Abstract
A glpQ ortholog was identified in DNA from Borrelia lonestari-positive Amblyomma americanum, providing further evidence that B. lonestari is more closely related to the relapsing fever group spirochetes than to borreliae that cause Lyme disease. This finding provides a basis for developing diagnostic assays to differentiate species of borrelia transmitted by hard ticks.
The medical and scientific communities are still searching for an explanation for the Lyme disease (LD) cases reported in the United States each year with no serologic or culture confirmation of infection with the agent of LD, Borrelia burgdorferi sensu stricto. Patients bitten by Ixodidae ticks in the southeastern or south-central United States may suffer from southern tick-associated rash illness (STARI) caused by an unknown etiologic agent or an inflammatory response (1-3, 5, 9, 10). Evidence suggests that persons in these regions bitten by the hard tick, Amblyomma americanum, who develop a red, expanding rash with central clearing (indistinguishable from erythema migrans, the hallmark rash of LD) are infected with a spirochete named Borrelia lonestari (3, 4, 8, 18).
Since Barbour et al. first characterized the 16S rRNA and flagellin (fla) genes of B. lonestari in 1996 (3), there have been few published reports extending our knowledge of this newfound spirochete. DNA evidence of B. lonestari infections has been documented in the following: (i) A. americanum ticks from Alabama, Arkansas, Delaware, Kansas, Kentucky, Maryland, Missouri, New Jersey, North Carolina, Tennessee, Texas, and Virginia (1, 3, 4, 17, 18); (ii) blood samples from white-tailed deer, Odocoileus virginianus, in Arkansas, Georgia, North Carolina, and South Carolina (11); and (iii) the skin rash biopsy sample and attached A. americanum tick from one human patient with possible exposure in Maryland or North Carolina (8). However, there have been no published reports of successful cultivation, additional gene targets, or serologic assays developed to aid clinicians in determining a diagnosis of B. lonestari as an etiologic agent of STARI.
In 1996, Schwan et al. reported the identification of an immunodominant enzyme in Borrelia hermsii called glycerophosphodiester phosphodiesterase (GlpQ) and demonstrated that serum from humans infected with relapsing fever group (RFG) spirochetes reacted positively by immunoblotting with GlpQ recombinant antigen, while serum from patients with LD did not (15). Later, Schwan et al. found by Southern blot analysis with B. hermsii glpQ probes that eight species of RFG spirochetes contained a glpQ ortholog and six LD Borrelia species did not (14). With these data, and given that B. lonestari is more closely related to RFG spirochetes than to LD spirochetes based on 16S ribosomal DNA (rDNA) and fla analyses (3, 4, 7, 8, 13), we decided to look for an ortholog of glpQ in B. lonestari-positive tick DNA. The 16S rRNA and fla genes are highly conserved and are present in all Borrelia species, making them ill suited for development of rapid, differential diagnostic assays. If present in B. lonestari, glpQ could provide a target for distinguishing B. lonestari- from B. burgdorferi-infected ticks and human samples.
In 2003, we reported the presence of B. lonestari DNA in A. americanum collected from southeast Missouri, confirmed by sequence analyses of 16S rRNA and the complete fla genes (GenBank accession no. AY166715 and AY166716) (1). The gene encoding outer surface protein A (OspA) was not detected by PCR using B. burgdorferi sensu lato-specific primers (6). With few exceptions, OspA is present in all LD-causing borreliae, indicating that the samples likely did not contain DNA from these species. This provided ample B. lonestari-positive tick DNA template for future studies. A subset of A. americanum ticks from the same Missouri field collection was sent to the University of California—Irvine and processed for DNA extraction as reported in detail elsewhere (3). An aliquot of this tick DNA extract was sent to the Rocky Mountain Laboratories for independent PCR and DNA sequence analysis (1).
