Abstract
Wnt4−/− XX gonads display features normally associated with testis differentiation, suggesting that WNT4 actively represses elements of the male pathway during ovarian development. Here, we show that follistatin (Fst), which encodes a TGFβ superfamily binding protein, is a downstream component of Wnt4 signaling. Fst inhibits formation of the XY-specific coelomic vessel in XX gonads. In addition, germ cells in the ovarian cortex are almost completely lost in both Wnt4 and Fst null gonads before birth. Thus, we propose that WNT4 acts through FST to regulate vascular boundaries and maintain germ cell survival in the ovary. Developmental Dynamics 230:210–215, 2004.
Keywords: sex determination, ovary, coelomic vessel, germ cells, follistatin, Wnt4
INTRODUCTION
The formation of a testis or an ovary from the bipotential gonad is the primary step in sex determination in mammals. Testis determination is controlled by the expression of the Y-chromosome gene Sry, which initiates the differentiation of Sertoli cells and the architectural arrangement of somatic cells in the gonad to form a testis (reviewed in Lovell-Badge, 1992; Capel, 1996). When Sry is expressed in the gonad of an XX embryo, a testis forms (Koopman et al., 1991). Conversely, when Sry is absent or carries a critical mutation, an ovary develops (Gubbay et al., 1992; Hawkins et al., 1992). These results suggested that ovarian fate be haves as a default pathway, arising in the absence of prior specification of the testis pathway. However, occasional XX individuals (in human and other mammalian species) develop as males in the absence of the Sry gene. The most parsimonious explanation for this finding is that the ovarian pathway operates by repressing the male pathway, such that loss of this repressor, called “Z,” leads to the activation of the complete male developmental program (McElreavey et al., 1993).
Few ovary-specific genes have been discovered, although several genes have been identified that are essential for early gonadal development in both sexes (Kriedberg et al., 1993; Luo et al., 1994; Miyamoto et al., 1997; Katoh-Fukui et al., 1998; Birk et al., 2000; Hammes et al., 2001). Only two genes that are specifically up-regulated in the ovary have been investigated so far, Dax1 and Wnt4.
DAX1 was initially a candidate ovary-determining gene based on the identification of human DAX1 within the region of the X chromosome known to cause male to female sex reversal when duplicated in XY individuals (Bardoni et al., 1994; Zanaria et al., 1994). However, genetic evidence in the mouse has indicated that Dax1 is not essential for ovary development and may instead play a role in the testis pathway (Yu et al., 1998; Meeks et al., 2003a–c).
Duplication of part of human chromosome 1p, including the WNT4 gene, was also shown to lead to 46,XY male-to-female sex reversal, suggesting that, like DAX1, WNT4 might also play a dose-sensitive role in mammalian sex determination (Jordan et al., 2001). The idea that Wnt4 could be an anti-testis gene was originally put forward as a result of the discovery that loss of Wnt4 leads to a partial female to male sex reversal in mice. In Wnt4−/− XX gonads, male-like steroid-secreting cells differentiate early. By the time of birth, germ cells are absent, and the XX tissue has reverted to cord-like structures (Vainio et al., 1999). Recent findings in Wnt4−/− mice indicated that the ectopic steroidogenic cells found in early Wnt4−/− XX gonads might be adrenal precursor cells that were misapportioned to the gonad at the stage when the adrenal and gonadal primordia separated (Heikkila et al., 2002). Further investigation of the phenotype of Wnt4−/− mutants revealed that the coelomic vessel that is normally specific to testis development forms ec-topically in XX gonads. The ectopic formation of this vessel in XX gonads occurs through the normal male mechanism of migration of endothelial cells from the mesonephros into the gonad (Jeays-Ward et al., 2003). These data indicate that a normal function of Wnt4 is to repress aspects of the male pathway by blocking the migration of endothelial cells into XX gonads.
How WNT4 controls this process in XX gonads is not known. Here, we show that Wnt4 controls the expression of follistatin (Fst), which encodes an activin binding protein known to regulate the hypothalamic–pituitary–gonadal axis (Phillips and de Kretser, 1998). Fst acts downstream of Wnt4 to inhibit the formation of the coelomic vessel and to maintain germ cell survival in the cortical domain of the ovary.
