SUMMARY
Background
Gut microbiota modifiers may have beneficial effects of non-alcoholic fatty liver disease (NAFLD) but randomised controlled trials (RCT) are lacking in children.
Aim
We performed a double-blind RCT of VSL#3 vs. placebo in obese children with biopsy-proven NAFLD.
Methods
Of 48 randomised children, 44 (22 VSL#3 and 22 placebo) completed the study. The main outcome was the change in fatty liver severity at 4 months as detected by ultrasonography. Secondary outcomes were the changes in triglycerides, insulin resistance as detected by the homoeostasis model assessment (HOMA), alanine transaminase (ALT), body mass index (BMI), glucagon-like peptide 1 (GLP-1) and activated GLP-1 (aGLP-1). Ordinal and linear models with cluster confidence intervals were used to evaluate the efficacy of VSL#3 vs. placebo at 4 months.
Results
At baseline, moderate and severe NAFLD were present in 64% and 36% of PLA children and in 55% and 45% of VSL#3 children. The probability that children supplemented with VSL#3 had none, light, moderate or severe FL at the end of the study was 21%, 70%, 9% and 0% respectively with corresponding values of 0%, 7%, 76% and 17% for the placebo group (P < 0.001). No between-group differences were detected in triglycerides, HOMA and ALT while BMI decreased and GLP-1 and aGLP1 increased in the VSL#3 group (P < 0.001 for all comparisons).
Conclusions
A 4-month supplement of VSL#3 significantly improves NAFLD in children. The VSL#3-dependent GLP-1 increase could be responsible for these beneficial effects. Trial identifier: NCT01650025 (www.clinicaltrial.gov)
INTRODUCTION
The most recent World Health Organization (WHO) estimate indicates that obesity is increasing rapidly, particularly in children (http://www.who.int/mediacentre/factsheets/fs311/en/index.html, access October 2013). Childhood obesity not only causes long-term health problems that become obvious in adulthood like cardiovascular diseases and cancer but also short-term secondary complications, including dyslipidemia, insulin resistance and non-alcoholic fatty liver disease (NA-FLD).1–3 NAFLD in the paediatric repertoire comprises different diseases, ranging from simple intra-hepatic fat accumulation (fatty liver or NAFL) to a more severe pattern of liver damage (non-alcoholic steatohepatitis or NASH), which exhibits steatosis together with inflammation and ballooning, and eventually fibrosis.4 The development of NAFL and its progression to NASH are the consequence of the interactions between a genetic background and progressive environment-driven epigenetic code changes and molecular alterations.5 Indeed, environmental factors, like unhealthy diet (particularly Western dietary patterns) and low levels of physical activity, can drive epigenomic reprogramming of the host genome with post-translational modifications of gene expression ultimately determining phenotypic changes in the organism.6, 7 For these reasons, lifestyle interventions currently represent the core of prevention and treatment of NAFLD in children.8
The understanding of the mechanisms related with NAFLD development and progression remains a major challenge. However, recent evidence demonstrated that the interaction between the liver and gut, the so called ‘gut-liver axis’, might play a major role among the factors leading to the phenotypic switching from the NAFL state to a more aggressive lesion including NASH and NASH-related fibrosis.9, 10 In fact, in humans, NAFLD is associated with increased intestinal permeability (IP) and small intestinal bacterial overgrowth (SIBO), and these factors are associated with the severity of hepatic steato-sis.11 In NAFLD patients, the gut has been demonstrated to be ‘leaky’ because of a tight junctions disruption process that might explain the intestinal bacterial contribution to liver disease progression, since there is an increased exposure of the liver to gut-derived bacterial products.12 Moreover, some recent studies have demonstrated an important role for aberrations in gut microbiota composition in promoting NAFLD in children.13 These findings mirror those seen in animal models suggesting that gut microbiota composition may influence intra-hepatic fat accumulation as an environmental factor by several mechanisms, including increased monosaccharide absorption from the intestinal lumen, and production/release of hepatotoxic products and other molecules, which in turn may lead to a chronic low-grade inflammatory state.14–16 Therefore, several authors have suggested the modulation of gut microbiota by probiotics, prebiotics and synbiotics as a possible approach for obesity and NAFLD. Their potential beneficial effects have been confirmed in several experimental studies.17–19 In fact, it has been reported in animal models that probiotics administration was able to reset the ‘leaky gut’, and offered protection against NAFL development and its progression to NASH by modulating the expression of nuclear receptors and correcting insulin resistance in the liver and the adipose tissues.18–20 VSL#3, a mixture of eight probiotic strains (Streptococcus thermophilus, bifidobacteria [B. breve, B. infantis, B. longum], Lactobacillus acidophilus, L. plantarum, L. paracasei, and L. delbrueckii subsp. bulgaricus), is the most studied probiotic in NA-FLD.19, 20 In mouse models of genetic dyslipidemia (Apo-E deficient mice) which fail to develop NASH-like lesions on a standard diet, it has been shown how destrane sulphate sodium (DSS)-induced intestinal inflammation and consequent increased IP, triggered the transition of steatosis to NASH, and how these disorders were efficiently prevented by a therapeutic intervention with VSL#3.19 Interesting results were also obtained in animal models of high fat diet-induced NAFL/NASH that resulted in attenuated liver fibrosis after VSL#3 supplementation.20 Despite a large number of animal data on the efficacy, as well as the well-known safety profile of the use of probiotics, randomised placebo-controlled trials in NAFLD are still lacking in humans.
On the basis of the possibility that VSL#3 could have disease-modifying effects in humans, we performed the first randomised controlled trial (RCT) of VSL#3 in children with NAFLD.
MATERIALS AND METHODS
Study design
We performed a parallel-arm double-blind RCT of VSL#3 vs. placebo, which were provided by VSL Pharmaceuticals Inc (Towson, MD, USA), in obese children with NAFLD enrolled at ‘Bambino Gesù Children’s Hospital’.