PCR primers that amplify the complete coding sequence of glpQ from B. lonestari and Borrelia miyamotoi were designed from B. hermsii noncoding sequences flanking the glpQ gene (forward, 5′GGTATGCTTATTGGTCTTC3′; reverse, 5′TTGTATCCTCTTGTAATTG3′). Identical coding sequences were determined for the B. lonestari glpQ gene by the Centers for Disease Control and Prevention and Rocky Mountain Laboratories. The complete coding sequence for B. miyamotoi glpQ was determined using genomic DNA of strain FR64B.
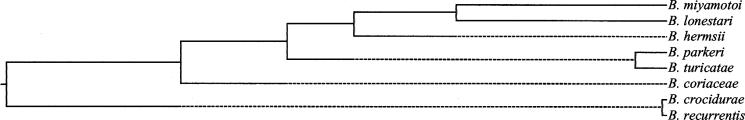
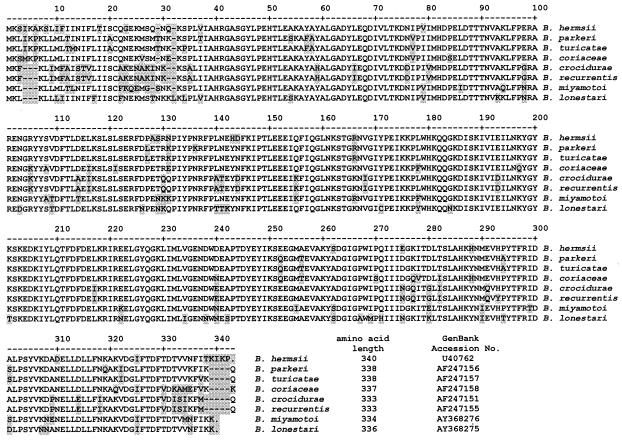
A comparison of the glpQ sequences from B. lonestari, B. miyamotoi, and orthologs in other Borrelia spp. determined previously (12, 15) was performed using the ClustalV (PAM250) algorithm of MegAlign (DNASTAR, Inc., Madison, Wis.) (Fig. 1). B. lonestari glpQ was most similar to B. miyamotoi glpQ (85% identical) and had less identity with orthologs found in B. hermsii (83.4%), Borrelia parkeri (81.9%), Borrelia turicatae (81.3%), Borrelia crocidurae (76.9%), Borrelia coriaceae (79.5%), and Borrelia recurrentis (76.6%). The dendrogram indicates a high level of similarity between B. crocidurae and B. recurrentis (99.7% identity) and between B. parkeri and B. turicatae (97.6% identity), as previously shown (12). The B. lonestari and B. miyamotoi glpQ genes encode 336 and 334 amino acids, respectively. Both have putative signal peptide sequences followed by a cysteine cleavage site at amino acid 21, as reported for orthologs from other borreliae (12, 15) (Fig. 2). B. lonestari contains a threonine at position 28 that is absent in B. miyamotoi and a leucine at position 32 that is absent in all other sequences aligned.
FIG. 1.
Dendrogram illustrating the relatedness of borrelia glpQ gene nucleotide sequences.
FIG. 2.
Deduced amino acid alignment of borrelia GlpQ. Residues differing from the consensus sequence are shaded. Gaps introduced to maximize alignment are indicated by dashes. Consensus sequence is not shown.
Our discovery of glpQ in B. lonestari strengthens the notion that B. lonestari is more closely related to the RFG spirochetes than to the LD-causing borreliae. A glpQ ortholog has never been found in spirochetes that cause LD (14). Others have shown that B. lonestari, B. miyamotoi, and the agent of bovine borreliosis, Borrelia theileri, form a sister group based on 16S rDNA and fla sequence analyses (13). DNA evidence of another RFG spirochete (Yale spirochete), also closely related to B. miyamotoi, has been obtained from Ixodes scapularis (16). Considering that these borreliae are the only RFG spirochetes known to infect hard ticks, we predict that B. theileri and the Yale spirochete also contain a glpQ ortholog. If so, screening field-collected hard ticks for glpQ and ospA by PCR could be more informative and faster than PCR, DNA analysis, or molecular subtyping based on 16S rDNA and/or fla, since these targets are common to all borrelia.