RESULTS AND DISCUSSION
Follistatin and Bmp2 Are Expressed Downstream of Wnt4 During Ovary Development
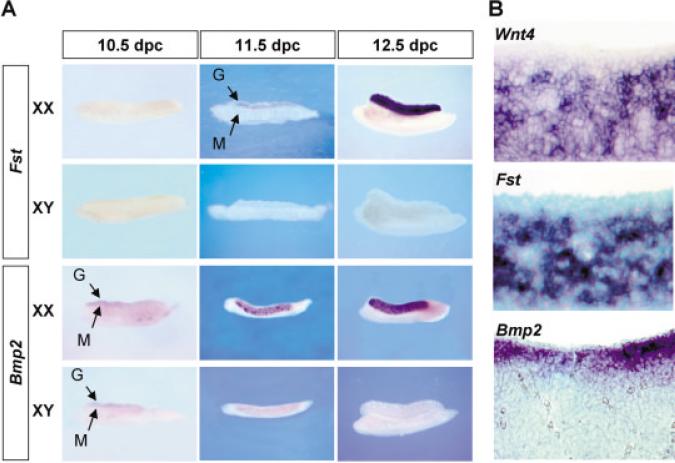
We performed a detailed investigation of the spatial and temporal expression patterns of two genes relative to Wnt4, follistatin (Fst; Menke and Page, 2002; Menke et al., 2003), and bone morphogenetic protein 2 (Bmp2; identified in our in situ hybridization screen of BMP family genes). Both genes are expressed specifically in the embryonic ovary. Fst first appears in the 11.5 days post coitum (dpc) XX gonad, reaches its peak at 12.5 dpc (Fig. 1A), and gradually decreases after 14.5 dpc (data not shown). Fst and Wnt4 are expressed in a similar pattern, in cells several layers beneath the coelomic epithelium of the XX gonad (Fig. 1B). Bmp2 is expressed in gonads of both sexes at 10.5 dpc, becomes XX-specific at 11.5 dpc, and is gradually extinguished after 14.5 dpc. In contrast to the expression domain for Wnt4 and Fst, Bmp2 is expressed in cells just beneath the coelomic epithelial layer of XX gonads (Fig. 1B). The expression patterns of Wnt4, Fst, and Bmp2 are not substantially altered in W/Wv gonads devoid of germ cells (data not shown), indicating that all three genes are expressed in somatic cell lineages and do not depend on the presence of germ cells in the early ovary.
Fig. 1.
Expression of Fst, Bmp2, and Wnt4 in mouse gonads. A: Temporal expression of Fst and Bmp2 in XX and XY gonads from 10.5 to 12.5 days post coitum (dpc) embryos by whole-mount in situ hybridization. The gonad (G, arrow) is a thin layer of cells on the top of the mesonephros (M, arrow) at early stages. In the whole-mount stains for Bmp2, the gonad is turned slightly toward the viewer. B: Spatial expression of Wnt4, Fst, and Bmp2 in a 12.5 dpc XX gonad by section in situ hybridization. The dark purple stain represents specific binding of the RNA antisense probes in all in situ hybridizations.
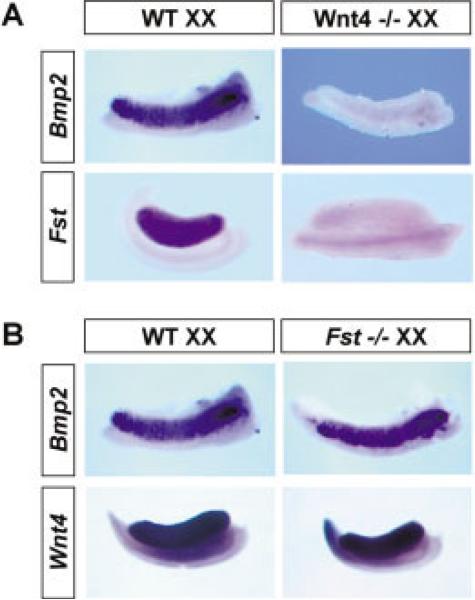
To investigate the epistatic relationship of these genes in the ovarian pathway, we analyzed the expression of Bmp2 and Fst in Wnt4−/− XX gonads. Neither Bmp2 nor Fst is expressed at any stage between 12.5 and 14.5 dpc in Wnt4−/− XX gonads (Fig. 2A), indicating that both genes are downstream of Wnt4 and could lie in pathways regulating the phenotypes observed in Wnt4−/− mutants. To determine whether loss of Fst affects the expression of Bmp2 and Wnt4, we investigated the expression profiles of these genes in Fst−/− XX gonads (Matzuk et al., 1995). Expression of both Bmp2 and Wnt4 is similar to patterns in wild-type gonads (Fig. 2B), confirming that Fst is activated downstream of Wnt4. It is worth noting that, although Bmp2 expression is absent in Wnt4−/− mutants (Fig. 2A), it is undisturbed in Fst−/− mutants (Fig. 2B), indicating a branch point in the Wnt4 pathway upstream of Fst. Unfortunately, Bmp2−/− mutants could not be investigated, because they do not survive to mid-gestation (Zhang and Bradley, 1996).
Fig. 2.
