Abstract
Pseudomonas aeruginosa is the predominant cause of chronic airway infection in cystic fibrosis (CF). CF airway isolates are often tested for antibiotic susceptibility but are rarely eradicated by the antibiotics identified as potentially effective. The growth state of P. aeruginosa in CF airways is probably different from that exhibited under conventional susceptibility testing conditions and may represent a bacterial biofilm. Biofilm susceptibility testing methods were adapted to create an assay for implementation in a clinical microbiology laboratory. This assay gave reproducible results when examined in 300 paired determinations with 12 antimicrobial agents, with a serious error rate of 5.7%. The biofilm assay was used retrospectively to test these 12 agents against 94 isolates from 41 CF patients. The biofilm inhibitory concentrations (BICs) were much higher than the corresponding conventionally determined MICs for the β-lactam antibiotics (median values: aztreonam, >128 μg/ml versus 4 μg/ml; ceftazidime, 128 μg/ml versus 2 μg/ml; piperacillin-tazobactam, 256 μg/ml versus 4 μg/ml; and ticarcillin-clavulanate, 512 μg/ml versus 16 μg/ml, respectively) and doxycycline (>64 μg/ml versus 16 μg/ml); and similar for meropenem (4 μg/ml versus ≤ 1 μg/ml), ciprofloxacin (0.5 μg/ml versus 1 μg/ml), and the aminoglycosides amikacin (32 μg/ml versus 16 μg/ml), gentamicin (16 μg/ml versus 8 μg/ml), and tobramycin (4 μg/ml versus 2 μg/ml). The median BIC for azithromycin was 2 μg/ml, whereas isolates were uniformly resistant when tested by standard methods. This demonstrates the feasibility of adapting biofilm susceptibility methods to the clinical microbiology laboratory and opens the way to examining whether biofilm testing might be used to select more effective antibiotic combinations for CF airway infections than methods in current use.
Chronic Pseudomonas aeruginosa airway infections remain the primary cause of morbidity and mortality in the cystic fibrosis (CF) population (22). Young children with CF may be infected as early as 6 months of age, based on cultures of bronchoalveolar lavage fluid and positive serology (3, 6, 26, 35), and P. aeruginosa becomes chronic in the first decade of life with pulmonary exacerbations increasing in frequency (10). Antibiotic therapy, directed by standardized susceptibility testing, has traditionally been used to treat symptomatic CF patients with chronic infection (22, 29). In the setting of pulmonary exacerbation, antipseudomonal therapy, frequently consisting of a β-lactam and an aminoglycoside, may result in clinical improvement and a decrease in bacterial burden; however, eradication of infection is quite rare.
Despite recommendations for the use of standard susceptibility testing in CF (29), the methods used for this testing were developed specifically to direct therapy for the treatment of acute bloodstream and urinary tract infections and have only been fully validated for these infections. The clinical utility of standard susceptibility testing in CF has been called into question based on data recently reported by Smith et al. (32). This is not surprising since CF chronic airway infections differ significantly from acute bloodstream and urinary tract infections; thus, it seems reasonable that testing strategies should reflect these differences.
Bacteria in CF airways appear to be very slow growing or even in stationary phase, are likely to be in an anaerobic or microaerophilic environment (39), and may even constitute a biofilm (11, 31). Bacterial biofilms are inherently resistant to antimicrobial agents, and sessile cells are much less susceptible than when growing planktonically (18, 20, 34). These findings are highly reminiscent of CF airway infections (21). Thus, the inability to eradicate P. aeruginosa chronic infections in CF may result, in part, from treatment regimens based on susceptibility testing that only poorly reflects the growth state of bacteria in CF airways. The response to antibiotic exposure of stationary-phase, microaerophilic bacteria growing as biofilms in the clinical laboratory may better predict the response of CF patients to antimicrobial therapy.
The present study was performed to examine the susceptibility of a large number of CF clinical isolates of P. aeruginosa growing as biofilms and to compare these results with standard MIC determinations. Evidence of in vitro antibiofilm activity and of different susceptibility patterns by the two methods would support further examination of the clinical utility of biofilm susceptibility testing in CF.
MATERIALS AND METHODS
Bacterial strains.