B. lonestari infecting A. americanum was first reported in 1996, but the impact of this organism on human health remains uncertain. One probable case of human infection with B. lonestari was reported in 2001, but all other published research has focused on finding evidence of B. lonestari in vector ticks and mammalian hosts. Although ecologic studies are important in understanding the zoonotic cycle of this organism, more research is needed to determine whether the bite of A. americanum is associated with human borreliosis and whether B. lonestari is an etiologic agent of STARI. Because of the similarity in rash illness resulting after the bite of Amblyomma and Ixodes ticks and the lack of early antibody responses in many of these patients, researchers must rely on PCR-based assays for differential diagnosis of borrelia in rash biopsy samples, emphasizing the importance of finding glpQ in B. lonestari. However, a need to cultivate B. lonestari to identify specific antigens to aid development of a serologic test still exists, since human antibody to B. lonestari GlpQ is likely to cross-react with other RFG orthologs.
Here we identify the glpQ gene of B. lonestari and present its sequence with that of B. miyamotoi glpQ. There is enough sequence divergence among the borrelia glpQ genes to design DNA-based assays for rapid identification of B. lonestari in tick or mammal surveys and in human samples from STARI patients. To this end, efforts to develop and validate a rapid, sequence-based assay specific for B. lonestari glpQ are in progress.
Nucleotide sequence accession numbers.
Sequences for the B. lonestari glpQ gene and the B. miyamotoi glpQ gene were submitted to GenBank under accession no. AY368275 and AY368276, respectively.
ADDENDUM IN PROOF
In March 2004, the first successful culture of B. lonestari was reported (A. S. Varela, M. P. Luttrell, E. W. Howerth, V. A. Moore, W. R. Davidson, D. E. Stallknecht, and S. E. Little, J. Clin. Microbiol. 42:1163-1169, 2004).
Acknowledgments
We thank Joe Piesman and Miquel Quintana for tick collections, Alan Barbour and Jonas Bunikis for DNA extraction, Masahito Fukunaga for B. miyamotoi strain FR64b, Steve Sviat for B. miyamotoi culture and maintenance, and Robert D. Gilmore, Jr., for thoughtful review of the manuscript.
REFERENCES
- 1.Bacon, R. M., R. D. Gilmore, Jr., M. Quintana, J. Piesman, and B. J. B. Johnson. 2003. DNA evidence of Borrelia lonestari in Amblyomma americanum (Acari:Ixodidae) in Southeast Missouri. J. Med. Entomol. 40:590-592. [DOI] [PubMed] [Google Scholar]
- 2.Barbour, A. G. 1996. Does Lyme disease occur in the South? A survey of emerging tick-borne infections in the region. Am. J. Med. Sci. 311:34-40. [DOI] [PubMed] [Google Scholar]
- 3.Barbour, A. G., G. O. Maupin, G. J. Teltow, C. J. Carter, and J. Piesman. 1996. Identification of an uncultivable Borrelia species in the hard tick Amblyomma americanum: possible agent of a Lyme disease-like illness. J. Infect. Dis. 173:403-409. [DOI] [PubMed] [Google Scholar]
- 4.Burkot, T. R., G. R. Mullen, R. Anderson, B. S. Schneider, C. M. Happ, and N. S. Zeidner. 2001. Borrelia lonestari DNA in adult Amblyomma americanum ticks, Alabama. Emerg. Infect. Dis. 7:471-473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Campbell, G. L., W. S. Paul, M. E. Schriefer, R. B. Craven, K. E. Robbins, and D. T. Dennis. 1995. Epidemiologic and diagnostic studies of patients with suspected early Lyme disease, Missouri, 1990-1993. J. Infect. Dis. 172:470-480. [DOI] [PubMed] [Google Scholar]