The epistatic relationship of Wnt4, Bmp2, and Fst in ovary development. A: Whole-mount in situ hybridization for Bmp2 (12.5 days post coitum [dpc]) and Fst (14.5 dpc) in wild-type (WT) and Wnt−/− XX gonads. B: Whole-mount in situ hybridization for Bmp2 (12.5 dpc) and Wnt4 (14.5 dpc) in wild-type and Fst−/− XX gonads.
Inactivation of Follistatin Leads to Formation of the Coelomic Vessel in the XX Gonad
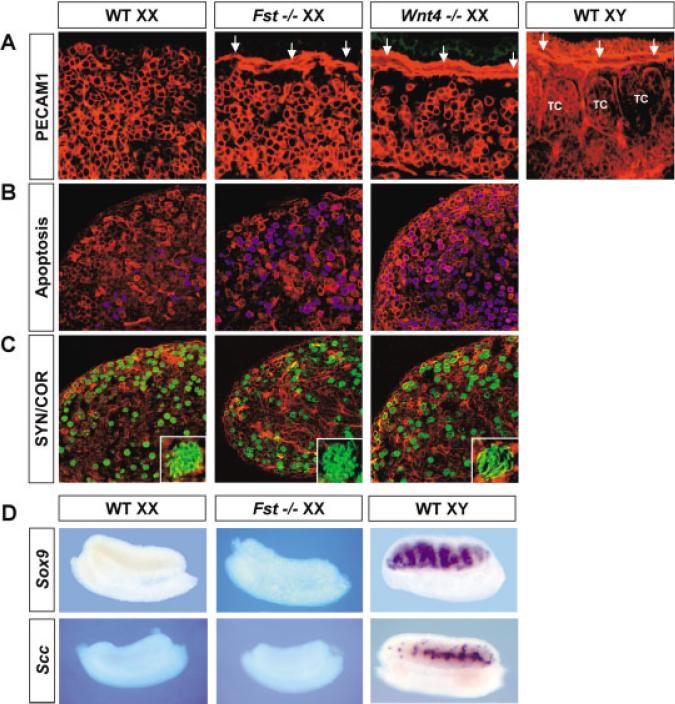
Examination of gonads from Fst−/− mice revealed the presence of the coelomic vessel on the surface of XX gonads at 12.5 dpc, a phenotype identical to that observed in Wnt4−/− XX gonads at the same stage (Fig. 3A, arrows). The coelomic vessels in Fst−/− and Wnt4−/− XX gonads are located in the same domain as in normal XY gonads, just beneath the coelomic epithelium. In both cases, the vessel lacks the branches that descend between testis cords in XY gonads (Fig. 3A). No defects in testis differentiation in XY gonads from Fst−/− embryos were identified (data not shown).
Fig. 3.
The coelomic vessel forms in Fst−/−, and Wnt4−/− XX gonads; meiotic germ cells undergo apoptosis. A: Immunostaining for PECAM-1 in 12.5 days post coitum (dpc) wild-type (WT) XX, Fst−/− XX, Wnt4−/− XX, and wild-type XY gonads. PECAM-1 (red) is a membrane marker for endothelial cells of the vasculature and for primordial germ cells. Arrows indicate formation of the coelomic vessel on the surface of Fst−/− XX, Wnt4−/− XX, and wild-type XY gonads (TC [H11005] testis cord). B: Double staining for PECAM-1 (red) and apoptosis (blue) by TUNEL assay in 16.5 dpc wild-type, Fst−/−, and Wnt4−/− XX gonads. C: Double immunostaining for PE CAM-1 (red) and SYN/COR (green) of adjacent 8-[H9262]m sections in B. Pictures in the insets are higher magnification (100[H11003]) of individual germ cells from the sections. Alignment of meiotic chromosomes can be seen clearly by SYN/COR staining. D: Whole-mount in situ hybridization for Sox9 and Scc in 12.5 dpc wild-type XX, Fst−/− XX, and wild-type XY gonads.
Because formation of the coelomic vessel is normally an event downstream of Sry and Sertoli cell differentiation, we investigated whether Sertoli differentiation is activated in Fst−/− XX gonads. We found that Sox9 (Fig. 3D) and anti-Müllerian hormone (Amh, data not shown), two Sertolispecific genes, are not expressed in Fst−/− XX gonads during early developmental stages, similar to results previously reported in Wnt4−/− XX gonads at these stages (Vainio et al., 1999). These results indicate that formation of the coelomic vessel does not require Sertoli cell differentiation nor does it require Sry, because this gene is not present in XX animals. Expression of Sertoli-specific genes such as desert hedgehog and Amh is activated after birth in Wnt4−/− XX gonads, possibly as the indirect result of germ cell loss (Vainio et al., 1999). We were not able to examine these patterns in Fst−/− XX gonads because of the perinatal lethality of Fst−/− embryos (Matzuk et al., 1995).