Obesity was diagnosed as body mass index (BMI) >85th percentile. The diagnosis of NAFLD was based on a combination of physical findings at examination, elevated aminotransferase (ALT) levels (up to 40 UI/L) of unknown origin and ultrasonographic) evidence of hepatic steatosis as well as histological evaluation of liver biopsies obtained at entry by an expert pathologist.
Exclusion criteria included the presence of liver disease due to any of the following: hypothyroidism, Wilson disease, viral hepatitis (HBV, HCV), acute systemic disease, cystic fibrosis, coeliac disease, suspicion of muscular dystrophy, alpha-1-antitrypsin deficiency, metabolic inherited diseases, autoimmune hepatitis, drug toxicity and drugs known to induce steatosis (e.g. valproate, amiodarone or prednisone).21 Patients were also excluded if body weight and carbohydrate metabolism were altered by the use of parenteral nutrition, protein malnutrition, previous gastrointestinal surgery, structural abnormalities of the gastrointestinal tract or neurological impairment. Finally, the use of nonsteroidal anti-inflammatory drugs, antibiotics, probiotics or anti-secretory drugs capable of causing achlorhydria within 2 months preceding enrolment were also considered exclusion criteria.
Sample size was calculated on the basis of the change in NAFLD severity observed with docosahexaenoic acid (DHA) in a previous RCT.22 Using proportional odds-logistic regression, we found that the log-odds of less severe vs. more severe NAFLD was 1.4 in the DHA group vs. the placebo group. This is the value associated with the treatment×time interaction in the proportional odds-logistic regression model used to analyse the DHA RCT.22 We decided to enrol a number of subjects sufficient to detect such effect also for VSL#3 vs. placebo. We calculated that 24 subjects per group would ensure a power of 83% to detect such log-odds at an alpha level of 0.05.23 Children were randomised to receive in blinded fashion, 1 sachet/day of VSL#3, or placebo, if the subject’s age was less than 10 years old. Two sachets of VSL#3 or placebo were administered in children older than 10 years of age. A computer-generated randomisation sequence assigned participants in a 1:1 ratio to treatment with VSL #3 or placebo. A statistician blinded to participants’ clinical data, and who did not perform the final analysis, generated the allocation sequence and randomly assigned participants to the VSL#3 or placebo group. Only the statistician had access to the treatment codes. Sachets were stored at the hospital pharmacy and dispensed at the baseline visit (randomisation) and bimonthly thereafter.
Treatment duration was for 4 months. Enrollees in both arms, treating physicians, and study coordinators were blinded to treatment as sachets provided were identical as was the visual aspect and taste of the study agent and the placebo. The envelopes were numbered, and all investigators were blinded for all the duration of the study.
A low calorie diet was prescribed to all patients during the entire study. Specifically, as recommended by the Italian Recommended Dietary Allowances, each patient received the following diet: carbohydrate, 50–60%; fat, 23–30%; fatty acid, two-thirds saturated, one-third unsaturated protein, 15–20%; for a total of 25–30 Kcal/kg body weight/day. In addition to the prescribed diet, a moderate programme of aerobic exercise (30–45 min at least 3 times a week) was also recommended and was tailored to individual preferences.21.
Compliance was monitored through monthly phone calls. Patients and patients’ families were not asked to take VSL#3/placebo sachets with them during the calls and visits since the sachets had to be kept at a temperature of 4°C before oral use. Therefore, compliance to the treatments was also evaluated every visit (month 0, 1, 2, 3 and 4) by review of medication records in a patients’ diary reporting sachets count and adverse events, and direct interview of patients by AA, GiBa and VG. Compliance was estimated as a percentage of sachets taken during the treatment and greater than 90% in both groups (mean values of 93% vs. 94%).
Complete medical histories were recorded for all participants. Any patients, parents or respective guardians reported about adverse events during the trial and the post-treatment follow-up.
Collection of anthropometrical data, biochemical and ultrasound (US) data were performed at baseline and after 4 months from the initiation of the trial.
The Hospital Ethics Research Committee approved the study, in accordance with the Declaration of Helsinki (as revised in Seoul, Korea, October 2008). Parents of the included patients gave their written informed consent for liver biopsy, treatment, diagnostic tests and publication of the results. The study was registered at Clinical Trials.gov (NCT01650025).
Anthropometry
Weight and height were measured following standard procedures.24 BMI was calculated and standard deviations scores (SDS) were calculated using Italian reference data.25
Blood tests
Venous blood samples, obtained after an overnight 12-h fast, were used to measure biochemical parameters including: fasting glucose and insulin, total cholesterol, triglycerides, high-density lipoprotein (HDL) and low-density lipoprotein cholesterol (LDL), and levels of alanine transaminase (ALT) and aspartate transaminase (AST). The degree of insulin resistance/sensitivity was estimated with the homoeostatic model assessment (HOMA) equation, as follows: .26
GLP-1 assay
Part of collected blood was centrifuged at 2000 g for 12 min and plasma was stored at −80°C pending further analysis. Plasma samples were used to perform specific sandwich enzyme-linked immunosorbent assay (ELISA) to determine the circulating levels of total and activated GLP-1 (Listarfish, Milan, Italy).
Liver ultrasound
Liver US was performed by the same experienced radiologist using an Acuson Sequoia C512 scanner equipped with a 15L8 transducer (Universal Diagnostic Solutions, Oceanside, CA, USA). Normal liver, absent steatosis (grade 0) was defined as having normal liver echo-texture; mild steatosis (grade 1) as slight and diffuse increase in fine parenchymal echoes with normal visualisation of diaphragm and portal vein borders; moderate steatosis (grade 2) was defined as moderate and diffuse increase in fine echoes with slightly impaired visualisation of diaphragm and portal vein borders; and severe steatosis (grade 3) was defined as fine echoes with poor or no visualisation of diaphragm, portal vein borders and posterior portion of the right lobe.