P. aeruginosa isolates (n = 94) were studied from 41 patients of the Cystic Fibrosis Center at Children's Hospital and Regional Medical Center, in Seattle, Wash. Approval for the study was obtained from the Institutional Review Board. The subjects met the following eligibility criteria: a diagnosis of CF; age of ≥ 6 years; maximum forced expiratory volume in 1 s of ≤ 90% predicted during the 12 months prior to culture; density of most abundant isolate of P. aeruginosa in sputum of ≥ 105 CFU/g of sputum; and no history of Burkholderia cepacia airway infection. Multiple colonial morphotypes were obtained from a single sputum sample from each subject at clinical baseline as part of routine CF care, cultured quantitatively by standard clinical microbiological methods (5), and stored at −80°C. Only those isolates with a density ≥ 1% that of the most abundant morphotype were tested, with a mean of 2.3 isolates per patient (range, 1 to 5) meeting this criterion. Strains used for quality control of antibiotic panels included strains ATCC 29212, ATCC 29213, and ATCC 25922 from the American Type Culture Collection.
Chemicals and bacterial media.
Bacterial strains were grown in minimal broth medium (M63) with 1 mM MgSO4 and 0.4% (wt/vol) arginine or in cation-adjusted Mueller-Hinton broth (CAMHB) with or without 0.4% arginine. Antibiotics were pharmaceutical grade.
Biofilm susceptibility assay.
Biofilm assays were generally performed as previously described (1, 9, 34), with variations introduced and tested at key steps to make the procedure more compatible with routine clinical microbiology laboratory practices. In addition, more established biofilms were encouraged through longer incubation periods, and specific modifications were implemented to accommodate the standard work cycle in a clinical microbiology laboratory, such as longer antibiotic exposures and automated reading of bacterial densities with a standard microtiter plate reader.
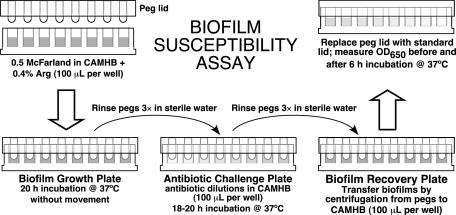
Isolates were passed twice on tryptic soy agar with 5% sheep blood after retrieval from −80°C storage and then grown overnight in CAMHB or minimal medium. After dilution of this culture to 0.5 McFarland in broth medium, 100 μl was transferred to all but the negative control wells of a flat-bottom 96-well microtiter plate (catalog no. 269787; Nalgene Nunc International, Rochester, N.Y.). Bacterial biofilms were formed by immersing the pegs of a modified polystyrene microtiter lid (catalog no. 445497; Nunc TSP system) into this biofilm growth plate (Fig. 1), followed by incubation at 37°C for 20 h with either rocking at 20Hz or no movement.
FIG. 1.
Biofilm susceptibility assay. See the text for details.
Peg lids were rinsed three times in sterile water, placed onto flat-bottom microtiter plates containing antibiotic twofold dilutions in 100 μl of CAMHB per well (antibiotic challenge plate), and incubated for 18 to 20 h at 37°C. Twelve antibiotics with known clinical efficacy in P. aeruginosa -infected CF patients were tested against all isolates (Table 1, group 1). Nine additional antibiotics were selected that are not commonly used in the United States for the treatment of P. aeruginosa in CF (Table 1, group 2). The group 2 agents were tested against a subset of 20 strains.
TABLE 1.
Antibiotics tested
| Antibiotic (no. of strains tested) | Concn range (μg/ml)
|
|
|---|---|---|
| BIC test | MIC test | |
| Group 1 agents (94) | ||
| Amikacin | 4-256 | 0.5-128 |
| Azithromycin | 0.5-32 | NAa |
| Aztreonam | 2-128 | 1-128 |
| Ceftazidime | 2-128 | 0.5-64 |
| Ciprofloxacin | 0.25-16 | 0.12-8 |
| Clarithromycin | 0.5-32 | NA |
| Doxycycline | 1-64 | 0.25-32 |
| Gentamicin | 1-64 | 1-64 |
| Meropenem | 1-64 | 0.5-32 |
| Piperacillin-tazobactamb | 16-512 | 0.5-1024 |
| Ticarcillin-clavulanateb | 16-512 | 2-4096 |
| Tobramycin | 1-64 | 1-512 |
| Group 2 agents (20) | ||
| Cefepime | 1-256 | 0.5-64 |
| Cefoxitin | 0.25-64 | 0.25-64 |
| Chloramphenicol | 1-256 | 2-64 |
| Clindamycin | 0.0625-16 | 0.0625-16 |
| Colistin | 0.5-128 | 0.5-128 |
| Levofloxacin | 0.25-64 | 0.25-64 |
| Rifampin | 0.125-32 | 0.125-32 |
| Trimethoprim-sulfamethoxazolec | 0.25-64 | 0.25-64 |
| Vancomycin | 0.5-128 | 0.5-128 |
NA, not available. MIC testing was not performed.
