Abstract
One hundred clinical isolates of Candida albicans were tested for amphotericin B and fluconazole susceptibilities by the National Committee for Clinical Laboratory Standards (NCCLS) broth microdilution test at center 1 (C1). The same isolates were tested blinded at center 2 (C2) by NCCLS and flow cytometry (FC) methods. The agreement between NCCLS and FC methods ranged from 96 to 99%.
In 2002, the National Committee for Clinical Laboratory Standards (NCCLS) published a standardized method for antifungal susceptibility testing of Candida spp. and Cryptococcus neoformans (M27-A2 [7]). This is a reliable method for susceptibility testing of yeasts for interlaboratory correlation; standardization of variables like medium, inoculum size, MIC endpoints, and temperature and duration of incubation; and correlation of the MICs (fluconazole and itraconazole) with clinical outcome in candidiasis (14). However, this method is somewhat labor-intensive, unreliable for detection of amphotericin B resistance, and prone to the trailing-growth phenomenon with azole antifungals (9, 14). Alternative methods such as spectrophotometry (1, 3), colorimetry (10, 12), agar-based assay (2, 11), and flow cytometry (FC) (4, 5, 6, 8, 13, 17) are being evaluated. The objectives of the present study were to evaluate the FC method by using isolates previously tested in another laboratory and to determine interlaboratory agreements between center 1 (C1; University of Iowa College of Medicine, Iowa City) and center 2 (C2; Mycology Laboratory, Wadsworth Center, Albany, N.Y.).
One hundred Candida albicans isolates were received blinded at C2 from C1. These isolates were routinely tested by the NCCLS M27-A2 protocol at C1. The cultures were maintained in sterile water at 4°C. Before the assays, the cultures were passaged twice on Sabouraud dextrose agar at 35°C. Amphotericin B was purchased from Sigma (St. Louis, Mo.), and fluconazole was a gift from Pfizer Pharmaceuticals (New York, N.Y.). Stock solutions of amphotericin B and fluconazole were prepared in dimethyl sulfoxide (Amresco, Solon, Ohio) and water, respectively, and were stored at −70°C. The broth microdilution test for amphotericin B (0.03 to 16.0 μg/ml) and fluconazole (0.06 to 64.0 μg/ml) was done according to the NCCLS M27-A protocol (7). The endpoints were a 100% optically clear well for amphotericin B and 50% growth inhibition compared to drug-free wells for fluconazole (7).
The FC assay was performed as described earlier (13). Briefly, serial twofold dilutions of two drugs were prepared in RPMI 1640 containing l-glutamine without bicarbonate, buffered to pH 7.0 with MOPS (morpholinepropanesulfonic acid). Yeast suspensions were prepared in 0.85% sterile saline and adjusted spectrophotometrically to match an 0.5 McFarland density. One-half milliliter of the yeast suspension was added to 0.5 ml of serial drug dilutions and incubated at 35°C for 2 h for amphotericin B and 4 h for fluconazole according to the rationale provided in an earlier publication from our laboratory (13). The growth control tube contained no drug. At the end of incubation, 200 μl of each mixture was placed in 12- by 75-mm polystyrene tubes. Two hundred microliters of 25 mM sodium deoxycholate (Sigma) and 10 μl of propidium iodide (PI; 1 μg/ml; Molecular Probes Inc., Eugene, Oreg.) were added, and the tubes were mixed by flicking with fingers. The samples were analyzed with a FACScan flow cytometer (Becton Dickinson, Lincoln, N.J.) and CellQuest software. Ten thousand yeast cells were analyzed for forward scatter (3.73 linear gain), side scatter (270-V log), log of red fluorescence, FL2 (457-V log), threshold value 52, and mean channel fluorescence (MCF; intensity of fluorescence of yeasts labeled withPI). The MIC was the lowest concentration of drug that produced a 50% increase in MCF compared to growth control. Candida parapsilosis ATCC 22019 and Candida krusei ATCC 6258 were included in each test run as controls.