Liver histology
We performed percutaneous liver biopsy and with subsequent histology on liver sections from an 18G biopsy needle using standard procedures. Histological assessment was performed by an experienced pathologist (Rita De Vito) according to criteria proposed by NAFLD Clinical Research Network.27 Briefly, steatosis was graded 0–3 as follows: 0 <5% steatosis; 1 = 5–33%; 2 = 33–66% and grade 3 >66%. Lobular inflammation was scored based on the number of inflammatory foci per 200X per field as follows: 0 = no inflammatory foci; 1 <2 foci; 2 = 2–4 foci and 3 >4 foci. Ballooning was scored as follows: 0 = none; 1 = few balloon cells present and 2 = prominent ballooning. Fibrosis was staged 0–4 as follows: 0 = no fibrosis; 1 = periportal or perisinusoidal; 1A = mild, Zone 3, perisinusoidal; 1B = moderate, Zone 3, perisinusoidal; 1C = portal/periportal; 2 = perisinusoidal and portal/periportal; 3 = bridging fibrosis and 4 = cirrhosis.
Statistical analysis
Most variables were not normally distributed and all are reported as medians and interquartile ranges (IQR). The effect of treatment on the main ordinal outcome (liver steatosis coded as 0 = none, 1 = light, 2 = moderate and 3 = severe), and the secondary continuous outcomes (triglycerides, ALT, BMI, HOMA, GLP-1 and aGLP-1) were evaluated using an ordinal generalised linear model (OGLM) and linear models (LM) employing treatment (0= placebo; 1 = VSL#3), time (0 = baseline; 1 = 4 months), a treatment×time interaction (discreteX-discrete) and the baseline value of the outcome as predictors.22, 28–31 When the treatment×time interaction equals 1 (treatment = 1 and time = 1), its size gives a measure of the change in the outcome of interest in the VSL#3 group relative to the placebo group. Repeated measures were taken into account by specifying cluster confidence intervals (CI) for each patient. The odds-ratio (OR) obtained from the OGLM is a measure of the odds of more severe vs. less severe steatosis. Probabilities estimated from the OGLM were plotted and continuous values estimated from the LMs tabulated to aid the clinical interpretation of the results. The analysis was intention-to-treat. Statistical significance was set to a P-value <0.05 and all statistical tests were two-tailed. Statistical analysis was performed using STATA 12.1 (Stata Corp, College Station, TX, USA) and StatXact 10 (Cytel Inc., Cambridge, MA, USA).
RESULTS
Patient characteristics at baseline
Between August 2012 and May 2013, we evaluated 116 Caucasian children with suspected NAFLD at our Out--patient Clinic. Fifty-seven of these children did not meet the inclusion criteria for the study. The remaining 59 children met the inclusion criteria and their parents agreed for respective children’s study participation. Just before randomisation, the parents of 11 of these children decided not to permit their children to participate in the study, leaving 48 children for randomisation, 24 for the VSL#3 group and 24 for the placebo group. As four of the children were lost to follow-up after the study began – two in the VSL#3 group and two in the placebo group – the final analysis was performed with a total of forty-four study participants, i.e. 92% of the planned sample size of 48 patients.
Table 1 gives the baseline anthropometric and biochemical measurements of the children randomised to VSL#3 and placebo. Table 2 gives the baseline US and histological features of the children randomised to VSL#3 and placebo.
Table 1.
Baseline measurements of the children randomised to VLS#3 and placebo
| Placebo (n = 22; males = 14)
|
VSL#3 (n = 22; males = 10)
|
|||||
|---|---|---|---|---|---|---|
| Median | IQR | Median | IQR | |||
| Age (years) | 11 | 10 | 12 | 10 | 9 | 12 |
|
| ||||||
| Weight (kg) | 53.9 | 47.8 | 65.0 | 60.5 | 55.7 | 70.5 |
|
| ||||||
| Height (cm) | 1.47 | 1.39 | 1.52 | 1.49 | 1.43 | 1.55 |
|
| ||||||
| BMI (kg/m2) | 25.6 | 23.2 | 27.9 | 27.3 | 24.7 | 28.6 |
|
| ||||||
| BMI (SDS) | 1.69 | 1.23 | 2.12 | 2.01 | 1.63 | 2.37 |
|
| ||||||
| Glucose (mg/dL) | 85 | 78 | 89 | 84 | 79 | 91 |
|
| ||||||
| Insulin (μU/mL) | 16 | 12 | 21 | 18 | 14 | 27 |
|
| ||||||
| HOMA | 3.1 | 2.3 | 4.7 | 3.9 | 2.7 | 5.4 |
|
| ||||||
| Cholesterol (mg/dL) | 156 | 135 | 179 | 156 | 137 | 175 |
|
| ||||||
| Triglycerides (mg/dL) | 94 | 64 | 118 | 86 | 63 | 121 |
|
| ||||||
| HDL cholesterol (mg/dL) | 48 | 42 | 54 | 45 | 38 | 53 |
|
| ||||||
| LDL cholesterol (mg/dL) | 92 | 80 | 111 | 83 | 71 | 101 |
|
| ||||||
| ALT (U/L) | 32 | 23 | 42 | 27 | 20 | 50 |
|
| ||||||
| AST (U/L) | 63 | 53 | 74 | 56 | 51 | 70 |
|
| ||||||
| GLP-1 (pmol/L) | 2.1 | 1.8 | 2.6 | 2.9 | 1.9 | 2.3 |
|
| ||||||
| aGLP-1 (pmol/L) | 1.3 | 1.1 | 1.8 | 1.3 | 1.0 | 2.1 |
BMI, body mass index; SDS, standard deviation scores; HOMA, homoeostasis model assessment of insulin resistance; HDL, high-density lipoprotein; LDL, low-density lipo-protein; ALT, alanine transaminase; AST, aspartate transaminase; GLP-1, glucagon-like peptide-1; aGLP-1, activated glucagon-like peptide-1.