For standard susceptibility testing (MIC) of the β-lactam and β-lactamase inhibitor combinations, only the β-lactam (without inhibitor) was tested. However, the MIC values should predict results for the combination.
Concentration represents the sulfamethoxazole component.
After antibiotic incubation, peg lids were again rinsed three times in sterile water and placed into antibiotic-free CAMHB in a flat-bottom microtiter plate (biofilm recovery plate, Fig. 1). To transfer biofilms from pegs to wells, each plate was sonicated at room temperature for 5 min (using a Bransonic 220; Branson Co., Shelton, Conn.) or centrifuged at 805 × g for 20 min. (Relatively low centrifugation speeds were used because microtiter plates tended to fracture at higher speeds, e.g., 1,811 × g.) The peg lid was discarded and replaced by a standard lid. The optical density at 650 nm (OD650) was measured on a microtiter plate colorimeter (Spectramax 190 Gemini ELISA plate reader; Molecular Devices Corp., Sunnyvale, Calif.) before and after incubation at 37°C for 6 h. Adequate biofilm growth for the positive control wells was defined as a mean OD650 difference (OD650 at 6 h minus the OD650 at 0 h) that is ≥ 0.05. The biofilm inhibitory concentrations (BICs) were defined as the lowest concentrations of drug that resulted in an OD650 difference at or below 10% of the mean of two positive control well readings. The 10% cutoff represents a 1-log10 difference in growth after 6 h of incubation.
The final assay conditions used for large-scale analysis of isolates, selected based on results of pilot experiments, were (i) overnight growth in CAMHB, (ii) dilution in CAMHB with 0.4% arginine, (iii) stationary overnight incubation of the growth plate, and (iv) transfer to the recovery plate by centrifugation.
Biofilm growth assays.
To assess the adequacy of growth, mature biofilms grown on pegs in the biofilm growth plates were rinsed, placed in a 0.1% (wt/vol) crystal violet solution for 15 min, rinsed again, and dried for several hours. To solubilize adsorbed crystal violet, pegs with stained biofilms were incubated in 95% ethanol (150 μl per well of a flat-bottom microtiter plate) for 15 min. The absorbance was read at 590 nm on a plate reader as described above. Alternatively, to determine CFU, pegs with mature biofilms were rinsed, placed into CAMHB with 0.1% Triton X-100 (100 μl per well of a flat-bottom microtiter plate), and sonicated for 5 min. Bacterial dilutions from each well were plated for enumeration. For experiments comparing alternative methods of transferring biofilms from pegs to media, peg lids with mature biofilms were placed onto flat-bottom 96-well plates with 100 μl of CAMHB in each well and either sonicated for 5 min or centrifuged at 805 × g for 20 min, followed by quantitation as described above.
Standard susceptibility assay.
MICs of the group 1 drugs indicated in Table 1 were determined for each isolate during clinical testing by using the semiautomated broth microdilution Sensititre System (AccuMed, Westlake, Ohio) (5). MICs of the group 2 drugs were determined in parallel with biofilm testing.
Data analysis.
Twelve P. aeruginosa isolates were tested in replicate (n = 8) to evaluate variations in biofilm assay conditions. For comparisons of two conditions, differences were evaluated by paired t test of mean replicate measurements. For comparisons of more than two conditions, repeated measures regression methods with robust variance estimates were used, followed by Wald tests to assess differences between pairs of conditions.
In order to assess reproducibility, biofilm assays were performed in duplicate with 25 isolates from 10 patients with the 12 group 1 antibiotics for a total of 300 possible paired determinations. The results of each pair of determinations were compared to determine interassay reproducibility (7, 8). “Serious errors” were defined as those that would result in misclassification of a resistant isolate as susceptible or vice versa, whereas “minor errors” were defined as those that would result in misclassification of an intermediate isolate as susceptible or resistant (18). For comparison of BIC and MIC results, the respective 50th percentile values (BIC50 and MIC50) and 90th percentile values (BIC90 and MIC90) were calculated, and ranges were compared.
RESULTS
Assay development.
In order to adapt previous biofilm assay methods to the clinical laboratory, as well as to mimic conditions in the CF lung more closely, several modifications of these methods were tested. Twelve CF P. aeruginosa isolates were used for this testing, six of which were mucoid.
