Heparanase rs4693608 SNP discrepancy between recipients and donors correlates with post transplantation engraftment and risk of acute GVHD.
Keywords: HPSE, allogeneic HSCT, acute GVHD, neutrophils, mononuclear cells
Abstract
Heparanase is an endo-β-glucuronidase that specifically cleaves the saccharide chains of HSPGs, important structural and functional components of the ECM. Cleavage of HS leads to loss of the structural integrity of the ECM and release of HS-bound cytokines, chemokines, and bioactive angiogenic- and growth-promoting factors. Our previous study revealed a highly significant correlation of HPSE gene SNPs rs4693608 and rs4364254 and their combination with the risk of developing GVHD. We now demonstrate that HPSE is up-regulated in response to pretransplantation conditioning, followed by a gradual decrease thereafter. Expression of heparanase correlated with the rs4693608 HPSE SNP before and after conditioning. Moreover, a positive correlation was found between recipient and donor rs4693608 SNP discrepancy and the time of neutrophil and platelet recovery. Similarly, the discrepancy in rs4693608 HPSE SNP between recipients and donors was found to be a more significant factor for the risk of aGVHD than patient genotype. The rs4693608 SNP also affected HPSE gene expression in LPS-treated MNCs from PB and CB. Possessors of the AA genotype exhibited up-regulation of heparanase with a high ratio in the LPS-treated MNCs, whereas individuals with genotype GG showed down-regulation or no effect on HPSE gene expression. HPSE up-regulation was mediated by TLR4. The study emphasizes the importance of rs4693608 SNP for HPSE gene expression in activated MNCs, indicating a role in allogeneic stem cell transplantation, including postconditioning, engraftment, and GVHD.
Introduction
Heparanase is an endoglycosidase that cleaves HS side-chains of HSPG, resulting among other effects, in release of HS-bound proinflammatory cytokines and chemokines, as well as proangiogenic factors, such as FGF and vascular endothelial growth factor [1–5]. Whereas heparanase is up-regulated in various solid and hematological malignancies, it is normally found mainly in platelets [6], mast cells [7], keratinocytes [8], DCs [9], macrophages [10], neutrophils [6], and activated T cells [11]. Up-regulation of heparanase was reported in different inflammatory conditions, often associated with degradation of HS and release of chemokines anchored within the ECM network and cell surface [12]. Moreover, remodeling of the ECM facilitates transmigration of inflammatory cells toward the injury sites [12]. Heparanase, locally expressed by the vascular endothelium at the site of inflammation, was found to be implicated directly in DTH inflammation [13]. High levels of heparanase were found in sinovial fluid from rheumatoid arthritis patients, suggesting an important role in promoting joint destruction [14]. Heparanase is also causally involved in inflammatory lung injury and sepsis [15].
Up-regulation of heparanase has also been identified in the colonic epithelium of patients with IBD [16]. Furthermore, we have demonstrated that heparanase induces activation of innate immune cells (i.e., macrophages), which in turn, may stimulate, via up-regulation of cytokines (i.e., TNF-α), further production of the enzyme by various cell types (i.e., epithelial cells, endothelial cells), thereby preserving chronic inflammation. It was found that macrophages not only represent a cellular target for heparanase action (i.e., macrophage activation) but can also up-regulate heparanase at the transcriptional and post-translational levels [16, 17].
We have determined previously genotype combinations of rs4693608 and rs4364254 SNPs, named HR, MR and LR groups, which correlate significantly with high, intermediate and low heparanase expression level among healthy individuals [18]. This finding led us to investigate the involvement of HPSE gene SNP combinations and heparanase in GVHD and allogeneic HSCT outcome [19]. Our study indicated a highly significant correlation between these SNPs combinations and the risk of aGVHD and extensive, chronic GVHD. Moreover, discrepancy between recipient and donor in these SNP combinations affected the risk of GVHD significantly. We suggested that possessors of genotype combination HR secrete high levels of heparanase compared with patients carrying the MR and LR genotype combinations [19].
In the present study, we analyzed the effect of conditioning regimens on heparanase expression levels in recipients before HSCT. Moreover, MNCs from PB of healthy adults and umbilical CB were used to mimic the influence of the proinflammatory milieu on donor transplant cells. In HSCT patients, we found that conditioning regimens led to up-regulation of heparanase. The expression level of the HPSE gene, before and after conditioning, remained in correlation with the rs4693608 SNP. The present study emphasizes the fact that the discrepancy in rs4693608 SNP between recipients and donors is a more important factor for predicting the risk of aGVHD development than patient genotype. Moreover, the difference in rs4693608 SNP between recipients and donors correlated with the time of neutrophil and platelet recovery.
In addition, the response to LPS strongly correlated with HPSE rs4693608 SNP. Individuals with the AA genotype demonstrated an elevation of HPSE gene expression in response to LPS, whereas those with the GG genotype showed down-regulation or no effect on HPSE gene expression. Notably, LPS treatment led to an increase of HPSE gene expression through TLR4. Altogether, the present study emphasizes the importance of rs4693608 SNP for HPSE gene expression in activated MNCs, indicating a role in allogeneic stem cell transplantation, including postconditioning, engraftment, and GVHD.
MATERIALS AND METHODS
Study population
Consecutive patients (198; men and women) with hematological malignancies were included in the study. Patients were transplanted at the Bone Marrow Transplantation Department of the Chaim Sheba Medical Center (Tel Hashomer, Israel). All patients gave written, informed consent, and the study was approved by the Institutional Review Board and the Israeli Ministry of Health (#7065-09-SMC). Patient characteristics are presented in Table 1. The median age was 52 years (range 18–76). Blood samples were collected 1 day prior to conditioning and 24 h post-last-dose chemotherapy pretransplantation. Transplant conditioning regimens varied according to the type and status of the disease. Pretransplantation conditioning regimens were: (1) classical myeloablative, including Cy and total body irradiation and i.v. busulfan and Cy and (2) RIC containing fludarabine with reduced doses of busulfan (Table 1). Anti-GVHD prophylaxis consisted of cyclosporine A and a short course of methotrexate. GVHD prophylaxis was administered for 3–6 months and tapered afterward in patients with no active GVHD. G-CSF was administered from Day 7 post-transplantation until engraftment. A standard regimen of antibiotic and antimycotic prophylaxis was used to prevent bacterial and fungal infections. Acyclovir, sulfumethoxasole, and trimethoprine were administered for prevention of viral and Pneumocystis carni infections, respectively.
Table 1. Characteristics of Transplant Recipients and Donors.
| Variable | Characteristic | No. of cases (%) |
|---|---|---|
| Age, year | Median, 52 | |
| Range, 18–76 | ||
| Gender | Recipient: | |
| M | 118 (59.6) | |
| F | 80 (40.4) | |
| Donor: | ||
| Male | 114 (57.6) | |
| Female | 84 (42.4) | |
| Recipient/donor | Male/male | 70 (35.4) |
| Male/female | 49 (24.7) | |
| Female/male | 45 (22.7) | |
| Female/female | 34 (17.2) | |
| Diagnosis | AML | 81 (40.9) |
| ALL | 18 (9.1) | |
| CML | 9 (4.6) | |
| CLL | 5 (2.5) | |
| MDS | 31 (15.7) | |
| HL | 4 (2.0) | |
| NHL | 25 (12.6) | |
| MM | 9 (4.6) | |
| MF | 8 (4.0) | |
| SAA | 8 (4.0) | |
| Donor | Sibling | 104 (52.5) |
| MUD/mm | 94 (47.5) | |
| Conditioning | Myeloablative | 131 (66.2) |
| regimen | Reduced-intensity conditioning | 67 (33.8) |
ALL, Acute lymphoblastic leukemia; CLL, chronic lymphocytic leukemia; F, female; HL, Hodgkin's lymphoma; M, male; MDS, myelodysplastic syndrome; MF, myelofibrosis; MM, multiple myeloma; MUD/mm, matched-unrelated or mismatched-unrelated donor; NHL, Non-Hodgkin's lymphoma; SAA, Severe aplastic anemia.
To analyze the timing of HPSE gene expression following HSCT, 20 PB samples were collected at five time-points: (1) before conditioning, Day −7; (2) 24 h post the last dose of chemotherapy, pretransplantation; and at (3) 1, (4) 2, and (5) 3 months after HSCT. In the case of aGVHD development (eight out of 20 patients developed aGVHD Grade II–IV), additional samples were collected. Expression level of the HPSE gene was examined in total PBLs.