Another defect reported in Wnt4−/− XX gonads was the incomplete sep aration of adrenal cells from the gonad primordium. Adrenal-specific steroid producing cells were found in the anterior portion of Wnt4−/− XY and XX gonads (Heikkila et al., 2002). We examined the expression of P450 side-chain cleavage (Scc), a marker for steroidogenic cells, in Fst−/− XX gonads to determine whether Fst−/− gonads share similar defects in steroidogenic cell sorting as occur in Wnt4−/− XX gonads. No Scc expression was found in Fst−/− XX gonads at any stage examined (Fig. 3D). This result indicates that Fst regulates only some of the pathways downstream of Wnt4.
Germ Cells Are Lost From the Ovarian Cortex in Both Wnt4−/− and Fst−/− Mutants
Germ cells are lost in Wnt4−/− XX gonads by the time of birth (Vainio et al., 1999); therefore, we compared the fate of germ cells in wild-type, Wnt4−/−, and Fst−/− XX gonads. We found that numbers of germ cells in Wnt4−/− and Fst−/− XX gonads are similar to numbers in wild-type XX gonads from 11.5 to 15.5 dpc (data not shown). However, at 16.5 dpc, more than 90% of germ cells in Wnt4−/− and Fst−/− XX gonads undergo apoptosis in contrast to ~30% in wild-type gonads (Fig. 3B). In wild-type XX gonads, apoptosis of germ cells is restricted to the medullary region; apoptosis is not seen in the coelomic domain where germ cells survive and accumulate. In contrast, in Wnt4−/− and Fst−/− XX gonads, germ cells undergo apoptosis throughout the gonad, in both the medulla and the coelomic domains.
To investigate whether germ cells in Wnt4 and Fst XX mutants enter meiosis normally, we assayed the appearance of components of the synaptonemal complex of meiotic chromosomes during the zygotene– pachytene transition using an antibody against SYN/COR (Dobson et al., 1994). In wild-type XX gonads, germ cells enter meiosis at 13.5 dpc and progress from leptotene to diplotene and meiotic arrest by 18.5 dpc. We found that the timing of the zygotene–pachytene transition and the number of germ cells positive for SYN/COR were similar among wild-type, Wnt4−/−, and Fst−/− XX gonads (Fig. 3C). The chromosomal localization of SYN/COR was also normal in germ cells of Wnt4−/− and Fst−/− XX gonads at 16.5 dpc (Fig. 3C, insets). Thus, Wnt4 and Fst are not essential for the initial phases of meiosis but are required for survival of meiotic germ cells in the coelomic domain at pachytene and diplotene stages.
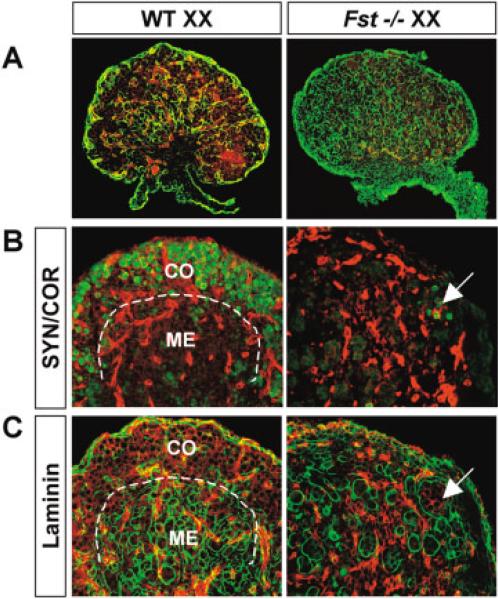
The massive germ cell apoptosis in Fst−/− XX gonads from 16.5 dpc onward leads to an almost complete loss of germ cells near birth, similar to data reported for Wnt4−/− XX gonads (Vainio et al., 1999). Immediately before birth (18.5–19.5 dpc), meiotic germ cells are located in the coelomic domain (cortex) of wild-type XX gonads (Fig. 4A,B). In wild-type gonads, a sharp division between the medulla and cortical regions is demarcated by the deposition of a laminin-rich boundary and the distinct tissue organizational differences between the two regions of the ovary (Fig. 4B). When Fst is absent, more than 99% of meiotic germ cells are lost and the boundary between cortical and medullary regions disappears (Fig. 4C). Cord-like structures fill the cortical region, and the few remaining meiotic germ cells are enclosed inside (Fig. 4B,C, arrows). This finding is similar to the phenotype previously described for Wnt4 XX mutants after birth (Vanio et al., 1999).
fig. 4.