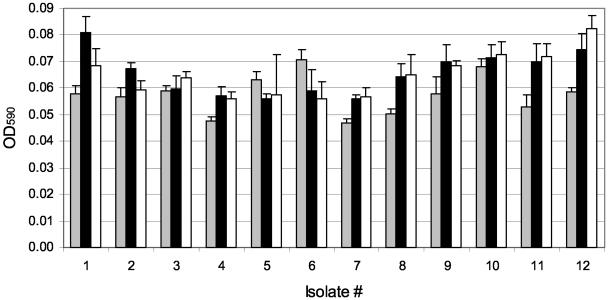
To test the effect of growth medium on biofilm formation, biofilms were grown on pegs for 20 h in three different medium formulations. A minimal medium broth supplemented with 0.4% arginine (which P. aeruginosa can utilize as an a terminal electron acceptor under hypoxic conditions) was compared to CAMHB (the NCCLS-approved medium for susceptibility testing). As shown in Fig. 2, CAMHB or CAMHB with 0.4% arginine resulted in more luxuriant biofilm growth than with minimal medium for the majority of isolates tested.
FIG. 2.
Effect of media on biofilm growth. Biofilms of 12 P. aeruginosa CF isolates were grown on pegs in three different media (M63 minimal medium plus 0.4% arginine, gray bars; CAMHB, solid bars; and CAMHB plus 0.4% arginine, open bars) and stained with crystal violet. Bars represent the mean OD590 values, and error bars represent the standard deviations. Isolates 1 to 6 were nonmucoid, and isolates 7 to 12 were mucoid, with eight replicates tested per isolate. The P values from pairwise comparisons included the following (unadjusted for multiple comparisons): CAMHB versus minimal (P = 0.02), CAMHB plus arginine versus minimal (P = 0.04), and CAMHB versus CAMHB plus arginine (P = 0.63).
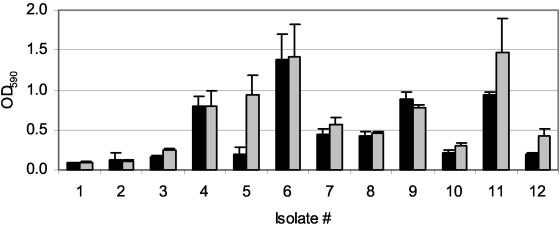
Biofilms are often grown in the presence of shear stress. In CF, the mucus rheological properties and mucociliary clearance are markedly altered. Thus, the extent to which biofilms in CF airways are exposed to shear forces is not known. To test the effect of shear stress on biofilm growth in this assay system, the formation of biofilms grown without movement of the surrounding medium was compared to those exposed to rocking at 20 Hz. Biofilm growth in CAMHB under each of these conditions was quantified by crystal violet staining. Incubation of the growth plates without motion consistently resulted in comparable growth compared to incubation in the presence of shear stress (Fig. 3).
FIG. 3.
Comparison of sheer stress versus static culture. P. aeruginosa biofilms of 12 isolates were grown under sheer stress (solid bars) and statically (gray bars), and bacteria on pegs were stained with crystal violet. Each value represents the mean OD590 and standard deviation of eight replicates of each isolate (P = 0.07).
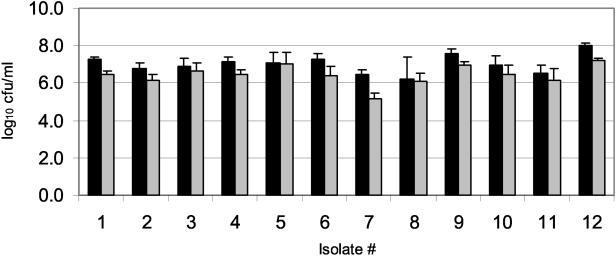
Several susceptibility protocols call for removing biofilms from the pegs by sonication for 5 min (9, 34). Although this removal method is effective, it is relatively impractical in the clinical laboratory setting, where multiple isolates must be processed simultaneously; most bath sonicators can only hold two plates, and it is possible that the water from the bath might enter and contaminate the plates. Thus, we evaluated centrifugation at 805 × g for 20 min as an alternative method of biofilm removal. Initially, these removal methods were compared by crystal violet staining of biofilm material remaining on pegs after processing. Centrifugation and sonication left comparable amounts on the pegs (results not shown). To examine this further, the two methods were compared quantitatively by counting CFU in recovery wells. Centrifugation at 805 × g for 20 min and sonication for 5 min gave similar results (Fig. 4) in that both methods were reproducible, with little variability. However, the mean CFU/well was 0.6 log10 lower for centrifugation compared to sonication. Whether this difference in bacterial recovery could translate to altered biofilm susceptibility and thus to altered drug selection in the clinical setting is unknown.
FIG. 4.