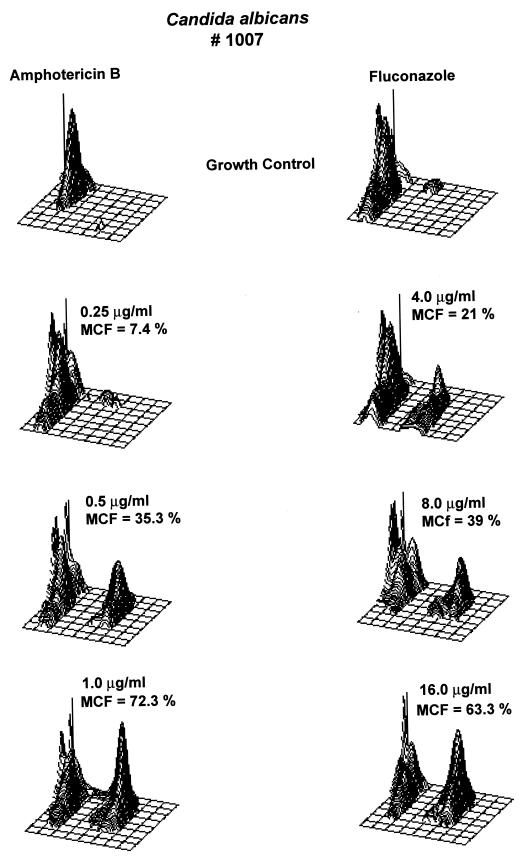
The geometric mean MICs of amphotericin B were similar at C1 and at C2 (Table 1). However, the geometric mean MIC of fluconazole was higher (2.12 μg/ml) at C1 than at C2 (1.11 μg/ml). C1 (NCCLS) and C2 (FC) results were 96% (±1 dilution) and 99% (±2 dilutions) in agreement for amphotericin B and 86% (±1 dilution) and 96% (±2 dilutions) in agreement for fluconazole. Table 2 shows the comparison of isolates classified as susceptible, susceptible dose-dependent, and resistant by the NCCLS criteria for fluconazole at C1 and C2. Representative three-dimensional plots of C. albicans (isolate 1007) for amphotericin B (MIC = 1.0 μg/ml) and fluconazole (MIC = 16.0 μg/ml) are illustrated in Fig. 1.
TABLE 1.
Antifungal susceptibility testing of C. albicans against amphotericin B and fluconazole at C1 and C2
| Drug | MIC (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| C1-NCCLS
|
C2-NCCLS
|
C2-FC
|
||||
| Range | Geomet- ric mean | Range | Geomet- ric mean | Range | Geomet- ric mean | |
| Amphotericin B | 0.5-2.0 | 0.986 | 0.12-1.0 | 0.676 | 0.12-2.0 | 0.841 |
| Fluconazole | 0.12->64.0 | 2.12 | 0.12->64.0 | 1.24 | 0.25->64.0 | 1.11 |
TABLE 2.
Interlaboratory agreement of fluconazole resultsa
| C1 | No. of C. albicans isolates
|
|||||||
|---|---|---|---|---|---|---|---|---|
| C2-NCCLS
|
2-FC
|
|||||||
| S | S-DD | R | Total | S | S-DD | R | Total | |
| S | 66 | 2 | 68 | 67 | 1 | 68 | ||
| S-DD | 4 | 4 | 8 | 3 | 4 | 1 | 8 | |
| R | 4 | 2 | 18 | 24 | 4 | 5 | 15 | 24 |
| Total | 74 | 10 | 18 | 100 | 74 | 10 | 16 | 100 |
S, susceptible; S-DD, susceptible dose-dependent; R, resistant.
FIG. 1.
Three-dimensional plots illustrating the increase in MCF of C. albicans (isolate 1007) for amphotericin B and fluconazole. The MIC of amphotericin B was 1.0 μg/ml; the MCF increased from 35.3% at 0.5 μg/ml to 72.3% at 1.0 μg/ml of amphotericin B. The MIC of fluconazole was 16.0 μg/ml; the MCF increased from 39% at 8.0 μg/ml to 63.3% at 16.0 μg/ml of fluconazole.
The results of this study revealed that the level of interlaboratory agreement between the NCCLS broth microdilution and FC methods is essentially the same as the level of agreement between C1 and C2 by the NCCLS broth microdilution method. For most isolates, the difference in the MICs determined by the two procedures was minimal. However, four isolates showed poor correlation for fluconazole MICs. Probably, this difference could be due to the trailing-growth phenomenon reported elsewhere (1), although we have no data to support this inference. It has been reported that about 18.2% of C. albicans isolates exhibit trailing growth against fluconazole (1). However, the trailing effect is usually minimal in the FC method, due to its shorter incubation and cumulative analysis of individual cells (13). A number of other methods, such as variations of agar diffusion tests (including Etest and disk diffusion), have demonstrated a good correlation with the NCCLS M27-A procedure (2, 11). Similarly, colorimetric assay with Alamar blue indicator (Sensititre YeastOne) and spectrophotometric determinations of ergosterol content and spectrophotometric determinations of growth (EUCAST method) are all potentially promising (1, 3, 10, 12, 14).
Previously, FC studies of Candida spp. have reported results comparable to those from the NCCLS broth microdilution method, but the number of isolates tested was low, ranging from 2 to 20 (5, 6, 17). Green and coworkers used PI to obtain MICs in 3.5 h, which showed good agreement between NCCLS and FC methods (5). Peyron and coworkers reported the comparison of NCCLS and FC susceptibilities of Candida spp. to amphotericin B through regression analysis (8). Favel and coworkers compared the FC method with Etest and NCCLS broth macrodilution for amphotericin B through regression analysis; they also reported better correlation between FC and Etest (4). The present study extends these observations, by demonstrating overall good intra- and interlaboratory agreement between the NCCLS and FC methods for C. albicans amphotericin B and fluconazole tests. FC still remains an esoteric technology possibly due to high capital costs and the need for specialized personnel. A number of refinements might overcome these obstacles to allow realization of the full potential of FC methods in routine testing (15, 16).