The structure of the ovarian cortex is lost in 18.5 days post coitum (dpc) Fst−/− XX gonads. A: A lower magnification view with double immunostaining for PECAM-1 (red) and laminin (green). Note that the size of the XX gonad is similar in wild-type (WT) and Fst−/−. Distinct cortex and medulla tissue boundaries can be seen at higher magnification in wild-type, but not in Fst−/− XX gonads (B,C). B: Double immunostaining for PECAM-1 (red) and SYN/COR (green): In the wild-type XX gonad, germ cells with SYN/COR staining were located in the cortical region (CO) of the gonad. In the Fst−/− XX gonad, >99% of germ cells were lost with rare germ cells enclosed inside cord structures (arrows in B,C). C: Double immunostaining for PECAM-1 and laminin1 in adjacent 8-μm sections: In the medullary region (ME) of the wild-type XX gonad, cord-like structures are delineated by laminin1 (a dotted line separates the cortical and medullary regions of the XX gonads). The cord-like structure extended throughout the cortical domain in the Fst−/− XX gonad.
On the basis of the similar pheno-types between Wnt4−/− and Fst−/− XX gonads and the absence of Fst expression in Wnt4−/− XX gonads, we propose that FST acts downstream of WNT4 to inhibit the migration of endothelial cells from the mesonephros and the formation of the coelomic vessel. Formation of the male-specific coelomic vessel in the ovary of these two knockouts is especially intriguing because key genes in the male pathway such as Sox9 are not expressed (Vidal et al., 2001). This observation suggests that, even though endothelial cell migration and formation of the coelomic vessel is normally XY-specific, it does not require Sry or the differentiation of Sertoli cells. Instead, formation of the coelomic vessel is actively inhibited in XX gonads through WNT4/FST signaling. These findings seem to be consistent with the “Z” theory that ovary-determining genes act as inhibitors of the testis pathway in the XX gonad (McElreavey et al., 1993). However, the theoretical ovary determining gene, “Z,” is classically defined as a master inhibitor of the initial step of the testis cascade (Vaiman and Pailhoux, 2000). We found that WNT4/FST signaling inhibits a specific downstream aspect of testis development, the formation of the coelomic vessel.
Fst also plays a critical role in the survival of female germ cells. In the case of Wnt4 mutant ovaries, the loss of germ cells might have resulted from the ectopic production of steroids in the XX gonad. However, steroid-producing cells are not present in Fst−/− XX gonads; thus, ec-topic steroid production cannot explain germ cell loss in this case. Because of the specific expression of Bmp2 in the cortical domain, it is a candidate for a gene promoting germ cell survival in this region. In Fst−/− XX gonads, expression of Bmp2 is normal by in situ analysis; however, disregulation at the protein level remains an open possibility.
One appealing hypothesis is that formation of the coelomic vessel alters the cellular and signaling environment of the ovarian cortex. The coelomic vessel forms in the cortical domain of Wnt4−/− and Fst−/− XX gonads, just beneath the coelomic epithelium, before the stage when germ cells normally begin to accumulate there. This cortical domain may provide a protective niche where meiotic germ cells escape from the extensive apoptosis that occurs in the medullary region of wild-type XX gonads. The ovary is not devoid of vasculature, but the major vessels are normally found in the medulla of the organ at this stage (Brennan and Capel, 2002). The formation of the coelomic vessel could introduce new signals into this protected domain that antagonize the survival of germ cells. Whether the coelomic vessel is directly involved in causing germ cell loss is not clear. It is possible that Fst controls pathways that affect germ cell survival independent of coelomic vessel formation.
Regulation of Fst expression by WNT3A was reported in human embryonic carcinoma cells (Willert et al., 2002). Our result provides the first physiological link between WNT4 and FST in an animal model. FST is known to modulate the activity of TGF-β family proteins such as activins and BMPs by direct binding (Chang et al., 2002). Recent work on hair follicle development revealed an intricate network among follistatin, activins, and BMPs (Nakamura et al., 2003). Of interest, TGF-β family proteins such as AMH and BMPs induce the formation of the coelomic vessel and cause loss of germ cells in the XX gonad in organ culture experiments (Ross et al., 2003; H.H-C. Yao and B. Capel, unpublished data). Furthermore, we and others had found that activins and many members of the BMP family are expressed in both embryonic XX and XY gonads (Feijen et al., 1994). These results suggest that activins and BMPs could be potential targets of FST in normal ovary development. This intricate control of a very specific aspect of vascular development and its association with altered organ structure and germ cell survival suggest that we have only begun to understand the role of vasculature in organogenesis and development.
In summary, we have identified a pathway downstream of WNT4 signaling that operates through FST to inhibit vascular development in the ovary. This signaling cascade is unique in that it possesses both anti-testis (inhibition of the coelomic vessel) and pro-ovary functions (survival of meiotic germ cells), and constitutes the first multigene signaling pathway defined in early development of the ovary.