Analysis of the correlation between time to neutrophils, lymphocytes, monocytes, and platelet recovery and rs4693608 SNP was performed in 449 recipient-donor pairs. Neutrophil and platelet engraftment was defined as the day on which the PB absolute neutrophil count exceeded 0.5 × 109/L, and the platelet count was 20 × 109/L after transplantation. The absolute lymphocyte and monocyte counts were determined on Day 30 post-HSCT and correlated with recipient and donor rs4693608 SNP genotypes. Additionally, 128 umbilical CB samples and 104 PB samples from healthy individuals were analyzed for HPSE gene expression in LPS-treated and untreated MNCs. Samples were chosen randomly and represent various Israeli ethnic groups of different origins. The average age of adults was 39 years (range 25–56 years). All normal subjects and maternal donors gave their written, informed consent, and the study was approved by the Institutional Review Board and the Israeli Ministry of Health (#2341-01-SMC).
rs4693608 SNP genotyping
Genomic DNA was prepared from PB and CB, applying the Wizard genomic DNA purification kit (Promega, Madison, WI, USA). Genotyping of rs4693608 SNP was carried out as described [20, 21]. Briefly, SNPs were identified by allele-specific amplification. The optimized PCR reaction mixture, in a final volume of 15 μl, consisted of Red Load Taq Master (Larova GmbH, Teltow, Germany), 10–20 pM of each forward and reverse primers, and 150–200 ng of the DNA template. All reactions were performed using a PTC-200 Peltier thermal cycler (MJ Research, Watertown, MS, USA). The cycle conditions were 94°C for 4 min, followed by 30 cycles of 94°C for 15 s (denaturation), 53°C for 30 s (annealing), and 72°C for 30 s (extension). The PCR products were separated by 2% agarose gel electrophoresis and visualized under UV light using ethidium bromide staining. Gels were photographed and analyzed by the EDAS 290 electrophoresis documentation and analysis system (Kodak, Rochester, NY, USA).
Isolation and stimulation of CB and PB
CB was obtained by venipuncture from umbilical cord veins immediately after delivery. MNCs from CB and PB were isolated on a standard Ficoll-Paque density gradient (Axis-Shield, Oslo, Norway). Cells were cultured further in RPMI-1640 medium and activated with LPS (100 ng/ml; Sigma-Aldrich, St. Louis, MO, USA) for 18 h. Monocytes and NK cells were separated by MACS separation columns (Miltenyi Biotec, Auburn, CA, USA). CD4+, CD8+, and B cell lymphocytes were enriched by RosetteSep human T/B cell enrichment cocktails (StemCell Technologies, Vancouver, BC, Canada). Neutrophils were separated by Histopaque-1119 (Sigma-Aldrich) and Histopaque-1077 (Sigma-Aldrich). Macrophages were derived by adhesion of MNCs to plastic tissue-culture flasks, followed by incubation at 37°C for 2 weeks in RPMI. Sample purity was detected by flow cytometric analysis using FACSCalibur (Becton Dickinson, San Jose, CA, USA). FITC-conjugated anti-CD8, -CD4, -CD14, -CD19, and -CD56 mAb and isotype control IgG1 were purchased from eBioscience (San Diego, CA, USA) and used according to the manufacturer's instructions.
Purification of total RNA, generation of cDNA, and real-time quantitative RT-PCR
Total RNA was extracted from LPS-activated and -nonactivated CB and PB MNCs and from total PBLs obtained from patients, using the TRIzol reagent (Invitrogen, Carlsbad, CA, USA), according to the manufacturer's instructions. The concentration of RNA was determined by measuring the OD at a wavelength of 260 nm, using the GenQuant instrument (Pharmacia Amersham Biosciences, Piscataway, NJ, USA). cDNA was synthesized using 1 μg total RNA, applying a high-capacity cDNA reverse transcription kit with RNase inhibitor (Applied Biosystems, Warrington, UK), as described by the manufacturer. SYBR Green (Applied Biosystems) real-time PCR reactions for amplification of the HPSE gene and the housekeeping gene L-19 ribosomal RNA were performed using the ABI PRISM 7700 sequence detector (Applied Biosystems) in a total volume of 20 μl. The sequences of primers and the detailed procedure for quantification were described previously [21]. Experiments were performed in triplicates for each sample, and a standard curve was generated for each gene. A dissociation curve was performed after each experiment to confirm that a single product was amplified. The CT method (ΔΔCT) was used to quantify HPSE gene expression, and RQ was calculated as 2−ΔΔCT. The ratio between HPSE mRNA expression before and after LPS treatment was calculated. The median of the ratio was 2.5. Values below and above 2.5 were defined as low and high ratios, respectively.
Immunocytochemistry
Cells were subjected to indirect immunofluorescence staining, essentially as described [22, 23]. Briefly, cells were fixed with cold methanol for 10 min. Cells were then washed with PBS and incubated in PBS containing 10% normal goat serum for 1 h at room temperature, followed by 2 h incubation with the indicated antiheparanase primary antibody. Cells were then washed extensively with PBS and incubated with AlexaFlour 488 (Cy2/Cy3-conjugated) secondary antibody (Invitrogen) for 1 h, washed, and stained with PI to view the cell nucleus (Vectashield; Vector Laboratories, Burlingame, CA, USA). Staining was visualized by confocal microscopy, as described [24].
Analysis of TLR4 involvement in HPSE gene expression
Two-color flow cytometry was performed on freshly isolated CB MNCs to investigate the expression levels of TLR4 on CB cell subsets. Briefly, MNCs were incubated (30 min, 4°C) with the following primary antibodies: PE-conjugated anti-TLR4 mAb; FITC-conjugated anti-CD3, anti-CD14, and anti-CD56 mAb; PerCP-Cy5-conjugated anti-CD20; and isotype control IgG1 (eBioscience), and used according to the manufacturer's instructions. Flow cytometric analysis was performed by FACSCalibur (Becton Dickinson) using CellQuest software (BD Biociences, San Jose, CA, USA).
Inhibitory peptide MyD88 was used for evaluation of TLR4 involvement in HPSE gene expression after LPS treatment. MyD88 homodimerization inhibitory peptide and control peptide were purchased from Imgenex (San Diego, CA, USA) and preincubated (24 h) with the cells at a final concentration of 25 μM.
Statistical analysis
Genotype and allele frequencies of the SNPs were calculated by direct counting. Cumulative incidence of aGVHD was calculated in relation to the rs4693608 SNP. Time to the clinical event was measured from the date of HSCT. In the analysis of the cumulative incidence of GVHD, relapse was considered a competing risk [25]. Analysis of discrepancy between recipient and donor was performed. All donor-recipient pairs were divided into three groups, according to the risk of aGVHD development: D1 group (AA-GG and AA-AG recipient-donor pairs) for high risk of aGVHD development; D2 group (AA-AA, AG-AG, AG-GG, and AG-AA recipient-donor pairs) for moderate risk; and D3 group (GG-GG, GG-AG, and GG-AA recipient-donor pairs) for low risk, respectively. Cumulative incidence analysis and the log-rank test were carried out.
Comparison between responsiveness to LPS activation and rs4693608 SNP was performed using the χ2 test. Mann-Whitney U- or Wilcoxon rank-sum test for differences in medians was used to evaluate the: (1) effect of LPS treatment on relative heparanase expression level before and after stimulation in correlation to rs4693608 SNP; (2) association between rs4693608 SNP and the relative heparanase expression, pre- and postconditioning; and (3) correlation between rs4693608 SNP and time of neutrophil and platelet reconstitution after HSCT. This correlation was analyzed in three newly designed recipient-donor pair groups (N1, N2, N3), according to their presumable differences in heparanase expression. The N1 group included AA-AG, AA-GG, and AG-GG recipient-donor pairs, in which recipient heparanase expression is presumably higher in comparison with the donor; the N2 group contained AA-AA, AG-AG, and GG-GG recipient-donor pairs with the same HPSE gene-expression levels; and the N3 group consisted of AG-AA, GG-AA, and GG-AG recipient-donor pairs with a low heparanase expression level in the patients relative to a high expression level in the donors. P ≤ 0.05 was regarded as statistically significant. Calculations were performed using NCSS software (NCSS Statistical Software, Kaysville, UT, USA).
RESULTS
Correlation between rs4693608 SNP and the risk of aGVHD
Patients (198) and their donors were included in the study. Fifty-four of them developed aGVHD (Grade II-IV). An additional 13 recipients developed relapse during the first 100 days post-transplantation and were calculated as a competing risk. Univariate analysis of cumulative incidence of Grade II–IV aGVHD revealed statistically significant correlation to rs4693608, thereby confirming our previous results [19]. The cumulative incidence of aGVHD (on Day 100 post-HSCT) was 46.8% (95% CI 34.5–63.5), 27.3% (95% CI 15.6–47.6), and 24.5% (95% CI 17.6–34.9) for recipients carrying genotype AA, GG, and AG of rs4693608 SNP, respectively (P=0.039; Fig. 1A). In our previous studies [18, 19], we found that an additional SNP rs4364254 identifies heterozygote AG individuals with low heparanase expression and low-risk of aGVHD. In the present study, we restricted the analyses to rs4693608 SNP, as complete analysis of differences between recipients and donors showed the most consistent correlation between rs4693608 SNP and the risk of aGVHD. Cumulative rate of aGVHD incidence was 69.2% (95% CI 53.6–89.5) in the D1 group (AA-AG and AA-GG pairs); 23.5% (95% CI 16.9–32.7) in the D2 group (AA-AA, AG-AA, AG-AG, and AG-GG pairs); and 27.3% (CI 95% 15.6–47.6) in the D3 group (GG-AA, GG-AG, and GG-GG pairs; χ2=18.04; P=0.0001; Fig. 1B). These results indicate that the discrepancy in rs4693608 SNP between recipients and donors is a more important factor for the risk of aGVHD development than patient genotype.
Figure 1. Cumulative incidence of aGVHD after HSCT.