Table 2.
Liver histopathology of the children randomised to VSL#3 and placebo
| Placebo
|
VSL#3
|
|||
|---|---|---|---|---|
| n | % | n | % | |
| Fatty liver at US | ||||
|
| ||||
| Moderate | 14 | 63.6 | 12 | 54.5 |
|
| ||||
| Severe | 8 | 36.4 | 10 | 45.5 |
|
| ||||
| Steatosis | ||||
|
| ||||
| 5–33% | 3 | 13.6 | 2 | 9.1 |
|
| ||||
| 33–66% | 9 | 40.9 | 10 | 45.5 |
|
| ||||
| >66% | 10 | 45.5 | 10 | 45.5 |
|
| ||||
| Inflammation | ||||
|
| ||||
| <2 foci | 7 | 31.8 | 7 | 31.8 |
|
| ||||
| 2–4 foci | 12 | 54.5 | 9 | 40.9 |
|
| ||||
| >4 foci | 3 | 13.6 | 6 | 27.3 |
|
| ||||
| Ballooning | ||||
|
| ||||
| Few balloon cells | 13 | 59.1 | 15 | 68.2 |
|
| ||||
| Prominent balloon cells | 9 | 40.9 | 7 | 31.8 |
|
| ||||
| Fibrosis | ||||
|
| ||||
| Periportal or perisinusoidal | 12 | 54.5 | 10 | 45.5 |
|
| ||||
| Perisinusoidal and portal/periportal | 8 | 36.4 | 9 | 40.9 |
|
| ||||
| Bridging fibrosis | 2 | 9.1 | 3 | 13.6 |
|
| ||||
| Non-alcoholic steatohepatitis score (NAS) | ||||
|
| ||||
| 3 | 1 | 4.5 | 2 | 9.1 |
|
| ||||
| 4 | 3 | 13.6 | 4 | 18.2 |
|
| ||||
| 5 | 5 | 22.7 | 3 | 13.6 |
|
| ||||
| 6 | 9 | 40.9 | 6 | 27.3 |
|
| ||||
| 7 | 4 | 18.2 | 5 | 22.7 |
|
| ||||
| 8 | 0 | 0.0 | 2 | 9.1 |
Effects of VSL#3 on fatty liver: main outcome
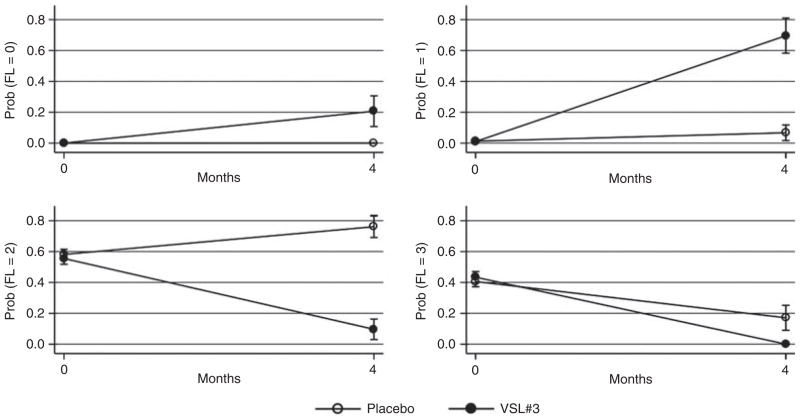
The change in fatty liver after 4 months of supplementation of VSL#3 was the main outcome of the study. At baseline, moderate and severe NAFLD were present in 64% and 36% of placebo children and in 55% and 45% of VSL#3 children. The OR of more severe vs. less severe steatosis at 4 months was 0.001 (95% CI 0.0001–0.02) for the VSL#3 vs. the placebo group (OGLM, P < 0.001). Figure 1 plots the corresponding probabilities of steatosis in the VSL#3 and placebo groups.
Figure 1.
Changes in the severity of fatty liver in the placebo and VSL#3 groups during the study. Values are mean probabilities and standard errors estimated from an ordinal logistic regression model for repeated measures (cluster confidence intervals). For this reason, probabilities at each time point sum to 100. Abbreviations: FL = fatty liver; 0= no fatty liver; 1 = light fatty liver; 2 = moderate fatty liver; 3 = severe fatty liver.
The probability that children supplemented with VSL#3 had none, light, moderate or severe FL at the end of the study was 21%, 70%, 9% and 0% in the VSL#3 group and 0%, 7%, 76% and 17% for the placebo group. These probabilities sum to 100 as they are obtained from an ordinal model and take into account the baseline degree of fatty liver.
Effects on triglycerides, HOMA, ALT, BMI and GLP-1
In Table 3, we reported the baseline and 4-month values of the secondary outcomes as estimated by the LMs for repeated measures. The changes in triglycerides, HOMA, ALT and BMI were similar in the VSL#3 and placebo groups. BMI values were decreased (P < 0.001) in VSL#3-supplemented children with respect to placebo group after the 4-month supplementation. Recently, Yadav et al. demonstrated that VSL#3 was able to suppress body weight gain and insulin resistance in mouse via modulation of the gut flora composition, which in turn enhanced the expression and the activity of the GLP-1 released by the intestinal L cells.32 Therefore, we assessed the plasma concentration of the total form (GLP-1) and activated form (aGLP-1) of this factor in both study groups (Table 3).
Table 3.