Comparison of sonication versus centrifugation. Quantification of bacteria removed from the pegs by sonication for 5 min (solid bars) and centrifugation for 20 min at 805 × g (gray bars), with the same set of 12 isolates examined in Fig. 3. Each value represents the mean log10 CFU/peg and standard deviation of eight replicates of each isolate. On average, the density was 0.6 log10 higher with sonication than with centrifugation (P = 0.0001). The 95% confidence interval for the log10 difference in density was 0.4 to 0.8.
To determine whether the removal method affects susceptibility results, a P. aeruginosa quality control strain was tested. Comparison of BIC values for 12 antibiotics against strain ATCC 27853 by each removal method demonstrated that 11 of 12 pairs of values were within one twofold dilution, and seven pairs were identical. The twelfth pair of values, for meropenem, was within two twofold dilutions, and both were consistent with susceptibility of the strain, based on NCCLS breakpoints (19).
In addition to an assessment of the effects of antibiotic exposure, the adequacy of biofilm formation was examined in the growth plates. Nonantibiotic exposed pegs were used as growth controls. Bacteria were centrifuged from pegs after antibiotic exposure and washing and growth over 6 h determined by OD650. The range of OD650 differences in the nonantibiotic growth controls was 0 to 0.3547 (mean, 0.1394), with 74% of isolates above the cutoff for adequate biofilm growth (0.05).
Reproducibility of BIC testing.
To determine reproducibility, the biofilm susceptibilities to the 12 group 1 agents were determined in duplicate for 25 isolates from nine of the subjects. Interpretable data were obtained for 260 of 300 isolate-antibiotic pairs; data for the remaining pairs was not available due to out-of-range values for quality control strains (n = 12 pairs, all with ticarcillin-clavulanate) or uninterpretable results for one of the pair (n = 28 pairs). The reproducibility for each pair of BIC determinations and the NCCLS-defined error rates were determined (Table 2). Overall, 87% of the paired BIC values were within two dilutions of each other, with the most reproducible results for doxycycline and tobramycin (100%) and the lowest results for meropenem (59%). No serious errors were identified for aztreonam, ciprofloxacin, clarithromycin, doxycycline, ticarcillin-clavulanate, and tobramycin. The highest percentages of serious errors were for piperacillin-tazobactam (20.0%) and meropenem (18.2%). The serious error rate for all agents was 5.7% overall.
TABLE 2.
Error rates by duplicate assay discordance for group 1 agents
| Antibiotic (no. of strains)a | No. (%)
|
||
|---|---|---|---|
| ±2 dilutions | Serious errors | Minor errors | |
| Amikacin (24) | 23 (96) | 1 (4.2) | 4 (16.6) |
| Azithromycin (25) | 21 (84) | 2 (8.0) | 1 (4.0) |
| Aztreonam (18) | 17 (94) | 0 | 0 |
| Ceftazidime (23) | 18 (78) | 2 (8.7) | 3 (13.0) |
| Ciprofloxacin (23) | 19 (83) | 0 | 1 (4.3) |
| Clarithromycin (22) | 20 (91) | 0 | 1 (4.5) |
| Doxycycline (22) | 22 (100) | 0 | 0 |
| Gentamicin (23) | 21 (91) | 1 (4.3) | 7 (30.4) |
| Meropenem (22) | 13 (59) | 4 (18.1) | 3 (13.6) |
| Piperacillin-tazobactam (20) | 16 (80) | 5 (20.0) | NAb |
| Ticarcillin-clavulanate (13) | 12 (92) | 0 | NA |
| Tobramycin (25) | 25 (100) | 0 | 4 (16.0) |
| Total (260) | 227 (87) | 15 (5.7) | 24 (9.2) |
A total of 25 strains were tested for each agent (possible 300 paired determinations).
NA, not applicable. There is no intermediate category for ticarcillin-clavulanate and piperacillin-tazobactam; thus, all errors are serious.
Comparison of MIC and BIC values.
BICs for the 12 group 1 antibiotics were determined for 94 clinical isolates of P. aeruginosa from 41 patients with CF by biofilm testing. Table 3 presents the MIC50, MIC90, BIC50, and BIC90 for these isolates. Percentile-based MIC and BIC values were similar for some drugs (amikacin, ciprofloxacin, and tobramycin), whereas other drugs had BIC percentiles that were somewhat higher than their MIC percentiles (gentamicin and meropenem). The β-lactam antibiotics (aztreonam, ceftazidime, piperacillin-tazobactam, and ticarcillin-clavulanate) and doxycycline had BIC percentiles much higher than their MICs. For two drugs (azithromycin and clarithromycin), no MICs were available because P. aeruginosa are uniformly considered to be resistant in standard susceptibility testing. However, azithromycin appeared quite active against P. aeruginosa biofilms under these assay conditions.