EXPERIMENTAL PROCEDURES
Animals
Wnt4−/− mice were obtained from The Jackson Laboratory (strain 129-Wnt4tm1Amc). Fst−/− mice were generated and genotyped as previously described (Matzuk et al., 1995). Timed matings were produced by housing female mice with males overnight and checking for vaginal plugs the next morning (0.5 dpc = noon of the day when a vaginal plug was found). The sex of each embryo was determined by Giemsa staining for X chromatin Barr bodies in cells of the amniotic sac (Palmer and Burgoyne, 1991).
In Situ Hybridization
Samples were fixed overnight in 4% paraformaldehyde in PBS at 4°C and processed according to Henrique et al. (1995). A digoxigenin-labeled RNA probe was detected by using an alkaline phosphatase conjugated anti-digoxigenin antibody.
Immunocytochemistry
For immunostaining against laminin, samples were fixed overnight in 4% paraformaldehyde in PBS at 4°C. For double immunostaining against PECAM-1 and SYN/COR, samples were fixed 1 hr in 1% paraformalde-hyde at 4°C. Samples were then processed and cut into 8-μm frozen sections as described (Karl and Capel, 1998). Primary antibody incubations were carried out overnight at 4°C in blocking solution (1:200 dilution of rabbit anti-laminin1 antibody, provided by Harold Erickson; 1:500 dilution of rat anti–PECAM-1 antibody, Pharmingen; 1:800 dilution of a rabbit polyclonal antibody against SYN1/COR1, provided by Peter Moens). Secondary antibody incubations were performed overnight at 4°C with a 1:500 dilution of fluorescently conjugated secondary antibodies (fluorescein isothiocya-nate [FITC]- or Cy5-conjugated goat anti-rabbit antibody and Cy3-conjugated goat anti-rat antibody, Jackson Immunochemicals). Sections were washed three times for 5 min each in washing solution and mounted on glass slides in DABCO. Images were collected on a Zeiss LSM confocal microscope and processed using Adobe Photoshop.
TUNEL Assay
Frozen sections were obtained and incubated in the reaction mix with 0.5 μl of TdT Enzyme from Sigma (35 U/μl) and 50 μl of FITC-dUTP/dNTP mix from Boehringer Mannheim for 1 hr at 37°C in a humid chamber. For control, only the nucleotide mix was added for reaction. Our standard immunocytochemistry protocol was followed to double stain the section with PECAM-1.
ACKNOWLEDGMENTS
We thank Harold Erickson and Peter Moens for antibodies against laminin and SYN1/COR1. We also greatly appreciate the contributions of members of the lab who helped with data (Jennifer Brennan and Christopher Tilmann), with mouse husbandry (Jordan Batchvarov), and with critical reading of the manuscript (Douglas Coveney and Andrea Ross). B.C., M.M.M., and D.C.P. were funded by NIH grants; D.C.P. was funded by the Howard Hughes Medical Institute; and H.H.C.Y. received a postdoctoral fellowship from the Lalor Foundation.
Footnotes
Dr. Yao's current address is Department of Veterinary Biosciences, University of Illinois at Urbana-Champaign, IL.
Dr. Menke's current address is Department of Developmental Biology, Stanford University School of Medicine, Stanford, CA.
REFERENCES
- Bardoni B, Zanaria E, Guioli S, Floridia G, Worley K, Tonini G, Ferrante E, Chiumello G, McCabe E, Fraccaro M, Zuffardi O, Camerino G. A dosage sensitive locus at chromosome Xp21 is involved in male to female sex reversal. Nat Genet. 1994;7:497–501. doi: 10.1038/ng0894-497. [DOI] [PubMed] [Google Scholar]
- Birk O, Casiano D, Wassif C, Cogliati T, Zhao L, Zhao Y, Grinberg A, Huang S, Kreidberg J, Parker K, Porter F, Westphal H. The LIM homeobox gene Lhx9 is essential for mouse gonad formation. Nature. 2000;403:909–913. doi: 10.1038/35002622. [DOI] [PubMed] [Google Scholar]
- Brennan J, Capel B. Divergent vascular mechanisms downstream of Sry establish the arterial system in the XY gonad. Dev Biol. 2002;244:418–428. doi: 10.1006/dbio.2002.0578. [DOI] [PubMed] [Google Scholar]
- Capel B. The role of Sry in cellular events underlying mammalian sex determination. In: Pedersen RA, editor. Current topics in developmental biology. Academic Press; New York: 1996. pp. 1–32. [DOI] [PubMed] [Google Scholar]