Changes in secondary outcomes during the study. Values are mean and standard errors estimated by a linear model for repeated measures (cluster confidence intervals)
| Placebo
|
VSL#3
|
P-value* | |||
|---|---|---|---|---|---|
| Baseline | 4 months | Baseline | 4 months | ||
| Triglycerides (mg/dL) | 98 (3) | 102 (10) | 99 (4) | 110 (9) | 0.575 |
|
| |||||
| HOMA | 4.7 (0.4) | 3.5 (0.6) | 4.3 (0.3) | 3.3 (0.3) | 0.693 |
|
| |||||
| ALT (U/L) | 42 (1) | 50 (5) | 34 (1) | 33 (1) | 0.170 |
|
| |||||
| BMI (kg/m2) | 25.6 (0.01) | 25.7 (0.24) | 27.1 (0.01) | 24.9 (0.2) | <0.001 |
|
| |||||
| BMI (SDS) | 1.68 (0.01) | 1.68 (0.04) | 1.94 (0.01) | 1.58 (0.04) | <0.001 |
|
| |||||
| GLP-1 (pmol/L) | 2.25 (0.03) | 2.17 (0.11) | 2.20 (0.02) | 3.24 (0.19) | <0.001 |
|
| |||||
| a-GLP1 (pmol/L) | 1.44 (0.01) | 1.42 (0.13) | 1.58 (0.02) | 2.20 (0.05) | <0.001 |
Tests whether there is a change in time of the outcomes of interest for the VSL#3 vs. placebo group taking into account baseline values of the outcome (Wald test for treatmentXtime interaction, linear model for repeated measures).
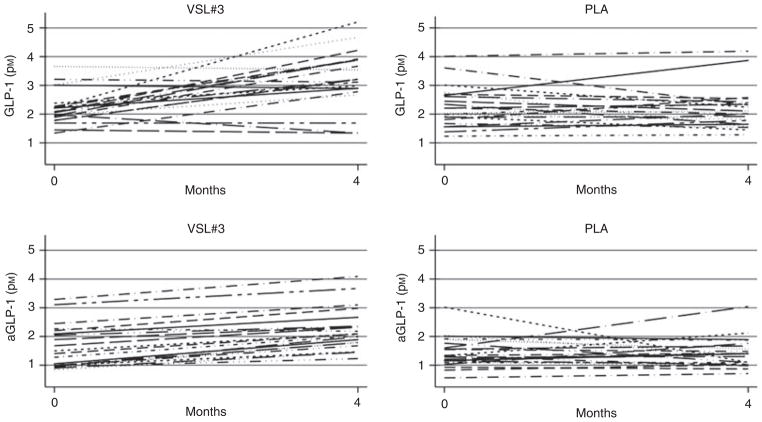
Figure 2 reports the individual before-after plots for GLP-1 and aGLP-1. There was a clear trend towards increasing values of GLP-1 and aGLP-1 in the VSL#3 group.
Figure 2.
Individual changes of GLP-1 and aGLP-1 in the placebo and VSL#3 groups during the study.
DISCUSSION
Current medical and lifestyle interventions offer modest efficacy in the treatment of paediatric NAFLD and other therapeutic interventions are not approved for children.3, 4 Clinical trials with safe and well-tolerated pharmacological agents that may improve insulin resistance and oxidative stress, Metformin and vitamin E respectively, were completed by different tertiary centres with controversial efficacy.21, 33, 34 Interestingly, Nobili et al. recently demonstrated that docosahexaenoic acid metabolically and histologically improves liver steatosis in children with NAFLD.4, 22, 35, 36 However, to date there are no clear specific therapeutics targeting NAFLD-associated liver damage and associated long-term risks in children.
The role of the intestinal microbiota in NAFLD has garnered significant attention during the last 5 years, and several reports in rodent models suggest a crucial role of the intestinal microbiota composition in NAFLD development and progression.37, 38 It is now well-recognised in humans that changes in microbiota composition, termed dysbiosis, is strongly associated with obesity and NAFLD.13, 39 Furthermore, as the microbiota and host response to changes in flora metabolism can be strongly influenced by diet, the ingestion of foods higher in refined sugar and fats, thus linking the overwhelming presence of gut-derived microbial products, activation of innate immunity an inflammation with consequential NAFLD development.40, 41
Therefore, based on these recent findings it would seem rational to study several therapeutic strategies to modify the gut microbiota composition (i.e. antibiotics, probiotics and prebiotics) – reverse dysbiosis, with the ultimate goal of reversing NAFLD-related liver injury. Until our present study, most studies have been conducted in pre-clinical models, while there are limited studies in paediatric human NAFLD-related disease.42–45
A recent double-blind clinical trial demonstrated that obese children with NAFLD treated with Lactobacillus GG resulted in a significant decrease (up to normalisation in 80% of cases) in serum ALT values; and in titres of anti-peptidoglycan-polysaccharide antibodies, which are a ubiquitously detected as a result of exposure to bacterial cell wall antigen. Consequently, these antibodies are suitable as an indirect indicator of intestinal bacterial overgrowth.44
Interestingly, Loguercio et al. demonstrated that VSL#3 supplementation in patients affected by several types of chronic liver diseases, including NAFLD, may reduce liver damage and improve serum levels of various biomarkers In that study, however, the only NAFLD-related study endpoint was ALT.45
According to the data we acquired here following 4 months of supplementation with VSL#3, we observed improvement of FL, as evaluated by US, as well as a significant decrease in the BMI ofVSL#3-supplemented children compared to the placebo group.
The dual beneficial effect on FL and body weight we observed could result from either direct or indirect consequences that result from VSL#3-dependent reversal of dysbiosis. The restoration of normal gut flora could result in reduced intestinal permeability, increased production of short chain fatty acids (SCFAs) and anorexogenic gut hormones (including GLP-1 and GLP-2), as well as enhancement of insulin sensitivity. Taken together, these beneficial effects would reduce the inflammatory state and insulin resistance, which are well known characteristics of human obesity.