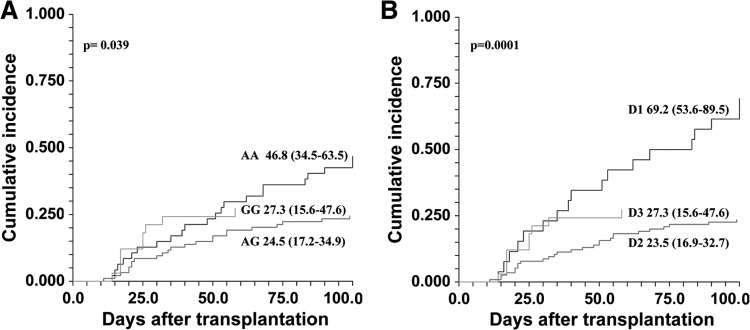
(A) Cumulative rate of aGVHD (Grade II–IV) incidence, analyzed according to the recipient rs4693608 SNP. The incidence was higher in possessors of the AA genotype compared with carriers of GG and AG genotypes (P=0.039). (B) Cumulative incidence of aGVHD (Grade II–IV), analyzed according to the discrepancy of rs4693608 SNP between recipient and donor. The D1 group consists of AA-GG and AA-AG recipient-donor pairs; the D2 group contains AA-AA, AG-AG, AG-GG, and AG-AA recipient-donor pairs; and the D3 group includes GG-GG, GG-AG, and GG-AA recipient-donor pairs. The incidence was higher in Group D1 compared with the D2 and D3 groups (P=0.0001).
Effect of pre-HSCT conditioning on heparanase expression, pre- and postconditioning, in 124 transplanted patients
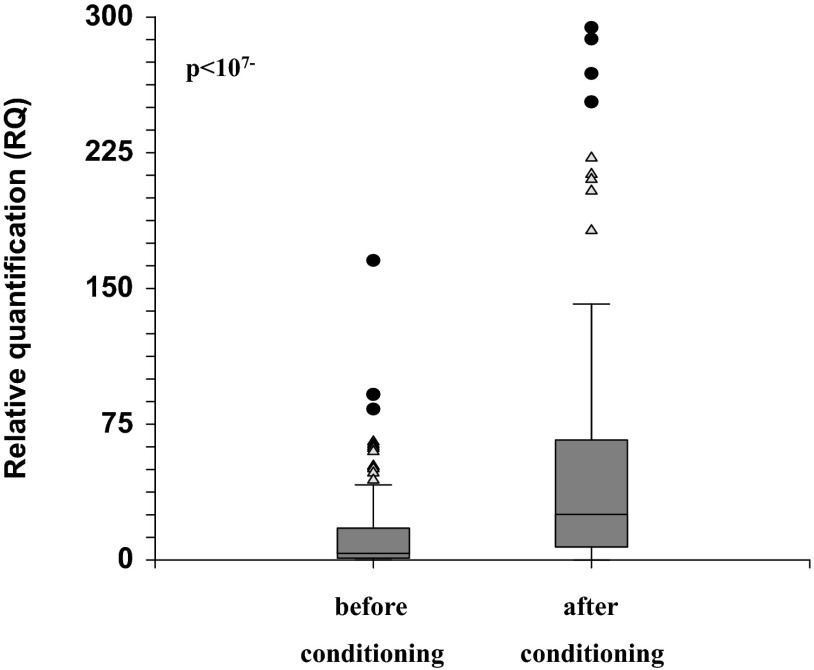
Our results indicate that the conditioning led to a significant increase in HPSE gene expressions (P<10−7; Fig. 2). There was no difference between myeloablative and RIC (not shown). Next, we examined the correlation between rs4693608 SNP and expression level of the HPSE gene, before and after conditioning. Recipients with the GG genotype showed relatively low HPSE mRNA levels, whereas patients with the AA genotype possessed a relatively high mRNA level, before and after conditioning (Table 2). Analysis of correlation between the incidence of aGVHD and HPSE gene expression level before HSCT did not reveal any association. Thus, high levels of heparanase before HSCT may not serve as a marker for prediction of aGVHD risk.
Figure 2. Expression levels of the HPSE gene in total PBLs before and after conditioning.
Expression level of HPSE mRNA was examined in total PBLs, pre- and postconditioning, in 124 transplanted patients. Blood samples were collected 1 day prior to conditioning and 24 h post last dose of chemotherapy, pretransplantation. Conditioning regimens led to a significant increase in heparanase expression levels. Triangles represent mild outliers, and circles represent severe outliers.
Table 2. Association between rs4693608 SNP and Relative HPSE mRNA Expression Levels in Transplant Recipients before and after Conditioning.
| Genotype | n | RQ median (95% LCL/UCL of median) | Comparison of carriers | Z value | P value | |
|---|---|---|---|---|---|---|
| Before conditioning | AA | 33 | 3.76 (1.15–12.04) | AA to GG | −1.8 | 0.036 |
| AG | 69 | 6.93 (3.79–13.36) | AA to AG+GG | −0.68 | NS | |
| GG | 22 | 1.2 (0.37–4.07) | GG to AA+AG | −2.98 | 0.003 | |
| After conditioning | AA | 33 | 47.54 (15.64–141.49) | AA to GG | −2 | 0.024 |
| AG | 69 | 39.26 (26.48–47.52) | AA to AG+GG | 1.1 | NS | |
| GG | 22 | 10.63 (2.85–39.88) | GG to AA+AG | −2.27 | 0.02 |
n, Sample size; LCL/UCL, lower/upper confidence limits for the median; Z value, a standard, normal deviation value; significant deviations (P<0.05) are in boldface.1
Modification of HPSE gene expression following allogeneic HSCT
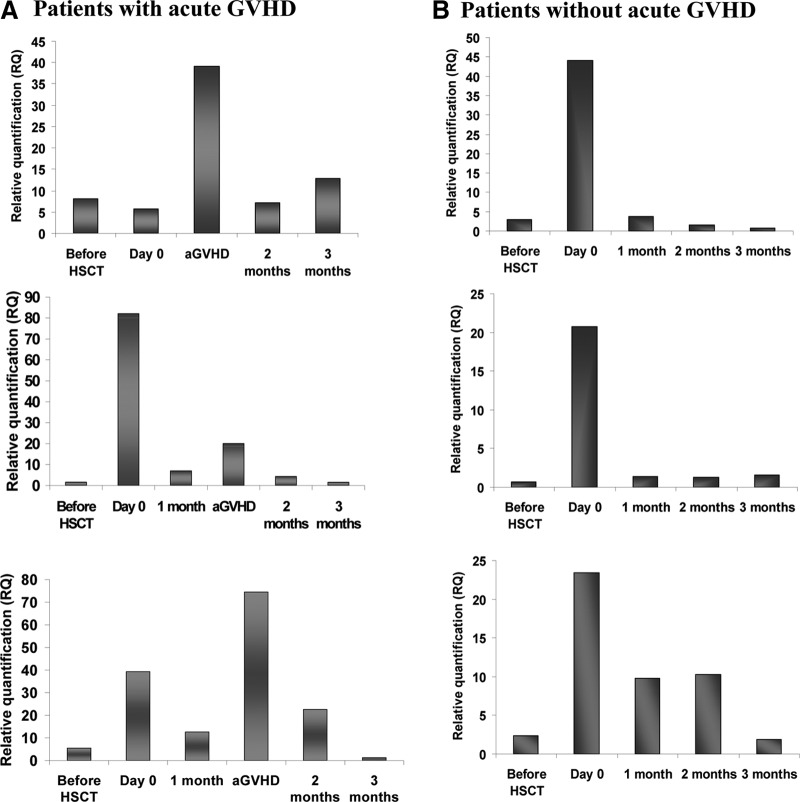
To analyze the timing of HPSE gene expression following HSCT, 20 PB samples (total leukocytes) were collected before conditioning; 24 h post the last dose of chemotherapy, pretransplantation; and at 1, 2, and 3 months after HSCT. Eight of the patients developed aGVHD (Grade II–IV). Figure 3 shows representative kinetics of HPSE gene expression in recipients who developed aGVHD versus patients without serious complications, up to Day 100 post-transplant. As depicted, pretransplant conditioning resulted in up-regulation of HPSE gene expression. Recipients who developed aGVHD exhibited elevated levels of HPSE expression, not only after conditioning but also during aGVHD development (Fig. 3A). In patients without serious complications, HPSE gene expression was increased after conditioning and before HSCT and was decreased thereafter (Fig. 3B), indicating that conditioning leads to elevation of heparanase gene expression. In patients without GVHD, HPSE levels decreased gradually to preconditioning levels, whereas in patients with aGVHD, a second peak of HPSE gene expression was noted.
Figure 3. Modification of HPSE gene expression following allogeneic HSCT.
Expression levels of the HPSE gene were examined in total PBLs, pre- and during the first 3 months after HSCT in 20 transplanted patients, with or without GVHD. Blood samples were collected at five time-points, up to Day 100. In patients with GVHD, HPSE expression levels were increased following conditioning and in GVHD (A). In patients with no GVHD, HPSE expression levels were increased following conditioning and decreased thereafter (B).
MNC response to inflammatory stimuli
Transplantation-conditioning regimens induce a proinflammatory environment with the generation of cytokines and release of bacterial products into the circulation following damage to the gastrointestinal blood barrier [26]. To mimic this process in vitro, MNCs from PB and umbilical CB were stimulated with LPS, known to activate signaling through CD14 and TLR4 [27].