- Chang H, Brown CW, Matzuk MM. Genetic analysis of the mammalian transforming growth factor-beta superfamily. Endocr Rev. 2002;23:787–823. doi: 10.1210/er.2002-0003. [DOI] [PubMed] [Google Scholar]
- Dobson M, Pearlman R, Karaiskakis A, Spyropoulos B, Moens P. Synaptonemal complex proteins: occurrence, epitope mapping and chromosome disjunction. J Cell Biol. 1994;107:2749–2760. doi: 10.1242/jcs.107.10.2749. [DOI] [PubMed] [Google Scholar]
- Feijen A, Goumans MJ, van den Eijndenvan Raaij AJ. Expression of activin subunits, activin receptors and follistatin in postimplantation mouse embryos suggests specific developmental functions for different activins. Development. 1994;120:3621–3637. doi: 10.1242/dev.120.12.3621. [DOI] [PubMed] [Google Scholar]
- Gubbay J, Vivian N, Economou A, Jackson D, Goodfellow P, Lovell BR. Inverted repeat structure of the Sry locus in mice. Proc Natl Acad Sci U S A. 1992;89:7953–7957. doi: 10.1073/pnas.89.17.7953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammes A, Guo JK, Lutsch G, Leheste JR, Landrock D, Ziegler U, Gubler MC, Schedl A. Two splice variants of the Wilms’ tumor 1 gene have distinct functions during sex determination and nephron formation. Cell. 2001;106:319–329. doi: 10.1016/s0092-8674(01)00453-6. [DOI] [PubMed] [Google Scholar]
- Hawkins JR, Taylor A, Berta P, Levilliers J, Van Der Auwere B, Goodfellow PN. Mutational analysis of SRY: nonsense and missense mutations in XY sex reversal. Hum Genet. 1992;88:471–474. doi: 10.1007/BF00215684. [DOI] [PubMed] [Google Scholar]
- Heikkila M, Peltoketo H, Leppaluoto J, Ilves M, Vuolteenaho O, Vainio S. Wnt-4 deficiency alters mouse adrenal cortex function, reducing aldosterone production. Endocrinology. 2002;143:4358–4365. doi: 10.1210/en.2002-220275. [DOI] [PubMed] [Google Scholar]
- Henrique D, Adam J, Myat A, Chitnis A, Lewis J, Ish-Horowicz D. Expression of a Delta homologue in prospective neurons in the chick. Nature. 1995;375:787–790. doi: 10.1038/375787a0. [DOI] [PubMed] [Google Scholar]
- Jeays-Ward K, Hoyle C, Brennan J, Dandonneau M, Alldus G, Capel B, Swain A. Endothelial and steroidogenic cell migration are regulated by WNT4 in the developing mammalian gonad. Development. 2003;130:3663–3670. doi: 10.1242/dev.00591. [DOI] [PubMed] [Google Scholar]
- Jordan BK, Mohammed M, Ching ST, Delot E, Chen XN, Dewing P, Swain A, Rao PN, Elejalde BR, Vilain E. Up-regulation of WNT-4 signaling and dosage-sensitive sex reversal in humans. Am J Hum Genet. 2001;68:1102–1109. doi: 10.1086/320125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karl J, Capel B. Sertoli cells of the mouse testis originate from the coelomic epithelium. Dev Biol. 1998;203:323–333. doi: 10.1006/dbio.1998.9068. [DOI] [PubMed] [Google Scholar]
- Katoh-Fukui Y, Tsuchiya R, Shiroishi T, Nakahara Y, Hashimoto N, Noguchi K, Higashinakagawa T. Male to female sex reversal in M33 mutant mice. Nature. 1998;393:688–692. doi: 10.1038/31482. [DOI] [PubMed] [Google Scholar]
- Koopman P, Gubbay J, Vivian N, Goodfellow P, Lovell-Badge R. Male development of chromosomally female mice transgenic for Sry. Nature. 1991;351:117–121. doi: 10.1038/351117a0. [DOI] [PubMed] [Google Scholar]
- Kriedberg JA, Sariola H, Loring JM, Maeda M, Pelletier J, Housman D, Jaenisch R. Wt-1 is required for early kidney development. Cell. 1993;74:679–691. doi: 10.1016/0092-8674(93)90515-r. [DOI] [PubMed] [Google Scholar]
- Lovell-Badge R. Testis determination: soft talk and kinky sex. Curr Opin Genet Dev. 1992;2:596–601. doi: 10.1016/s0959-437x(05)80178-9. [DOI] [PubMed] [Google Scholar]
- Luo X, Ikeda Y, Parker K. A cell-specific nuclear receptor is essential for adrenal and gonadal development and sexual differentiation. Cell. 1994;77:481–490. doi: 10.1016/0092-8674(94)90211-9. [DOI] [PubMed] [Google Scholar]
- Matzuk MM, Lu N, Vogel H, Sellheyer K, Roop DR, Bradley A. Multiple defects and perinatal death in mice deficient in follistatin. Nature. 1995;374:360–363. doi: 10.1038/374360a0. [DOI] [PubMed] [Google Scholar]