Acknowledgments
We thank Andrea Doney of the Mycology Laboratory, Wadsworth Center, and S. A. Messer of the Fungus Testing Laboratory, University of Iowa College of Medicine, for excellent technical assistance. We thank Robert Dilwith of the Immunology Core, Wadsworth Center, for his skillful operation of the flow cytometer.
This study was supported in part with an educational award from Pfizer, Inc. (V.C.).
REFERENCES
- 1.Arthington-Skaggs, B. A., W. Lee-Yang, M. A. Ciblak, J. P. Frade, M. E. Brandt, R. A. Hajjeh, L. H. Harrison, A. N. Sofair, and D. W. Warnock. 2002. Comparison of visual and spectrophotometric methods of broth microdilution MIC end point determination and evaluation of a sterol quantitation method for in vitro susceptibility testing of fluconazole and itraconazole against trailing and nontrailing Candida isolates. Antimicrob. Agents Chemother. 46:2477-2481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barry, A. L., and S. D. Brown. 1996. Fluconazole disk diffusion procedure for determining susceptibility of Candida species. J. Clin. Microbiol. 34:2154-2157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Clancy, C. J., and M. H. Nguyen. 1997. Comparison of a photometric method with standardized methods of antifungal susceptibility testing of yeasts. J. Clin. Microbiol. 35:2878-2882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Favel, A., F. Peyron, M. De Meo, A. Michel-Nguyen, J. Carriere, C. Chastin, and P. Regli. 1999. Amphotericin B susceptibility testing of Candida lusitaniae isolates by flow cytofluorometry: comparison with the Etest and the NCCLS broth macrodilution method. J. Antimicrob. Chemother. 43:227-232. [DOI] [PubMed] [Google Scholar]
- 5.Green, L., B. Petersen, L. Steimel, P. Haeber, and W. Current. 1994. Rapid determination of antifungal activity by flow cytometry. J. Clin. Microbiol. 32:1088-1091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kirk, S. M., S. M. Callister, L. C. Lim, and R. F. Schell. 1997. Rapid susceptibility testing of Candida albicans by flow cytometry. J. Clin. Microbiol. 35:358-363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 8.Peyron, F., A. Favel, H. Guiraud-Dauriac, M. El Mzibri, C. Chastin, G. Dumenil, and P. Regli. 1997. Evaluation of a flow cytofluorometric method for rapid determination of amphotericin B susceptibility of yeast isolates. Antimicrob. Agents Chemother. 41:1537-1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller, M. A., and W. L. Yu. 2001. Antifungal susceptibility testing. New technology and clinical applications. Infect. Dis. Clin. N. Am. 15:1227-1261. [DOI] [PubMed] [Google Scholar]
- 10.Pfaller, M. A., S. Arikan, M. Lozano-Chiu, Y. Chen, S. Coffman, S. A. Messer, R. Rennie, C. Sand, T. Heffner, J. H. Rex, J. Wang, and N. Yamane. 1998. Clinical evaluation of the ASTY colorimetric microdilution panel for antifungal susceptibility testing. J. Clin. Microbiol. 36:2609-2612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pfaller, M. A., S. A. Messer, A. Bolmstrom, F. C. Odds, and J. H. Rex. 1996. Multisite reproducibility of the Etest MIC method for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 34:1691-1693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pfaller, M. A., and A. L. Barry. 1994. Evaluation of a novel colorimetric broth microdilution method for antifungal susceptibility testing of yeast isolates. J. Clin. Microbiol. 32:1992-1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ramani, R., and V. Chaturvedi. 2000. Flow cytometry antifungal susceptibility testing of pathogenic yeasts other than Candida albicans and comparison with the NCCLS broth microdilution test. Antimicrob. Agents Chemother. 44:2752-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shapiro, H. M. 2001. Microbiology. Clin. Lab. Med. 21:897-909. [PubMed] [Google Scholar]
- 16.Veal, D. A., D. Deere, B. Ferrari, J. Piper, and P. V. Attfield. 2000. Fluorescence staining and flow cytometry for monitoring microbial cells. J. Immunol. Methods 243:191-210. [DOI] [PubMed] [Google Scholar]
- 17.Wenisch, C., K. F. Linnau, B. Parschalk, K. Zedtwitz-Liebenstein, and A. Georgopoulos. 1997. Rapid susceptibility testing of fungi by flow cytometry using vital staining. J. Clin. Microbiol. 35:5-10. [DOI] [PMC free article] [PubMed] [Google Scholar]