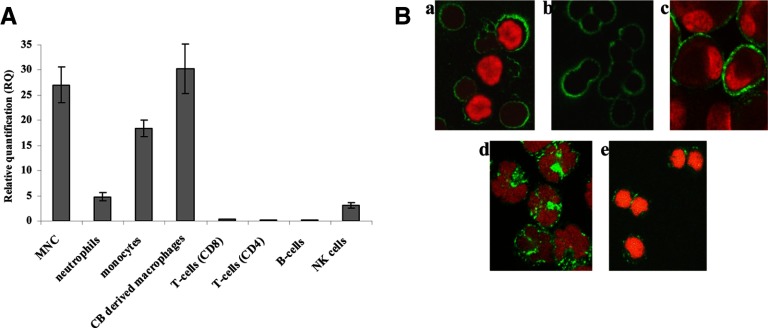
Previous studies showed that heparanase is normally expressed in PB neutrophils, monocytes, macrophages, and activated T lymphocytes [11], whereas no data are available for CB cells. As demonstrated in Fig. 4, heparanase (both mRNA and protein) is expressed primarily in CB-derived neutrophils, monocytes, and macrophages. Heparanase expression in T and B lymphocytes was very low. In addition, we found, for the first time, that heparanase is expressed in NK cells (Fig. 4B). The pattern of heparanase expression in NK cells differs from its expression in other hematopoietic cells. Whereas in NK cells, heparanase appears on the cell membrane in clusters and is widely expressed in the nucleus (Fig. 4B, e), in neutrophils (Fig. 4B, b) and monocytes (Fig. 4B, d), heparanase is distributed more uniformly on the cell membrane and is hardly detected in the cell nucleus. Analysis of heparanase expression in resting MNCs did not reveal differences among individuals with various HPSE gene genotypes in PB and CB samples (Tables 3 and 4). Our previous study [18] showed statistically significant differences between heparanase expression levels and rs4693608 SNPs among healthy individuals in total PBLs. Hereby, we therefore assume that this type of correlation in resting leukocytes is restricted to neutrophils.
Figure 4. Analysis of heparanase expression in umbilical CB cells.
(A) mRNA. MNCs were isolated on a standard Ficoll-Paque density gradient. Enrichment of MNC subpopulations was performed, as described in Materials and Methods. Monocyte purity was 95%; NK cells, 83%; macrophages, 92%; and neutrophils, 89%. Enrichment of CD4 was 89%; CD8, 82%; and CD19, 72% (n=3). Heparanase is expressed primarily in CB-derived MNCs, monocytes, and macrophages and to a lesser extent, in neutrophils and NK cells. There was little or no expression of heparanse in nonactivated T and B lymphocytes. (B) Immunostaining. Cells were subjected to immunostaining, as described in Materials and Methods. After fixation and PBS washing, cells were incubated with antiheparanase primary antibody (green). Cells were then washed extensively with PBS and incubated with AlexaFlour 488 (Cy2/Cy3-conjugated) secondary antibody, washed, and stained with PI to view the cell nucleus (red). (a) MNC; (b) neutrophils; (c) macrophages; (d) monocytes; and (e) NK cells. Original magnification, ×40. In neutrophils, CB-derived macrophages, and monocytes, heparanase is distributed uniformly in the cell membrane (B, a–d), whereas in NK cells, it appears in clusters and is also found in the nucleus (B, e).
Table 3. Association between rs4693608 SNP and Heparanase mRNA Expression Levels in MNCs from Healthy Individuals before and after LPS Treatment.
| Genotype | n | RQ median (95% LCL/UCL of median) | Comparison of carriers | Z value | P value | |
|---|---|---|---|---|---|---|
| Pretreatment | AA | 31 | 5.28 (3.06–8.98) | AA to GG | −0.56 | NS |
| AG | 50 | 6.03 (3.78–8.94) | AA to AG+GG | 0.79 | NS | |
| GG | 23 | 4.08 (2.2–9.1) | GG to AA+AG | 0.26 | NS | |
| Post-treatment | AA | 31 | 16.34 (7.47–34.46) | AA to GG | −2.47 | 0.014 |
| AG | 50 | 19.27 (10.87–23.89) | AA to AG+GG | 1.13 | NS | |
| GG | 23 | 6.37 (3.27–19.94) | GG to AA+AG | −2.87 | 0.004 | |
| Ratio (post-treatment RQ/pretreatment RQ) | AA | 31 | 3.11 (1.78–5.34) | AA to GG | −3.29 | 0.001 |
| AG | 50 | 2.83 (1.96–3.26) | AA to AG+GG | 1.87 | 0.03 | |
| GG | 23 | 1.56 (1.19–2.39) | GG to AA+AG | −3.26 | 0.001 |
See descriptions in Table 2.
Table 4. Association between rs4693608 SNP and Relative HPSE mRNA Expression Levels in LPS-Treated CB MNCs.
| Genotype | n | RQ median (95% LCL/UCL of median) | Comparison of carriers | Z value | P value | |
|---|---|---|---|---|---|---|
| Pretreatment | AA | 47 | 5.72 (3.77–7.77) | AA to GG | 0.64 | NS |
| AG | 56 | 4.68 (3.9–5.38) | AA to AG + GG | 0.36 | NS | |
| GG | 25 | 6.85 (3.31–11.12) | GG to AA + AG | 0.98 | NS | |
| Post-treatment | AA | 47 | 24.92 (15.22–40.49) | AA to GG | −3.42 | 0.0006 |
| AG | 56 | 8.64 (5.72–16.46) | AA to AG + GG | 3.21 | 0.001 | |
| GG | 25 | 5.37 (3.11–13.03) | GG to AA + AG | −2.58 | 0.009 | |
| Ratio (post-treatment RQ/pretreatment RQ) | AA | 47 | 5.13 (2.39–8.33) | AA to GG | −3.41 | 0.0006 |
| AG | 56 | 2.13 (1.08–4.03) | AA to AG + GG | 2.92 | 0.003 | |
| GG | 25 | 0.99 (0.57–2.26) | GG to AA + AG | −2.82 | 0.004 |
See descriptions in Table 2.
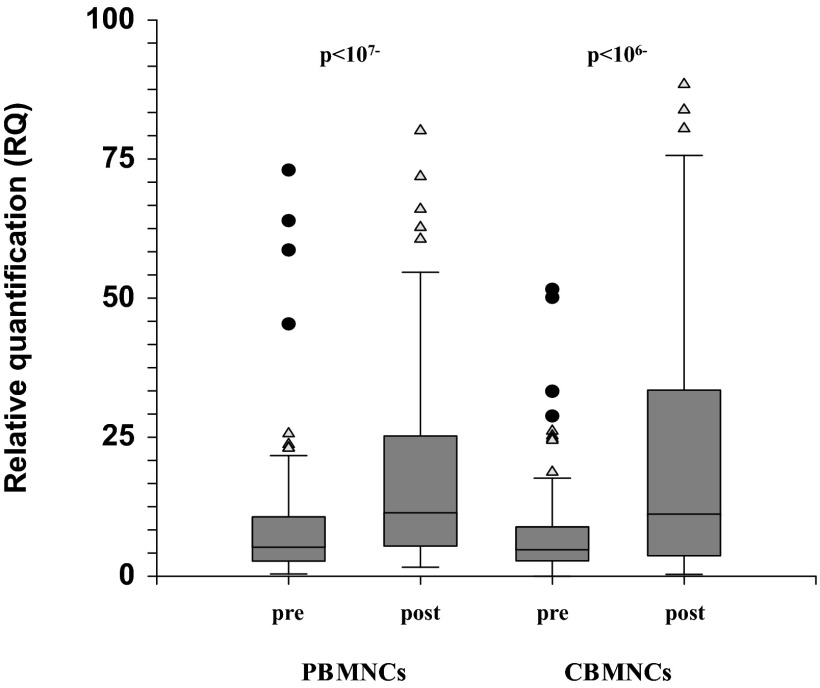
Examination of heparanase expression levels in PB MNCs after treatment with LPS disclosed a significant increase in HPSE gene expression (P<10−7; Fig. 5). Analysis of correlation between rs4693608 SNP and HPSE gene expression after LPS treatment revealed a highly significant association. Heparanase expression was relatively high in carriers of AA and AG genotypes and relatively low in possessors of the GG genotype (Table 3). A ratio test revealed a strong correlation between the rs4693608 SNP genotype and the increase in heparanase expression level in response to LPS stimulation. Healthy individuals with the AA genotype increased the level of heparanase to a higher extent than their counterparts with the GG genotype (P<0.001; Table 3). Analysis of HPSE gene expression in MNCs of umbilical CB showed similar results (Fig. 5 and Table 4). LPS stimulation elevated the expression of heparanase significantly in carriers of the AA genotype compared with possessors of the GG genotype (P<0.0006; Table 4). The ratio of heparanase expression before and after LPS treatment was significantly higher in CB samples with genotype AA compared with CB samples with genotype GG (P<0.0006; Table 4).
Figure 5. HPSE gene expression in PB and CB before and after treatment with LPS.
MNCs from CB and PB were isolated on a standard Ficoll-Paque density gradient. Cells/ml (1×106) were treated with 100 ng/ml LPS, as described in Materials and Methods, for 18 h. Expression level of heparanase mRNA was examined in 104 PB and 128 CB samples, pre- and post-LPS treatment. LPS led to a significant increase in HPSE gene expression level (P<10−7 for PB; P<10−6 for CB). Triangles represent mild outliers, and circles represent severe outliers.