In fact, interestingly, Yadav et al. recently demonstrated that VSL#3 in rats may modulate the gut flora composition (i.e. decreased firmicutes and increased bacteriodetes and bifidobacteria) and stimulate differential production of SCFAs, like butyrate, that have a beneficial effect on weight loss if administered to diet-induced obese mice32 Therefore, it is quite conceivable that the effects of VSL#3 in our patients could be dependent on the restoration of normal gut microbiota as well as a consequent production of SCFAs. These findings merit further investigation by metagenomic and metabolomic approaches in VSL#3-supplemented obese children with biopsy-proven NAFLD. Yadav and colleagues also demonstrated that increased gut microbiota butyrate could enhance the expression and the activity of the GLP-1. GLP-1 has long been known to be an incretin secreted by the enterochromaffin cells of the small intestine and proximal colon.26 Following ingestion of food, GLP-1 is released by these cells and stimulates the endocrine pancreas thus promoting insulin sensitivity, and therefore aids both glucose and fat metabolism. Indeed, circulating levels of GLP-1, both in total and active form are significantly increased in our VSL#3 patients after the 4-month supplementation.
Interestingly, over time evolutionary changes in the biology of GLP-1 have rendered its half-life in mammals rather short since the peptide is inactivated by dipeptidyl peptidase IV (DPPIV). Recently the role of GLP-1 and respective analogues – used currently for the treatment of type 2 diabetes mellitus (T2DM) – has been challenged. GLP-1 receptors are found ubiquitously in various mammalian organs, but controversy exists as to their presence on hepatocytes.46 Importantly, however, there is an emerging consensus, following the production of long-acting GLP-1 analogues, that GLP-1 may have insulino-mimetic effects. Recent studies in animal models and NAFLD adults showed an effective role of GLP-1 receptor agonists (such as exenatide and liraglutide) as a new promising therapy in NAFLD for their ability in modulating fatty acid oxidation, decreasing lipogenesis and improving hepatic glucose metabolism.47, 48 The cells responsible for synthesising the pre-propetide for GLP-1/GLP-2 are found in the distal small intestine and proximal colon and are termed L cells. L cells have G protein coupled receptors (GPCRs) which are known to avidly bind bile salts. While the recent FLINT trial appears promising, the effects of the long-term use of bile salt agonists in patients, especially children, is not currently known. A safer pharmacologic mechanism to enhance GLP protein secretion, as we have observed in this study raises the spectra of a safe alternative. Furthermore, a recent randomised, double-blind, placebo-controlled, multicentre clinical, demonstrated that a well-tolerated exenatide administered to obese adolescents resulted in a significant reduction in BMI compared with placebo that was confirmed during the open-label extension of the study.49 Although, the long-term hepato-metabolic effects of GLP-1 agonists are still unknown, this latter study suggests that exenatide could be a plausible new potentially safe and efficacious drug that could in combination with VSL#3, be a treatment for obese children with NAFLD.
In summary, our human data corroborate findings in previous pre-clinical studies. Our clinical trial provides the first human clinical evidence demonstrating that a brief-course of supplementation with VSL#3 significantly improves fatty liver and BMI in children with NAFLD. The effect of long-term supplementation with VSL#3 with respect to reversal of hepatic injury in NASH, as well as metabolomic, analysis of gut microflora remain to be defined in a possible extension of this trial.
Acknowledgments
We are indebted to Prof Claudio De Simone who provided VSL#3 with verified composition and indistinguishable placebo, and intellectual contribution to the study. We also thank Dr. Rita De Vito for her help in liver histology assessment.
Footnotes
Declaration of personal interests: None.
AUTHORSHIP
Guarantor of the article: Valerio Nobili.
Author contributions: Obtained funding: Nobili V. Study design: Nobili V, Alisi A. Data collection: Alisi A, Baviera G, Giorgio V. Statistical analysis: Bedogni G, Alisi A. Data interpretation: Porro E, Paris C, Giammaria P and Reali E. Drafting of MS: Alisi A, Bedogni G, Baviera G. Revision of manuscript for important intellectual content: Anania F. All authors approved the final version of the article, including the authorship list.
Declaration of funding interests: This study was funded by the Italian Ministry of Health (Fondi di Ricerca Corrente and 5*1000) to Prof. Valerio Nobili. Prof. Anania is supported by US Public Health Service Grant DK062092 and Departments of Veterans’ Affairs Grant BX001746.