- 6.Demaerschalck, I., A. Ben Messaoud, M. De Kesel, B. Hoyois, Y. Lobet, P. Hoet, G. Bigaignon, A. Bollen, and E. Godfroid. 1995. Simultaneous presence of different Borrelia burgdorferi genospecies in biological fluids of Lyme disease patients. J. Clin. Microbiol. 33:602-608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Fukunaga, M., K. Okada, M. Nakao, T. Konishi, and Y. Sato. 1996. Phylogenetic analysis of Borrelia species based on flagellin gene sequences and its application for molecular typing of Lyme disease borreliae. Int. J. Syst. Bacteriol. 46:898-905. [DOI] [PubMed] [Google Scholar]
- 8.James, A. M., D. Liveris, G. P. Wormser, I. Schwartz, M. A. Montecalvo, and B. J. B. Johnson. 2001. Borrelia lonestari infection after a bite by an Amblyomma americanum tick. J. Infect. Dis. 183:1810-1814. [DOI] [PubMed] [Google Scholar]
- 9.Kirkland, K. B., T. B. Klimko, R. A. Meriwether, M. Schriefer, M. Levin, J. Levine, W. R. MacKenzie, and D. T. Dennis. 1997. Erythema migrans-like rash illness at a camp in North Carolina: a new tick-borne disease? Arch. Intern. Med. 157:2635-2641. [PubMed] [Google Scholar]
- 10.Masters, E., S. Granter, P. Duray, and P. Cordes. 1998. Physician-diagnosed erythema migrans and erythema migrans-like rashes following Lone Star tick bites. Arch. Dermatol. 134:955-960. [DOI] [PubMed] [Google Scholar]
- 11.Moore, V. A., IV, A. S. Varela, M. J. Yabsley, W. R. Davidson, and S. E. Little. 2003. Detection of Borrelia lonestari, putative agent of southern tick-associated rash illness, in white-tailed deer (Odocoileus virginianus) from the southeastern United States. J. Clin. Microbiol. 41:424-427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Porcella, S. F., S. J. Raffel, M. E. Schrumpf, M. E. Schriefer, D. T. Dennis, and T. G. Schwan. 2000. Serodiagnosis of louse-borne relapsing fever with glycerophosphodiester phosphodiesterase (GlpQ) from Borrelia recurrentis. J. Clin. Microbiol. 38:3561-3571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rich, S. M., P. M. Armstrong, R. D. Smith, and S. R. Telford III. 2001. Lone star tick-infecting borreliae are most closely related to the agent of bovine borreliosis. J. Clin. Microbiol. 39:494-497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schwan, T. G., J. M. Battisti, S. F. Porcella, S. J. Raffel, M. E. Schrumpf, E. R. Fischer, J. A. Carroll, P. E. Stewart, P. Rosa, and G. A. Somerville. 2003. Glycerol-3-phosphate acquisition in spirochetes: distribution and biological activity of glycerophosphodiester phosphodiesterase (GlpQ) among Borrelia species. J. Bacteriol. 185:1346-1356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schwan, T. G., M. E. Schrumpf, B. J. Hinnebusch, D. E. J. Anderson, and M. E. Konkel. 1996. GlpQ: an antigen for serological discrimination between relapsing fever and Lyme borreliosis. J. Clin. Microbiol. 34:2483-2492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Scoles, G. A., M. Papero, L. Beati, and D. Fish. 2001. A relapsing fever group spirochete transmitted by Ixodes scapularis ticks. Vector Borne Zoonotic Dis. 1:21-34. [DOI] [PubMed] [Google Scholar]
- 17.Stegall-Faulk, T., D. C. Clark, and S. M. Wright. 2003. Detection of Borrelia lonestari in Amblyomma americanum (Acari:Ixodidae) from Tennessee. J. Med. Entomol. 40:100-102. [DOI] [PubMed] [Google Scholar]
- 18.Stromdahl, E. Y., P. C. Williamson, T. M. Kollars, Jr., S. R. Evans, R. K. Barry, M. A. Vince, and N. A. Dobbs. 2003. Evidence of Borrelia lonestari DNA in Amblyomma americanum (Acari: Ixodidae) removed from humans. J. Clin. Microbiol. 41:5557-5567. [DOI] [PMC free article] [PubMed] [Google Scholar]