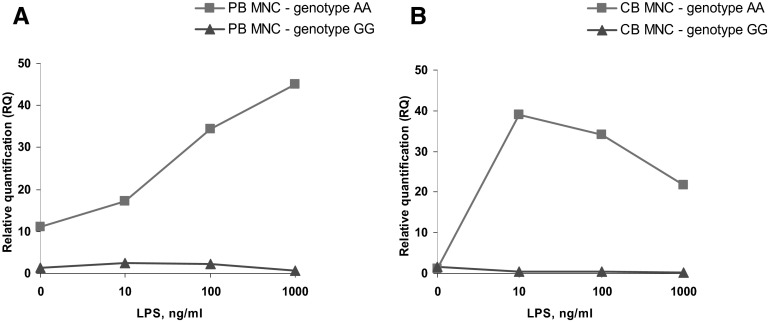
Detailed analysis of CB MNC data revealed that although in most of the samples, HPSE expression was elevated in response to LPS, in a minority of the samples (26.6%), LPS stimulation led to down-regulation of heparanase gene expression, whereas in 10.9%, nonresponsiveness to LPS treatment was noted. We therefore analyzed the correlation between responsiveness to LPS stimulation and the rs4693608 SNP genotype. Strong association was found between genotype AA and allele A and up-regulation of HPSE gene expression after LPS treatment. In contrast, down-regulation and nonresponsiveness were associated with genotype GG and allele G (P=0.002 for genotype frequencies; P<0.001 for allele frequency; Table 5). This observation was found in PB MNCs with low frequency (down-regulation in 8.7% and nonresponsiveness in 6.7% of the samples). Similarly, in PB MNCs, analysis of the correlation between rs4693608 SNP and responsiveness to LPS stimulation disclosed a statistically significant correlation between genotype GG and allele G and heparanase down-regulation or nonresponsiveness to LPS (Table 5). With the use of a previously published approach [18], we identified that 11 out of 17 individuals, who showed down-regulation or nonresponsiveness of the HPSE gene to LPS, had genotype LR (GG-CC, GG-TC, GG-TT, and AG-CC genotypes), and the association was highly significant (χ2=16.66; P<0.001). Next, PB and CB MNCs with genotypes AA and GG were exposed to increasing concentrations of LPS for 18 h, and RQ of the HPSE gene expression was determined. MNCs with the AA genotype exhibited up-regulation of the HPSE gene, whereas MNCs with the GG genotype disclosed nonresponsiveness to increasing amounts of LPS in PB and CB samples (Fig. 6).
Table 5. Comparison of HPSE Gene rs4693608 SNP Genotype and Allele Frequencies among CB and PB MNCs, According to Their Responsiveness to LPS Stimulation.
| MNCs | Genotype and allele | Down-regulation (D) |
Nonresponsiveness (N) |
Up-regulation (U) |
Comparison of D and U |
Comparison of D + N and U |
|||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| n | Frequency, % | n | Frequency, % | n | Frequency, % | χ2 | P | χ2 | P | ||
| CB | AA | 8 | 23.53 | 1 | 7.14 | 38 | 47.5 | 7.57 | 0.023 | 12.84 | 0.002 |
| AG | 16 | 47.06 | 8 | 57.14 | 32 | 40.0 | |||||
| GG | 10 | 29.41 | 5 | 35.71 | 10 | 12.5 | |||||
| A | 32 | 47.06 | 10 | 35.71 | 108 | 67.5 | 8.41 | 0.004 | 13.95 | <0.001 | |
| G | 36 | 52.94 | 18 | 64.29 | 52 | 32.5 | |||||
| PB | AA | 1 | 11.11 | 0 | 0 | 30 | 34.48 | 4.45 | NS | 9.62 | 0.008 |
| AG | 4 | 44.44 | 4 | 50.0 | 42 | 48.28 | |||||
| GG | 4 | 44.44 | 4 | 50.0 | 15 | 17.24 | |||||
| A | 6 | 33.33 | 4 | 25.0 | 102 | 58.62 | 4.24 | 0.04 | 9.76 | 0.002 | |
| G | 12 | 66.67 | 12 | 75.0 | 72 | 41.38 | |||||
Significant deviations (P<0.05) are in boldface.
Figure 6. Expression level of the HPSE gene in LPS-treated PB and CB derived from individuals with AA versus GG.
MNCs and PB and CB were isolated on a standard Ficoll-Paque density gradient. Cells/ml (1×106) were treated with increasing concentrations of LPS, as described in Materials and Methods, for 18 h. Expression levels of heparanase mRNA were examined. MNCs with the AA genotype exhibited up-regulation of the HPSE gene, whereas MNCs with the GG genotype disclosed nonresponsiveness to increasing amounts of LPS in PB (A) and CB (B) samples.
TLR4 involvement in heparanase gene expression
To assess the contribution of TLR4 to the observed increase in HPSE gene expression following exposure to LPS, MNCs from healthy individuals were treated with the MyD88 homodimerization inhibitory peptide [17]. Preincubation with the inhibitory peptide, but not with control nonspecific peptide, led to a marked decrease in HPSE gene expression, indicating that LPS elevates HPSE gene expression through TLR4 (Fig. 7A).
Figure 7. Effect of TLR4 on HPSE gene expression.
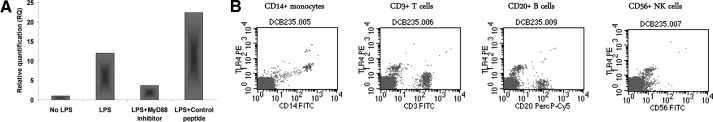
CB MNCs were subjected to FACS analysis, as described in Materials and Methods. PE-conjugated anti-TLR4 mAb; FITC-conjugated anti-CD3, anti-CD14, and anti-CD56 mAb; and PerCP-Cy5-conjugated anti-CD20 mAb were used for the analysis. (A) Treatment of LPS-stimulated MNCs with the MyD88 homodimerization inhibitory peptide (25 μM; 24 h; Imgenex), but not with a control, nonspecific peptide, led to a marked decrease in HPSE gene expression, indicating that LPS elevates HPSE gene expression through the TLR4. (B) Fluorescence histograms demonstrating TLR4 expression on CB monocytes and other CB subsets.
Previously published studies showed that TLR4 is highly expressed in adult monocytes, as opposed to very low expression in T, B, and NK cells and DCs [27, 28]. Having found that TLR4 is involved in LPS-induced elevation of HPSE in CB cells, we next analyzed TLR4 expression in various CB cell subsets. As indicated in Fig. 7B, TLR4 was found to be expressed in CB monocytes but not in CB T and B lymphocytes and NK cells. It is therefore conceivable that CB monocytes are the key cells involved in the observed LPS, TLR4, and HPSE cascade.
Correlation between rs4693608 SNP and time to neutrophil and platelet recovery
Our results indicate that interindividual variations in heparanase expression among healthy individuals were restricted to neutrophils. Therefore, the association between rs4693608 SNP and time of neutrophil and platelet recovery post-HSCT was investigated in 449 recipient-donor pairs. As detailed in Table 6, the trend to association was found between patient genotypes and time of neutrophil and platelet reconstitution. Analysis of association between recipient-donor discrepancy and time of neutrophil and platelet recovery disclosed that reconstitution of neutrophils and platelets occurred more quickly in patients from the D1 group compared with patients from the D2 and D3 groups. As our results indicated a strong correlation between heparanase expression and the rs4693608 SNP, we divided all recipient-donor pairs into three newly designed groups (N1, N2, and N3), according to their presumable differences in heparanase expression. The N1 group included AA-AG, AA-GG, and AG-GG recipient-donor pairs, in which recipient heparanase expression is presumably higher compared with the donor. The N2 group contained AA-AA, AG-AG, and GG-GG recipient-donor pairs with the same HPSE gene expression levels. The N3 group consisted of AG-AA, GG-AA, and GG-AG recipient-donor pairs with a relatively low heparanase expression level in the patients relative to a high expression level in the donors. The mean time to neutrophil and platelet recovery in the N1 group was 13 (range 4–30) and 16 (range 9–37) days after transplantation, respectively. In the N2 group, it was 14 (range 3–69) and 18 (range 9–80) days, respectively. In the N3 group, it was 14 (range 10–26) and 18 (range 9–45) days after transplantation, respectively. Analysis of correlation among these groups and time of neutrophil and platelet recovery revealed that patients from the N1 group reconstituted the neutrophils and platelets significantly quicker than patients from the N2 and N3 groups (P=0.003 for neutrophils, and P=0.029 for platelets, respectively; Table 6).