TABLE 3.
Comparison of biofilm and standard susceptibility testing of P. aeruginosa isolates for group 1 agents
| Antibiotic (no. of strains)a | Concn (μg/ml)
|
|||||
|---|---|---|---|---|---|---|
| BIC
|
MIC
|
|||||
| BIC50 | BIC90 | Range | MIC50 | MIC90 | Range | |
| Amikacin (90) | 32 | 256 | 4->256 | 16 | 128 | ≤0.5->128 |
| Azithromycin (90) | 2 | 32 | ≤0.5->32 | NA | NA | NA |
| Aztreonam (85) | >128 | >128 | ≤2->128 | 4 | 32 | ≤2->64 |
| Ceftazidime (88) | 128 | >128 | ≤2->128 | 2 | 16 | ≤0.5-512 |
| Ciprofloxacin (90) | 0.5 | 4 | <0.25->16 | 1 | 4 | ≤0.25-16 |
| Clarithromycin (90) | 32 | >32 | ≤0.5->32 | NA | NA | NA |
| Doxycycline (86) | >64 | >64 | ≤1->64 | 16 | 32 | ≤1->32 |
| Gentamicin (90) | 16 | >64 | ≤1->64 | 8 | >32 | ≤1->32 |
| Meropenem (87) | 4 | 64 | ≤1->64 | ≤1 | 8 | ≤1-16 |
| Piperacillin-tazobactamb (85) | 256 | >512 | ≤16->512 | 4 | 128 | ≤1-1,024 |
| Ticarcillin-clavulanateb (72) | 512 | >512 | ≤16->512 | 16 | 256 | ≤2->4,096 |
| Tobramycin (92) | 4 | 32 | ≤1->64 | 2 | 32 | ≤0.25->512 |
A total of 94 strains were tested.
For standard susceptibility testing (MIC) of the β-lactam and β-lactamase inhibitor combinations, only the β-lactam (without inhibitor) was tested. However, the MIC values should predict results for the combination.
In addition to testing the antibiofilm activity of 12 agents that are widely used in the United States for the treatment of CF-associated P. aeruginosa airway infections (group 1 agents), 9 additional antimicrobial agents (group 2 agents) were tested against a subset of isolates (n = 20). This included antibiotics that lack conventional activity against P. aeruginosa but that might have antibiofilm effects (e.g., anaerobic agents), as well as antipseudomonal antibiotics that are not commonly used either because of their recent introduction to clinical practice (e.g., cefepime) or toxicity (e.g., chloramphenicol and colistin). Of these, only levofloxacin displayed significant antibiofilm activity (Table 4) comparable to that of ciprofloxacin. Cefepime displayed antipseudomonal activity in the conventional MIC assay, as expected, but like the other β-lactam antibiotics tested, displayed generally poor antibiofilm activity. Colistin also displayed conventional antipseudomonal activity but not significant antibiofilm activity, although this may have been due to inoculum size differences between the methods, an effect to which colistin susceptibility testing is sensitive.
TABLE 4.
Comparison of biofilm and standard susceptibility testing results for group 2 agents
| Drug (no. of strains)a | BIC50 (μg/ml) | BIC range (μg/ml) | MIC50 (μg/ml) | MIC range (μg/ml) |
|---|---|---|---|---|
| Cefepime (20) | >256 | 8->256 | 8 | ≤1->256 |
| Cefoxitin (18) | >64 | 32->64 | >64 | 64->64 |
| Chloramphenicol (19) | >256 | 2->256 | >256 | 32->256 |
| Clindamycin (20) | >16 | >16 | >16 | 16->16 |
| Colistin (18) | 128 | 8->128 | ≤0.5 | ≤0.5-8 |
| Levofloxacin (20) | 4 | ≤0.25-32 | 2 | ≤0.25-16 |
| Rifampin (14) | 16 | 4->32 | 8 | 0.5-16 |
| Trimethoprim-sulfamethoxazoleb (18) | >64 | 8->64 | 32 | 2->64 |
| Vancomycin (18) | >128 | 128->128 | >128 | 128->128 |
A total of 20 strains were tested.
The concentrations represent the sulfamethoxazole component of the drug.