- McElreavey K, Vilain E, Abbas N, Herskowitz I, Fellous M. A regulatory cascade hypothesis for mammalian sex determination: SRY represses a negative regulator of male development. Proc Natl Acad Sci U S A. 1993;90:3368–3372. doi: 10.1073/pnas.90.8.3368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meeks JJ, Crawford SE, Russell TA, Morohashi K, Weiss J, Jameson JL. Dax1 regulates testis cord organization during gonadal differentiation. Development. 2003a;130:1029–1036. doi: 10.1242/dev.00316. [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Russell TA, Jeffs B, Huhtaniemi I, Weiss J, Jameson JL. Leydig cell specific-expression of Dax1 improves fertility of the Dax1-deficient mouse. Biol Reprod. 2003b;69:154–160. doi: 10.1095/biolreprod.102.011429. [DOI] [PubMed] [Google Scholar]
- Meeks JJ, Weiss J, Jameson JL. Dax1 is required for testis determination. Nat Genet. 2003c;34:32–33. doi: 10.1038/ng1141. [DOI] [PubMed] [Google Scholar]
- Menke D, Page D. Sexually dimorphic gene expression in the developing mouse gonad. Gene Expr Pattern. 2002;2:359–367. doi: 10.1016/s1567-133x(02)00022-4. [DOI] [PubMed] [Google Scholar]
- Menke DB, Koubova J, Page DC. Sexual differentiation of germ cells in XX mouse gonads occurs in an anterior-to-posterior wave. Dev Biol. 2003;262:303–312. doi: 10.1016/s0012-1606(03)00391-9. [DOI] [PubMed] [Google Scholar]
- Miyamoto N, Yoshida M, Kuratani S, Matsuo I, Aizawa S. Defects of urogenital development in mice lacking Emx2. Development. 1997;124:1653–1664. doi: 10.1242/dev.124.9.1653. [DOI] [PubMed] [Google Scholar]
- Nakamura M, Matzuk MM, Gerstmayer B, Bosio A, Lauster R, Miyachi Y, Werner S, Paus R. Control of pelage hair follicle development and cycling by complex interactions between follistatin and activin. FASEB J. 2003;17:497–499. doi: 10.1096/fj.02-0247fje. [DOI] [PubMed] [Google Scholar]
- Palmer S, Burgoyne PS. XY follicle cells in the ovaries of XO/XY and XO/XY/XYY mosaic mice. Development. 1991;111:1017–1020. doi: 10.1242/dev.111.4.1017. [DOI] [PubMed] [Google Scholar]
- Phillips DJ, de Kretser DM. Follistatin: a multifunctional regulatory protein. Front Neuroendocrinol. 1998;19:287–322. doi: 10.1006/frne.1998.0169. [DOI] [PubMed] [Google Scholar]
- Ross AJ, Tilmann C, Yao H, MacLaughlin D, Capel B. AMH induces mesonephric cell migration in XX gonads. Mol Cell Endocrinol. 2003;211:1–7. doi: 10.1016/j.mce.2003.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vaiman D, Pailhoux E. Mammalian sex reversal and intersexuality: deciphering the sex-determination cascade. Trends Genet. 2000;16:488–494. doi: 10.1016/s0168-9525(00)02126-0. [DOI] [PubMed] [Google Scholar]
- Vanio S, Heikkila M, Kispert A, Chin N, McMahon A. Female development in mammals is regulated by Wnt-4 signaling. Nature. 1999;397:405–409. doi: 10.1038/17068. [DOI] [PubMed] [Google Scholar]
- Vidal VP, Chaboissier MC, de Rooij DG, Schedl A. Sox9 induces testis development in XX transgenic mice. Nat Genet. 2001;28:216–217. doi: 10.1038/90046. [DOI] [PubMed] [Google Scholar]
- Willert J, Epping M, Pollack JR, Brown PO, Nusse R. A transcriptional response to Wnt protein in human embryonic carcinoma cells. BMC Dev Biol. 2002;2:8. doi: 10.1186/1471-213x-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu R, Ito T, Saunders S, Camper S, Jameson J. Role of Ahch in gonadal development and gametogenesis. Nat Genet. 1998;20:353–357. doi: 10.1038/3822. [DOI] [PubMed] [Google Scholar]
- Zanaria E, Muscatelli F, Bardoni B, Strom T, Guioli S, Guo W, Lalli E, Moser C, Walker A, McCabe E, Meitinger T, Monaco A, Sassone-Corsi P, Camerino G. An unusual member of the nuclear hormone receptor superfamily responsible for X-linked adrenal hypoplasia congenita. Nature. 1994;372:635–641. doi: 10.1038/372635a0. [DOI] [PubMed] [Google Scholar]
- Zhang H, Bradley A. Mice deficient for BMP2 are nonviable and have defects in amnion/chorion and cardiac development. Development. 1996;122:2977–2986. doi: 10.1242/dev.122.10.2977. [DOI] [PubMed] [Google Scholar]