Table 6. Association between rs4693608 SNP and Time of Neutrophil and Platelet Reconstitution after HSCT.
| Group of analysis | Genotype | n | Comparison of carriers | Neutrophils |
Platelets |
||
|---|---|---|---|---|---|---|---|
| Z value | P value | Z value | P value | ||||
| Patients | AA | 124 | AA to GG | 1.29 | NS | 1.0 | NS |
| AG | 227 | AA to AG + GG | −1.56 | .059 | −1.53 | 0.063 | |
| GG | 98 | GG to AA + AG | 0.56 | NS | 0.26 | NS | |
| Donors | AA | 110 | AA to GG | −0.49 | NS | −1.31 | NS |
| AG | 207 | AA to AG + GG | −0.16 | NS | 1.15 | NS | |
| GG | 81 | GG to AA + AG | −0.91 | NS | −1.0 | NS | |
| Discrepancy | D1 | 54 | D1 to D2 | −1.66 | 0.049 | −1.67 | 0.047 |
| D2 | 120 | D1 to D3 | −1.84 | 0.033 | −1.32 | NS | |
| D3 | 235 | D2 to D3 | 0.27 | NS | −0.29 | NS | |
| Differences in heparanase levels | N1 | 89 | N1 to N2 | −2.17 | 0.015 | −2.04 | 0.021 |
| N2 | 191 | N1 to N3 | −2.7 | 0.003 | −1.9 | 0.029 | |
| N3 | 104 | N2 to N3 | 0.75 | NS | 0.2 | NS | |
Discrepancy according to the risk of aGVHD development: D1, AA-AG and AA-GG recipient-donor pairs; D2, AG-AG, AG-AA, AG-GG, and AA-AA recipient-donor pairs; and D3, GG-GG, GG-AA, and GG-AG recipient-donor pairs. Three newly designed recipient-donor pair groups, according to their presumable differences in heparanase expression: N1, AA-AG, AA-GG, and AG-GG recipient-donor pairs (recipient heparanase expression is presumably higher compared with the donor); N2, AA-AA, AG-AG, and GG-GG recipient-donor pairs (the same HPSE gene expression levels); and N3, GG-AG, GG-AA, and AG-AA recipient-donor pairs (low heparanase expression in the patients relative to high expression level in the donors). Significant deviations (P<0.05) are in boldface.
Additionally, we assessed whether recipient and donor rs4693608 SNP genotypes correlate with absolute lymphocyte and monocyte recovery on Day 30 post-HSCT. No significant association was detected. Similarly, no correlation between rs4693608 SNP and nonengraftment (25 patients did not engraft) was found (data not shown). SNP rs4693608 is thus associated only with neutrophil, but not lymphocyte and monocyte engraftment, further emphasizing our findings published previously [18]—that interindividual variations in heparanase expression are restricted to neutrophils, not only in healthy individuals but also in patients post-HSCT.
DISCUSSION
In the present study, we correlated heparanase gene expression and rs4693608 SNP with transplantation outcome. Specifically, we investigated the effect of pretransplant-conditioning regimens on heparanase expression levels in transplant recipients, as well as the predictive value of rs4693608 SNP for post-transplant engraftment and GVHD occurrence. In an attempt to mimic the influence of the peritransplant proinflammatory state on the donor-engrafted cells, we have also assessed heparanase levels in LPS-treated normal MNCs maintained in vitro and the putative involvement of TLR4. In addition, the pretransplant conditioning led to a significant overexpression of the HPSE gene, followed by a gradual decrease thereafter. Notably, the increase in HPSE gene expression, postconditioning, was higher in patients harboring the AA genotype compared with those with the GG genotype. These results corroborated our previous assumption [19] that a strong, proinflammatory process may lead to heparanase overexpression and that the level of heparanase strongly correlates with the rs4693608 SNP.
In addition, we have demonstrated that patients who developed aGVHD exhibited elevated HPSE gene expression, after conditioning and during aGVHD. We suggest that elevation of HPSE after conditioning and during aGVHD is an independent process.
A proinflammatory environment is involved in the pathogenesis of postconditioning gastrointestinal tract injury and GVHD [26]. To mimic this process in vitro, MNCs were stimulated with LPS, revealing a significant increase in HPSE gene expression, which was mediated by TLR4. Possessors of the AA genotype exhibited up-regulation of heparanase with a high ratio in the LPS-treated MNCs, whereas individuals with genotype GG showed down-regulation or no effect on HPSE gene expression.
The present observations confirmed our results published previously [19] on the association between HPSE gene rs4693608 SNP and the risk of aGVHD. Importantly, the study emphasizes that the discrepancy in rs4693608 SNP between recipients and donors is more significant for the risk of aGVHD development than patient genotype. As no correlation between HPSE gene expression pretransplant and the incidence of post-transplant aGVHD was found, it appears that rather than a SNP, discrepancy in rs4693608 SNP between recipient and donor best correlates with GVHD and hence, may serve as a predictive marker for aGVHD risk.
Up-regulation of heparanase in response to inflammatory and autoimmune stimuli was noted in various pathologies, including arthritis [14], colitis [16, 25], autoimmune diabetes [29], sepsis [15], and experimental encephalomyelitis [30]. Recently, a correlation between increased expression of the HPSE gene and the rs4693608 AA genotype was demonstrated in human atherosclerosis associated with inflammation [31].
In a recently published study [32], Chien et al. analyzed 40 previously reported SNPs for genetic association with aGVHD using the Affymetrix GeneChip Genome-Wide Human SNP Array 5.0 in 1298 allogeneic hematopoietic cell transplantation donors and recipients. The key rs4693608 SNP was included in the study but not analyzed. In contrast to our previously published results [19], the second rs4364254 SNP did not correlate with the incidence of GVHD [32]. The rs4364254 SNP is only an additional SNP, which may help to identify heterozygote AG individuals with low heparanase expression and low risk of aGVHD development. The inconsistency between these two studies may be a result of the fact that the rs4693608 SNP is located between two relatively frequent rs4693083 and rs4693084 SNPs that are only 59 bp apart (http://www.ncbi.nlm.nih.gov/projects/SNP/snp_ref.cgi?showRare=on&chooseRs=all&go=Go&locusId=10855), leading to a potential pitfall in hybridization of a specific probe and a low call rate. Indeed, although correlation of the rs4364254 SNP with hematological malignancies [21] and gastric cancer [33] was reported previously, the role of this polymorphism remains unclear.
In subsequent experiments, we tried to mimic the influence of the recipient proinflammatory milieu on heparanase expression in donor cells by stimulation of PB and CB MNCs with LPS. We found that heparanase expression is modified differently in MNCs, in accordance with their rs4693608 SNP genotype. Possessors of the AA genotype disclosed up-regulation of heparanase with a high ratio in PB and CB MNCs, whereas individuals with genotype GG showed a nonresponsiveness or down-regulation of the HPSE gene in response to LPS (some GG genotype carriers showed a minimal increase in heparanase after LPS treatment, with a ratio close to 1). Moreover, exposure of MNCs with genotype GG to increasing amounts of LPS revealed a very low elevation of heparanase expression or even unresponsiveness, the significance of which is unclear.
A number of studies demonstrated the involvement of heparanase in inflammatory processes [12–15]. Recently, Schmidt et al. [15] investigated the involvement of heparanase in experimental sepsis. It was found that sepsis induced activation of heparanase, which cleaves HS from the pulmonary endothelial glycocalyx and thereby, increases neutrophil recognition and adhesion to the endothelial surface. Inhibition of heparanase activity was suggested as a potential lung-protective therapy during sepsis [15]. Our results indicate that the level of heparanase varies among healthy individuals and correlates with rs4693608 SNP in neutrophils. Moreover, we found that patients with the AA genotype had a high level of HPSE gene expression and a higher risk for aGVHD. It is of interest to assess whether possessors of the AA genotype have a higher risk to develop acute lung injury compared with carriers of the GG genotype. Neutrophils are one of the first lines of defense against invading microbes. These cells are terminally differentiated and they have a short lifespan and low levels of gene expression. When they reach the circulation, they are already equipped with the proteins required to kill microorganisms [34]. The observed correlation between rs4693608 SNP and heparanase expression in neutrophils and in LPS-treated MNCs suggests a yet-undefined, specific switch occurring only in activated cells.
Successful immune reconstitution post-transplant is important for decreasing infection incidence and relapse rate without increasing GVHD. The first recovered cells are neutrophils. Despite rapid recovery of neutrophil counts, neutrophil function (e.g., chemotaxis, phagocytosis, superoxide production, and killing of bacteria) is subnormal in the immediate post-transplant period, gradually normalizing by 2 months [35]. The present study reveals that differences in rs4693608 SNP between recipient and donor affect the time of neutrophil recovery. It is conceivable that a high heparanase expression level in the bone marrow microenvironment of AA recipients promotes faster recovery of AG/GG donor neutrophils (AA-AG, AA-GG, and AG-GG recipient-donor pairs).
In a previously published study, Mohty et al. [36] analyzed the role of inflammatory cytokines in aGVHD development. They found that IL-12 p70 levels were significantly associated with Grade II–IV aGVHD development. Moreover, blood monocytes, the main precursor pool of IL-12p70-secreting DCs, recovered more rapidly in patients with aGVHD [36]. Indeed, it has been shown that immature DCs contain a resting pool of nuclear and cytoplasmic heparanase, which can be actively translocated to cell-surface extensions upon LPS stimulation and promote cellular transmigration [9] and thereby, GVHD development.