DISCUSSION
We have utilized a previously developed technique to evaluate biofilm susceptibility testing of CF clinical isolates (1, 9, 34). Some of the growth and testing conditions that are used in antibiotic susceptibility assays deliberately reflect in vivo conditions during human infection, whereas other aspects of these methods reflect practical considerations or are purely arbitrary. Recognizing that many aspects of existing methods are arbitrary, we adapted the published methods to (i) reflect conditions in the CF lung more accurately and (ii) facilitate performance in a clinical laboratory setting. These methodological modifications appear to affect the results of the test only minimally and, importantly, improve its acceptability in the clinical microbiology laboratory.
Validation of the reproducibility of the modified methodology, as well as determination of NCCLS-defined error rates, was deemed important in considering this assay as a potential clinical test. The concordance between duplicate determinations and the observed error rates for BIC testing was similar to that previously reported for standard methods. The totals of 5.7% serious errors and 9.2% minor errors are within an acceptable range when viewed in light of our previous examination of susceptibility testing methods in CF isolates of P. aeruginosa (8). Also, as previously reported for MIC testing, the greatest numbers of serious errors for BIC testing were seen with β-lactam antibiotics, and the greatest numbers of minor errors with the aminoglycosides. Of particular note, some of the serious errors represented a difference of only two twofold dilutions.
No predominant pattern of P. aeruginosa CF biofilm susceptibility emerged among the 94 isolates tested. The ranges of BIC values for most drugs were broad, and it was not possible to select a single best antibiofilm agent or combination of agents based on biofilm susceptibility testing of this group of isolates. This indicates that the optimal combination of antibiofilm agents must be determined through antibiotic susceptibility testing of individual isolates. This is similar to the results reported for synergy testing, where it is not possible to predict the most active combinations by studying populations of organisms (28).
Studies of biofilm susceptibility testing have been performed on a small scale for years, with reports of increased clinical activity of drugs known to have activity against biofilms in both in vitro and in vivo models compared to standard therapy. Infections on indwelling devices such as intravenous, urinary, and peritoneal dialysis catheters and endotracheal tubes are perhaps the best studied (2, 30). In these studies, both standard antibiotics delivered at higher doses and agents not previously described as having antibacterial activity have been demonstrated to be effective against biofilms. In addition, there are numerous anecdotes of patients responding to therapy based on either direct biofilm susceptibility testing or extrapolated from in vitro testing of different drug classes (Howard Ceri, University of Calgary [unpublished data]).
Biofilm susceptibility testing of nearly 100 P. aeruginosa strains from individuals with CF demonstrated diminished activity of several antipseudomonal antibiotics, compared to standard in vitro susceptibility testing. With recent reports of biofilm formation in CF airway infections (11, 17, 31, 39), these data raise the possibility that current antimicrobial regimens based on standard susceptibility testing may result in suboptimal drug combinations. This may explain the failure of treatment regimens based upon standard susceptibility testing to eradicate P. aeruginosa from CF airways. Studies of young patients with CF who have recent acquisition of P. aeruginosa and of older patients with non-CF bronchiectasis have shown evidence of eradication (4, 13, 14, 24, 37). In contrast, although antibiotic therapy may improve clinical status and reduce bacterial burden by up to 2 log10 in older CF patients (23, 25, 33), efforts to eradicate P. aeruginosa in the setting of more established CF airway infections have generally failed. Among a group of CF patients with chronic persistent P. aeruginosa infection, 28 days of tobramycin inhalation decreased the geometric mean bacterial density by only 1.9 log10 CFU/g (23).
A tendency toward biofilm formation in chronic CF airway infection may also contribute to this difference in eradicability. Biofilm formation has been demonstrated in CF patients with chronic P. aeruginosa infection (31), and P. aeruginosa biofilm properties, including antibiotic resistance, have been correlated with mucoidy (15). This association is more likely related to shared regulatory control (36) than to involvement of alginate itself in biofilm formation (40). Both mucoidy and biofilm formation are associated with chronic but not transient P. aeruginosa infection of CF airways, since only 17% of CF patients have mucoid isolates in the first 3 years of life (6), whereas up to 94% of older patients may have them (5).
In contrast to the finding that many drugs with good activity in conventional testing did not have antibiofilm activity, at least one agent, azithromycin, appeared much more active against biofilm-grown P. aeruginosa. This has previously been reported in non-CF isolates (16). The antibiofilm activity of azithromycin is particularly significant in the face of recent reports of the clinical efficacy of azithromycin in CF patients with P. aeruginosa infection (12, 27, 38).