References
- 1.King B, Jiang Y, Su X, et al. Weight control, endocrine hormones and cancer prevention. Exp Biol Med (Maywood) 2013;238:502–8. doi: 10.1177/1535370213480695. [DOI] [PubMed] [Google Scholar]
- 2.Vazzana N, Santilli F, Sestili S, Cuccurullo C, Davi G. Determinants of increased cardiovascular disease in obesity and metabolic syndrome. Curr Med Chem. 2011;18:5267–80. doi: 10.2174/092986711798184299. [DOI] [PubMed] [Google Scholar]
- 3.Nobili V, Svegliati-Baroni G, Alisi A, Miele L, Valenti L, Vajro P. A 360-degree overview of paediatric NAFLD: recent insights. J Hepatol. 2013;58:1218–29. doi: 10.1016/j.jhep.2012.12.003. [DOI] [PubMed] [Google Scholar]
- 4.Alisi A, Feldstein AE, Villani A, Raponi M, Nobili V. Pediatric nonalcoholic fatty liver disease: a multidisciplinary approach. Nat Rev Gastroenterol Hepatol. 2012;9:152–61. doi: 10.1038/nrgastro.2011.273. [DOI] [PubMed] [Google Scholar]
- 5.Alisi A, Cianfarani S, Manco M, Agostoni C, Nobili V. Non-alcoholic fatty liver disease and metabolic syndrome in adolescents: pathogenetic role of genetic background and intrauterine environment. Ann Med. 2012;44:29–40. doi: 10.3109/07853890.2010.547869. [DOI] [PubMed] [Google Scholar]
- 6.Oddy WH, Herbison CE, Jacoby P, et al. The Western dietary pattern is prospectively associated with nonalcoholic fatty liver disease in adolescence. Am J Gastroenterol. 2013;108:778–85. doi: 10.1038/ajg.2013.95. [DOI] [PubMed] [Google Scholar]
- 7.Cordero P, Campion J, Milagro FI, Martinez JA. Transcriptomic and epigenetic changes in early liver steatosis associated to obesity: effect of dietary methyl donor supplementation. Mol Genet Metab. 2013;110:388–95. doi: 10.1016/j.ymgme.2013.08.022. [DOI] [PubMed] [Google Scholar]
- 8.Nobili V, Alisi A, Raponi M. Pediatric non-alcoholic fatty liver disease: preventive and therapeutic value of lifestyle intervention. World J Gastroenterol. 2009;15:6017–22. doi: 10.3748/wjg.15.6017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park JS, Seo JH, Youn HS. Gut Microbiota and Clinical Disease: obesity and Nonalcoholic Fatty Liver Disease. Pediatr Gastroenterol Hepatol Nutr. 2013;16:22–7. doi: 10.5223/pghn.2013.16.1.22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Frasinariu OE, Ceccarelli S, Alisi A, Moraru E, Nobili V. Gut-liver axis and fibrosis in nonalcoholic fatty liver disease: an input for novel therapies. Dig Liver Dis. 2013;45:543–51. doi: 10.1016/j.dld.2012.11.010. [DOI] [PubMed] [Google Scholar]
- 11.Sabaté JM, Jouët P, Harnois F, et al. High prevalence of small intestinal bacterial overgrowth in patients with morbid obesity: a contributor to severe hepatic steatosis. Obes Surg. 2008;18:371–7. doi: 10.1007/s11695-007-9398-2. [DOI] [PubMed] [Google Scholar]
- 12.Miele L, Valenza V, La Torre G, et al. Increased intestinal permeability and tight junction alterations in nonalcoholic fatty liver disease. Hepatology. 2009;49:1877–87. doi: 10.1002/hep.22848. [DOI] [PubMed] [Google Scholar]
- 13.Zhu L, Baker SS, Gill C, et al. Characterization of gut microbiomes in nonalcoholic steatohepatitis (NASH) patients: a connection between endogenous alcohol and NASH. Hepatology. 2013;57:601–9. doi: 10.1002/hep.26093. [DOI] [PubMed] [Google Scholar]
- 14.Bäckhed F, Ding H, Wang T, et al. The gut microbiota as an environmental factor that regulates fat storage. Proc Natl Acad Sci USA. 2004;101:15718–23. doi: 10.1073/pnas.0407076101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cope K, Risby T, Diehl AM. Increased gastrointestinal ethanol production in obese mice: implications for fatty liver disease pathogenesis. Gastroenterology. 2000;119:1340–7. doi: 10.1053/gast.2000.19267. [DOI] [PubMed] [Google Scholar]
- 16.Rivera CA, Adegboyega P, van Rooijen N, Tagalicud A, Allman M, Wallace M. Toll-like receptor-4 signaling and Kupffer cells play pivotal roles in the pathogenesis of non-alcoholic steatohepatitis. J Hepatol. 2007;47:571–9. doi: 10.1016/j.jhep.2007.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li Z, Yang S, Lin H, et al. Probiotics and antibodies to TNF inhibit inflammatory activity and improve nonalcoholic fatty liver disease. Hepatology. 2003;37:343–50. doi: 10.1053/jhep.2003.50048. [DOI] [PubMed] [Google Scholar]
- 18.Mencarelli A, Cipriani S, Renga B, et al. VSL#3 resets insulin signaling and protects against NASH and atherosclerosis in a model of genetic dyslipidemia and intestinal inflammation. PLoS ONE. 2012;7:e45425. doi: 10.1371/journal.pone.0045425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mencarelli A, Distrutti E, Renga B, et al. Probiotics modulate intestinal expression of nuclear receptor and provide counter-regulatory signals to inflammation-driven adipose tissue activation. PLoS ONE. 2011;6:e22978. doi: 10.1371/journal.pone.0022978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Velayudham A, Dolganiuc A, Ellis M, et al. VSL#3 probiotic treatment attenuates fibrosis without changes in steatohepatitis in a diet-induced nonalcoholic steatohepatitis model in mice. Hepatology. 2009;49:989–97. doi: 10.1002/hep.22711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nobili V, Manco M, Devito R, et al. Lifestyle intervention and antioxidant therapy in children with nonalcoholic fatty liver disease: a randomized, controlled trial. Hepatology. 2008;48:119–28. doi: 10.1002/hep.22336. [DOI] [PubMed] [Google Scholar]
- 22.Nobili V, Alisi A, Della Corte C, et al. Docosahexaenoic acid for the treatment of fatty liver: randomized controlled trial in children. Nutr Metab Cardiovasc Dis. 2013;23:1066–70. doi: 10.1016/j.numecd.2012.10.010. [DOI] [PubMed] [Google Scholar]
- 23.Hilton JF, Mehta R. Power and sample size calculations for exact conditional tests with ordered categorical data. Biometrics. 1993;49:609–16. [PubMed] [Google Scholar]