Recently published studies revealed involvement of TLR4 and HS in the development of aGVHD [37, 38]. Imado et al. [37] found that host TLR4 is crucial for the induction of tissue-protective factors and for protection against intestinal cell apoptosis during aGVHD. In contrast, Brennan et al. [38] identified that HS, an endogenous TLR4 agonist, promotes aGVHD following allogeneic stem cell transplantation. Previously, Ferrara and coworkers [39] have demonstrated, using TLR4 mutant donor mice, that donor resistance to endotoxin reduced the development of aGVHD by attenuating early intestinal damage. This effect was mediated by TNF-α, derived from donor macrophages and DCs [39]. HS, a major constituent of the ECM and the endothelial barrier [40], is known to control inflammatory responses at multiple levels, including sequestration of cytokines/chemokines in the extracellular space, modulation of leukocyte interactions with the endothelium and ECM, and initiation of innate immune responses through interactions with TLR4 [12, 41]. Thus, HS enzymatic remodeling by heparanase is expected to affect several aspects of inflammatory reactions, such as leukocyte recruitment, extravasation, and migration, toward inflammation sites and release of cytokines and chemokines anchored within the ECM or cell surfaces, as well as activation of innate immune cells, possibly via activation of TLR4 and TLR2 [12, 17, 41].
Constraint of TLR4 signaling by intact HS may help avert unwanted activation of innate immunity and can thus be viewed as a signal of “well being” [42]. On the other hand, as currently demonstrated, postconditioning tissue injury that leads to up-regulation and release of heparanase degrades HS, thereby relieving the constraint on TLR4 function and contributing to the inflammatory process associated with GVHD. The intestinal flora and LPS have long been implicated in the regulation of immunological processes, including GVHD. Recently, Jenq et al. [43] demonstrated the relevance of gut commensals to the severity of GVHD following allogeneic transplantation. As shown in the present and previous studies, the resulting large amounts of LPS up-regulate heparanase (via a TNF-α-dependent mechanism), thereby further promoting GVHD following HSCT. In fact, a similar scenario was found previously in IBD, closely resembling GVHD, in that activated cells of the immune system secrete TNF, which up-regulates heparanase in neighboring cells [16]. Notably, heparanase itself has been shown to activate macrophages and stimulate cytokine (i.e., TNF, IL-1, MIP-1) secretion [17], thereby fueling a self-sustaining, inflammatory circuit that could partially explain the so-called “multiplier” effect in IBD, in which a small elevation in initiating inflammatory stimuli leads to a large increase in downstream cytokines. The same mechanism appears relevant to GVHD as well.
In a recently published article, He et al. [44] found that heparanase regulates histone H3K4 and H3K9 methylation by binding to target gene control regions of several hundred genes in resting and activated T cells. This new function of heparanase may further contribute to T cell-mediated inflammatory responses, such as aGVHD. Moreover, the heparanase-stimulated T cells may also pave the way for myeloid cell engraftment [45], a further explanation to the faster engraftment that we observed in correlation with high heparanase expression.
Taken together, our results emphasize the clinical relevance of heparanase rs4693608 SNP to allogeneic stem cell transplantation outcome, including pretransplant conditioning regimens, post-transplant engraftment, and GVHD incidence. Future studies should validate our results in other data sets and in a multicenter study, aiming at establishing a heparanase-based clinical test for predicting the risk of aGVHD development and transplantation outcome.
ACKNOWLEDGMENTS
This work was supported by The Guy Weinshtock Foundation and the Jacqueline Seroussi Grant (to A.N.) and by grants from the Israel Science Foundation (grant 593/10), National Cancer Institute (RO1-CA106456), and the Israel Ministry of Health (to I.V.). I.V. is a Research Professor of the Israel Cancer Research Fund (ICRF).
Footnotes
- aGVHD
- acute graft-versus-host disease
- CB
- cord blood
- CI
- confidence interval
- CT
- comparative threshold
- Cy
- cyclophosphamide
- GVHD
- graft-versus-host disease
- HPSE
- heparanase gene
- HS
- heparan sulfate
- HSCT
- hematopoietic stem cell transplantation
- HSPG
- heparan sulfate proteoglycans
- IBD
- inflammatory bowel disease
- MNC
- mononuclear cell
- PB
- peripheral blood
- PBL
- peripheral blood leukocyte
- PI
- propidium iodide
- RIC
- reduced intensity conditioning
- RQ
- relative quantification
- SNP
- single nucleotide polymorphism
AUTHORSHIP
O.O. designed and performed experiments, analyzed and interpreted data, and wrote the manuscript. A.S. collected data and cowrote the manuscript. P.B. performed experiments (LPS treatment of MNCs). Y.M. collected samples and data. M.M. collected samples. K.B. performed FACS analysis. A.S. and N.I. performed immunocytochemistry. I.V. codirected the study and cowrote the manuscript. A.N. directed the study and cowrote the manuscript.
REFERENCES
- 1. Vlodavsky I., Friedmann Y. (2001) Molecular properties and involvement of heparanase in cancer metastasis and angiogenesis. J. Clin. Invest. 108, 341–347 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Sanderson R. D., Yang Y., Suva L. J., Kelly T. (2004) Heparan sulfate proteoglycans and heparanase—partners in osteolytic tumor growth and metastasis. Matrix Biol. 23, 341–352 [DOI] [PubMed] [Google Scholar]
- 3. Vlodavsky I., Miao H. Q., Medalion B., Danagher P., Ron D. (1996) Involvement of heparan sulfate and related molecules in sequestration and growth promoting activity of fibroblast growth factor. Cancer Met. Rev. 15, 177–186 [DOI] [PubMed] [Google Scholar]
- 4. Parish C. R., Freeman C., Hulett M. D. (2001) Heparanase: a key enzyme involved in cell invasion. Biochim. Biophys. Acta 1471, M99–M108 [DOI] [PubMed] [Google Scholar]
- 5. Ilan N., Elkin M., Vlodavsky I. (2006) Regulation, function and clinical significance of heparanase in cancer metastasis and angiogenesis. Inter. J. Biochem. Cell Biol. 38, 2018–2039 [DOI] [PubMed] [Google Scholar]
- 6. Vlodavsky I., Eldor A., Haimovitz-Friedman A., Matzner Y., Ishai-Michaeli R., Lider O., Naparstek Y., Cohen I. R., Fuks Z. (1992) Expression of heparanase by platelets and circulating cells of the immune system: possible involvement in diapedesis and extravasation. Invasion Metastasis 12, 112–127 [PubMed] [Google Scholar]
- 7. Wang B., Jia J., Zhang X., Zcharia E., Vlodavsky I., Pejler G., Li J. P. (2011) Heparanase affects secretory granule homeostasis of murine mast cells through degrading heparin. J. Allergy Clin. Immunol. 128, 1310–1317 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Kobayashi M., Naomoto Y., Nobuhisa T., Okawa T., Takaoka M., Shirakawa Y., Yamatsuji T., Matsuoka J., Mizushima T., Matsuura H., Nakajima M., Nakagawa H., Rustgi A., Tanaka N. (2006) Heparanase regulates esophageal keratinocytes differentiation through nuclear translocation and heparan sulfate cleavage. Differentiation 74, 235–243 [DOI] [PubMed] [Google Scholar]
- 9. Benhamron S., Nechushtan H., Verbovetski I., Krispin A., Abboud-Jarrous G., Zcharia E., Edovitsky E., Nahari E., Peretz T., Vlodavsky I., Mevorach D. (2006) Translocation of active heparanase to cell surface regulates degradation of extracellular matrix heparan sulfate upon transmigration of mature monocyte-derived dendritic cells. J. Immunol. 176, 6417–6424 [DOI] [PubMed] [Google Scholar]
- 10. Sasaki N., Higashi N., Taka T., Nakajima M., Irimura T. (2004) Cell surface localization of heparanase on macrophages regulates degradation of extracellular matrix heparan sulfate. J. Immunol. 172, 3830–3835 [DOI] [PubMed] [Google Scholar]
- 11. Bitan M., Polliack A., Zecchina G., Nagler A., Friedmann Y., Nadav L., Deutsch V., Pecker I., Eldor A., Vlodavsky I., Katz B. Z. (2002) Heparanase expression in human leukemias is restricted to acute myeloid leukemias. Exp. Hemat. 30, 34–41 [DOI] [PubMed] [Google Scholar]
- 12. Vlodavsky I., Beckhove P., Lerner I., Pisano C., Meirovitz A., Ilan N., Elkin M. (2012) Significance of heparanase in cancer and inflammation. Cancer Microenviron. 5, 115–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Edovitsky E., Lerner I., Zcharia E., Peretz T., Vlodavsky I., Elkin M. (2006) Role of endothelial heparanase in delayed-type hypersensitivity. Blood 107, 3609–3616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Li R. W., Freeman C., Yu D., Hindmarsh E. J., Tymms K. E., Parish C. R., Smith P. N. (2008) Dramatic regulation of heparanase activity and angiogenesis gene extression in synovium from patients with rheumatoid arthritis. Arthritis Rheum. 58, 1590–1600 [DOI] [PubMed] [Google Scholar]
