain protein interacts with ASC-1 to inhibit host inflammatory and apoptotic responses to infection. Immunity 23, 587–598 [DOI] [PubMed] [Google Scholar]
- 122. Dorfleutner A., Talbott S. J., Bryan N. B., Funya K. N., Rellick S. L., Reed J. C., Shi X., Rojanasakul Y., Flynn D. C., Stehlik C. (2007) A Shope fibroma virus PYRIN-only protein modulates the host immune response. Virus Genes 35, 685–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Stasakova J., Ferko B., Kittel C., Sereinig S., Romanova J., Katinger H., Egorov A. (2005) Influenza A mutant viruses with altered NS1 protein function provoke caspase-1 activation in primary human macrophages, resulting in fast apoptosis and release of high levels of interleukins 1β and 18. J. Gen. Virol. 86, 185–195 [DOI] [PubMed] [Google Scholar]