- 24.Lohman TG, Roche AF, Martorell R. Anthropometric standardization reference manual. Champaign, IL: Human Kinetics Books; 1988. [Google Scholar]
- 25.Cacciari E, Milani S, Balsamo A, et al. Italian crosssectional growth charts for height, weight and BMI (2 to 20 yr) J Endocrinol Invest. 2006;29:581–93. doi: 10.1007/BF03344156. [DOI] [PubMed] [Google Scholar]
- 26.Matthews DR, Hosker JP, Rudenski AS. Homeostasis model assessment: insulin resistance and β-cell function from fasting plasma glucose and insulin concentrations in man. Diabetologia. 1985;28:412–9. doi: 10.1007/BF00280883. [DOI] [PubMed] [Google Scholar]
- 27.Kleiner DE, Brunt EM, Van Natta M, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–21. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 28.Agresti A. Analysis of ordinal categorical data. Hoboken, NJ: Wiley; 2010. [Google Scholar]
- 29.Fitzmaurice GM, Laird NM, Ware JH. Applied longitudinal analysis. Hoboken, N.J: Wiley; 2011. [Google Scholar]
- 30.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. 3. College Station: Stata Press; 2012. [Google Scholar]
- 31.Williams R. Fitting heterogeneous choice models with oglm. Stata Journal. 2010;10:540–67. [Google Scholar]
- 32.Yadav H, Lee JH, Lloyd J, Walter P, Rane SG. Beneficial metabolic effects of a probiotic via butyrate-induced GLP-1 hormone secretion. J Biol Chem. 2013;288:25088–97. doi: 10.1074/jbc.M113.452516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nobili V, Manco M, Ciampalini P, et al. Metformin use in children with nonalcoholic fatty liver disease: an open label, 24-month, observational pilot study. Clin Ther. 2008;30:1168–76. doi: 10.1016/j.clinthera.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 34.Lavine JE, Schwimmer JB, Van Natta ML, et al. Effect of vitamin E or metformin for treatment of nonalcoholic fatty liver disease in children and adolescents: the TONIC randomized controlled trial. JAMA. 2011;305:1659–68. doi: 10.1001/jama.2011.520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Nobili V, Bedogni G, Alisi A, et al. Docosahexaenoic acid supplementation decreases liver fat content in children with non-alcoholic fatty liver disease: double-blind randomized controlled clinical trial. Arch Dis Child. 2011;96:350–3. doi: 10.1136/adc.2010.192401. [DOI] [PubMed] [Google Scholar]
- 36.Nobili V, Carpino G, Alisi A, et al. Role of docosahexaenoic Acid treatment in improving liver histology in pediatric nonalcoholic Fatty liver disease. PLoS ONE. 2014;9:e88005. doi: 10.1371/journal.pone.0088005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Compare D, Coccoli P, Rocco A, et al. Gut–liver axis: the impact of gut microbiota on non alcoholic fatty liver disease. Nutr Metab Cardiovasc Dis. 2012;22:471–6. doi: 10.1016/j.numecd.2012.02.007. [DOI] [PubMed] [Google Scholar]
- 38.Aron-Wisnewsky J, Gaborit JB, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19:338–48. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 39.Spencer MD, Hamp TJ, Reid RW, Fischer LM, Zeisel SH, Fodor AA. Association between composition of the human gastrointestinal microbiome and development of fatty liver with choline deficiency. Gastroenterology. 2011;40:976–86. doi: 10.1053/j.gastro.2010.11.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Brown K, DeCoffe D, Molcan E, Gibson DL. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients. 2012;4:1095–119. doi: 10.3390/nu4081095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Mehal WZ. The Gordian Knot of dysbiosis, obesity and NAFLD. Nat Rev Gastroenterol Hepatol. 2013;10:637–44. doi: 10.1038/nrgastro.2013.146. [DOI] [PubMed] [Google Scholar]
- 42.Iacono A, Raso GM, Canani RB, Calignano A, Meli R. Probiotics as an emerging therapeutic strategy to treat NAFLD: focus on molecular and biochemical mechanisms. J Nutr Biochem. 2011;22:699–711. doi: 10.1016/j.jnutbio.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 43.Wong VW, Won GL, Chim AM, et al. Treatment of nonalcoholic steatohepatitis with probiotics. A proof-of-concept study. Ann Hepatol. 2013;12:256–62. [PubMed] [Google Scholar]
- 44.Vajro P, Mandato C, Licenziati MR, et al. Effects of Lactobacillus rhamnosus strain GG in pediatric obesity-related liver disease. J Pediatr Gastroenterol Nutr. 2011;52:740–3. doi: 10.1097/MPG.0b013e31821f9b85. [DOI] [PubMed] [Google Scholar]
- 45.Loguercio C, Federico A, Tuccillo C, et al. Beneficial effects of a probiotic VSL#3 on parameters of liver dysfunction in chronic liver diseases. J Clin Gastroenterol. 2005;39:540–3. doi: 10.1097/01.mcg.0000165671.25272.0f. [DOI] [PubMed] [Google Scholar]
- 46.Gallwitz B. Emerging DPP-4 inhibitors: focus on linagliptin for type 2 diabetes. Diabetes Metab Syndr Obes. 2013;6:1–9. doi: 10.2147/DMSO.S23166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Lee J, Hong SW, Rhee EJ, Lee WY. GLP-1 Receptor Agonist and Non-Alcoholic Fatty Liver Disease. Diabetes Metab J. 2012;36:262–7. doi: 10.4093/dmj.2012.36.4.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Suzuki D, Toyoda M, Kimura M, et al. Effects of liraglutide, a human glucagon-like peptide-1 analogue, on body weight, body fat area and body fat-related markers in patients with type 2 diabetes mellitus. Intern Med. 2013;52:1029–34. doi: 10.2169/internalmedicine.52.8961. [DOI] [PubMed] [Google Scholar]
- 49.Kelly AS, Rudser KD, Nathan BM, et al. The effect of glucagon-like peptide-1 receptor agonist therapy on body mass index in adolescents with severe obesity: a randomized, placebo-controlled, clinical trial. JAMA Pediatr. 2013;167:355–60. doi: 10.1001/jamapediatrics.2013.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]