- 15. Schmidt E. P., Yang Y., Janssen W. J., Gandjeva A., Perez M. J., Barthel L., Zemans R. L., Bowman J. C., Koyanagi D. E., Yunt Z. X., Smith L. P., Cheng S. S., Overdier K. H., Thompson K. R., Geraci M. W., Douglas I. S., Pearse D. B., Tuder R. M. (2012) The pulmonary endothelial glycocalyx regulates neutrophil adhesion and lung injury during experimental sepsis. Nat. Med. 18, 1217–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Lerner I., Hermano E., Zcharia E., Rodkin D., Bulvik R., Doviner V., Rubinstein A. M., Ishai-Michaeli R., Atzmon R., Sherman Y., Meirovitz A., Peretz T., Vlodavsky I., Elkin M. (2011) Heparanase powers a chronic inflammatory circuit that promotes colitis-associated tumorigenesis in mice. J. Clin. Invest. 121, 1709–1721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Blich M., Golan A., Arvatz G., Sebbag A., Shafat I., Sabo E., Cohen-Kaplan V., Petcherski S., Avniel-Polak S., Eitan A., Hammerman H., Aronson D., Axelman E., Ilan N., Nussbaum G., Vlodavsky I. (2013) Macrophage activation by heparanase is mediated by TLR-2 and TLR-4 and associates with plaque progression. Arterioscler. Thromb. Vasc. Biol. 33, e56–e65 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Ostrovsky O., Korostishevsky M., Shafat I., Mayorov M., Ilan N., Vlodavsky I., Nagler A. (2009) Inverse correlation between HPSE gene single nucleotide polymorphisms (SNPs) and heparanase expression: possibility of multiple levels of heparanase regulation. J. Leukoc. Biol. 86, 445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Ostrovsky O., Shimoni A., Rand A., Vlodavsky I., Nagler A. (2010) Genetic variations in the heparanase gene (HPSE) associate with increased risk of GVHD following allogeneic stem cell transplantation: effect of discrepancy between recipients and donors. Blood 115, 2319–2328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Ostrovsky O., Korostishevsky M., Levite I., Leiba M., Galski H., Gazit E., Vlodavsky I., Nagler A. (2007) Characterization of HPSE gene single nucleotide polymorphisms in Jewish populations of Israel. Acta Haemat. 177, 57–64 [DOI] [PubMed] [Google Scholar]
- 21. Ostrovsky O., Korostishevsky M., Levite I., Leiba M., Galski H., Vlodavsky I., Nagler A. (2007) Association of heparanase gene (HPSE) single nucleotide polymorphisms with hematological malignancies. Leukemia 21, 2296–2303 [DOI] [PubMed] [Google Scholar]
- 22. Zetser A., Levy-Adam F., Kaplan V., Gingis-Velitski S., Bashenko Y., Schubert S., Flugelman M. Y., Vlodavsky I., Ilan N. (2004) Processing and activation of latent heparanase occurs in lysosomes. J. Cell Sci. 117, 2249–2258 [DOI] [PubMed] [Google Scholar]
- 23. Gingis-Velitski S., Zetser A., Kaplan V., Ben-Zaken O., Cohen E., Levy-Adam F., Bashenko Y., Flugelman M. Y., Vlodavsky I., Ilan N. (2004) Heparanase uptake is mediated by cell membrane heparan sulfate proteoglycans. J. Biol. Chem. 279, 44084–44092 [DOI] [PubMed] [Google Scholar]
- 24. Levy-Adam F., Feld S., Suss-Toby E., Vlidavsky I., Ilan N. (2008) Heparanase facilitates cell adhesion and spreading by clustering of cell surface heparan sulfate proteoglycans. PLoS One 3, e2319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gooley T. A., Leisenring W., Crowley J., Storer B. E. (1999) Estimation of failure probabilities in the presence of competing risk: new representations of old estimators. Stat. Med. 18, 695–706 [DOI] [PubMed] [Google Scholar]
- 26. Ferrara J. L., Reddy P. (2006) Pathophysiology of graft-versus-host disease. Semin. Hematol. 43, 3–10 [DOI] [PubMed] [Google Scholar]
- 27. Palsson-McDermott E. M., O'Neill L. A. (2004) Signal transduction by the lipopolysaccharide receptor, Toll-like receptor-4. Immunology 113, 153–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hornung V., Rothenfusser S., Britsch S., Krug A., Jahrsdörfer B., Giese T., Endres S., Hartmann G. (2002) Quantative expression of Toll-like receptor 1–10 mRNA in cellular subsets of human peripheral blood mononuclear cells and sensitivity to CpG oligodeoxynucleotides. J. Immunol. 168, 4531–4537 [DOI] [PubMed] [Google Scholar]
- 29. Ziolkowski A. F., Popp S. K., Freeman C., Parish C. R., Simeonovic C. J. (2012) Heparan sulfate and heparanase play key roles in mouse β cell survival and autoimmune diabetes. J. Clin. Invest. 122, 132–141 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lider O., Baharav E., Mekori Y. A., Miller T., Naparstek Y., Vlodavsky I., Cohen I. R. (1989) Suppression of experimental autoimmune diseases and prolongation of allograft survival by treatment of animals with low doses of heparins. J. Clin. Invest. 83, 752–756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Osterholm C., Folkersen L., Lengquist M., Pontén F., Renné T., Li J., Hedin U. (2013) Increased expression of heparanase in symptomatic carotid atherosclerosis. Atherosclerosis 226, 67–73 [DOI] [PubMed] [Google Scholar]
- 32. Chien J. W., Zhang X. C., Fan W., Wang H., Zhao L. P., Martin P. J., Storer B. E., Boeckh M., Warren E. H., Hansen J. A. (2012) Evaluation of published single nucleotide polymorphisms associated with acute graft versus host disease. Blood 119, 5311–5319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Li A. L., Song Y. X., Wang Z. N., Gao P., Miao Y., Zhu J. L., Yue Z. Y., Xu H. M. (2012) Polymorphisms and a haplotype in heparanase gene associations with the progression and prognosis of gastric cancer in a northern Chinese population. PLoS One 7, e30277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Fuchs T. A., Abed U., Goosmann C., Hurwitz R., Schulze I., Wahn V., Weinrauch Y., Brinkmann V., Zychlinsky A. (2007) Novel cell death program leads to neutrophil extracellular traps. J. Cell Biol. 176, 231–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Bosch M., Khan F. M., Storek J. (2012) Immune reconstitution after hematopoietic cell transplantation. Curr. Opin. Hematol. 19, 324–335 [DOI] [PubMed] [Google Scholar]
- 36. Mohty M., Blaise D., Faucher C., Vey N., Bouabdallah R., Stoppa A.-M., Viret F., Gravis G., Olive D., Gaugler B. (2005) Inflammatory cytokines and acute graft-versus-host disease after reduced-intensity conditioning allogeneic stem cell transplantation. Blood 106, 4407–4411 [DOI] [PubMed] [Google Scholar]
- 37. Imado T., Iwasaki T., Kitano S., Satake A., Kuroiwa T., Tsunemi S., Sano H. (2010) The protective role of host Toll-like receptor-4 in acute graft-versus-host disease. Transplantation 90, 1063–1070 [DOI] [PubMed] [Google Scholar]
- 38. Brennan T. V., Lin L., Huang X., Cardona D. M., Li Z., Dredge K., Chao N. J., Yang Y. (2012) Heparan sulfate, an endogenous TLR4 agonist, promotes acute GVHD following allogeneic stem cell transplantation. Blood 120, 2899–2908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Cooke K. R., Gerbitz A., Grawford J. M., Bungard D., Brinson Y. S., Delmonte J., Ferrara J. L. (1998) Tumor necrosis factor-α production to lipopolysaccharide stimulation by donor cells predicts the severity of experimental acute graft-versus-host disease. J. Clin. Invest. 102, 1882–1891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Iozzo R. V., Sanderson R. D. (2011) Proteoglycans in cancer biology, tumour microenvironment and angiogenesis. J. Cell. Mol. Med. 15, 1013–1031 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Hermano E., Lerner I., Elkin M. (2012) Heparanase enzyme in chronic inflammatory bowel disease and colon cancer. Cell. Mol. Life Sci. 69, 2501–2513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Brunn G. J., Bungum M. K., Johnson G. B., Platt J. L. (2005) Conditional signaling by Toll-like receptor 4. FASEB J. 19, 872–874 [DOI] [PubMed] [Google Scholar]
- 43. Jenq R. R., Ubeda C., Taur Y., Menezes C. C., Khanin R., Dudakov J. A., Liu C., West M. L., Singer N. V., Equinda M. J., Gobourne A., Lipuma L., Young L. F., Smith O. M., Ghosh A., Hanash A. M., Goldberg J. D., Aoyama K., Blazar B. R., Pamer E. G., van den Brink M. R. (2012) Regulation of intestinal inflammation by microbiota following allogeneic bone marrow transplantation. J. Exp. Med. 209, 903–911 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. He Y. Q., Sutcliffe E. L., Bunting K. L., Li J., Goodall K. J., Poon I. K., Hulett M. D., Freeman C., Zafar A., McInnes R. L., Taya T., Parish C. R., Rao S. (2012) The endoglycosidase heparanase enters the nucleus of T lymphocytes and modulates H3 methylation at actively transcribed genes via the interplay with key chromatin modifying enzymes. Transcription 3, 130–145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Childs R., Chernoff A., Contentin N., Bahceci E., Schrump D., Leitman S., Read E. J., Tisdale J., Dunbar C., Linehan W. M., Young N. S., Barrett A. J. (2000) Regression of metastatic renal-cell carcinoma after nonmyeloablative allogeneic peripheral-blood stem-cell transplantation. N. Engl. J. Med. 343, 750–758 [DOI] [PubMed] [Google Scholar]