Although antibiotic susceptibility testing is recommended for CF isolates of P. aeruginosa (29) and special techniques are advised (7, 8), there are no prospective data to demonstrate the clinical efficacy of using the results to direct patient care. A recent retrospective analysis of data from the placebo group from the phase III clinical trials of inhaled tobramycin suggests the lack of utility of standard testing (32). In that study, 76 of the 262 patients in the placebo arm experienced a pulmonary exacerbation during the trial for which they received intravenous tobramycin and ceftazidime therapy. The results of standard in vitro susceptibility testing for both the most prevalent strain and the most resistant strain from each patient did not correlate with clinical improvement. These results suggest that the use of standard broth microdilution susceptibility testing to guide therapy may not improve clinical outcomes and raises the question of whether alternative methods that more accurately simulate growth conditions in the CF airway might guide therapy more effectively.
The results of the present study demonstrate marked differences between the results of susceptibility testing performed according to standard NCCLS guidelines and testing performed with biofilm-grown CF isolates of P. aeruginosa. Whether these in vitro differences will translate to improved bacterial killing in vivo and better clinical response to therapy is not known. Nonetheless, the results of the present study suggest the utility of conducting a clinical trial in CF airway infections to compare the efficacy of antibiotic therapy based on biofilm susceptibility testing with that based on standard testing.
Acknowledgments
This study was funded by a grant to S.M.M. from the Research Endowment Fund at Children's Hospital and Regional Medical Center and by the CF Research Development Program at the University of Washington.
We thank Marcella Harris for assistance with data management.
REFERENCES
- 1.Aaron, S. D., W. Ferris, K. Ramotar, K. Vandemheen, F. Chan, and R. Saginur. 2002. Single and combination antibiotic susceptibilities of planktonic, adherent, and biofilm-grown Pseudomonas aeruginosa isolates cultured from sputa of adults with cystic fibrosis. J. Clin. Microbiol. 40: 4172-4179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adair, C. G., S. P. Gorman, L. M. Byers, D. S. Jones, B. Feron, M. Crowe, H. C. Webb, G. J. McCarthy, and K. R. Milligan. 2002. Eradication of endotracheal tube biofilm by nebulized gentamicin. Intensive Care Med. 28: 426-431. [DOI] [PubMed] [Google Scholar]
- 3.Armstrong, D. S., K. Grimwood, J. B. Carlin, R. Carzino, J. P. Gutierrez, J. Hull, A. Olinsky, E. M. Phelan, C. F. Robertson, and P. D. Phelan. 1997. Lower airway inflammation in infants and young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 156: 1197-1204. [DOI] [PubMed] [Google Scholar]
- 4.Barker, A. F., L. Couch, L., S. B. Fiel, M. H. Gotfried, J. Ilowite, K. C. Meyer, A. O'Donnell, S. A. Sahn, L. J. Smith, J. O. Stewart, T. Abuan, H. Tully, J. Van Dalfsen, C. D. Wells, and J. Quan. 2000. Tobramycin solution for inhalation reduces sputum Pseudomonas aeruginosa density in bronchiectasis. Am. J. Respir. Crit. Care Med. 162: 481-485. [DOI] [PubMed] [Google Scholar]
- 5.Burns, J. L., J. Emerson, J. R. Stapp, D. L. Yim, J. Krzewinski, L. Louden, B. W. Ramsey, and C. R. Clausen. 1997. Microbiology of sputum from patients at cystic fibrosis centers in the United States. Clin. Infect. Dis. 27: 158-163. [DOI] [PubMed] [Google Scholar]
- 6.Burns, J. L., R. L. Gibson, S. McNamara, D. Yim, J. Emerson, M. Rosenfeld, P. Hiatt, K. McCoy, R. Castile, A. L. Smith, and B. W. Ramsey, B. W. 2001. Longitudinal assessment of Pseudomonas aeruginosa in young children with cystic fibrosis. J. Infect. Dis. 183: 444-452. [DOI] [PubMed] [Google Scholar]
- 7.Burns, J. L., L. Saiman, S. Whittier, J. Krzewinski, Z. Liu, D. Larone, S. A. Marshall, and R. N. Jones. 2001. Comparison of two commercial systems (Vitek and MicroScan WalkAway) for antimicrobial susceptibility testing of Pseudomonas aeruginosa isolates from cystic fibrosis patients. Diagn. Microbiol. Infect. Dis. 39: 257-260. [DOI] [PubMed] [Google Scholar]
- 8.Burns, J. L., L. Saiman, S. Whittier, D. Larone, J. Krzewinski, Z. Liu, S. A. Marshall, and R. N. Jones. 2000. Comparison of agar diffusion methodologies for antimicrobial susceptibility testing of Pseudomonas aeruginosa isolates from cystic fibrosis patients. J. Clin. Microbiol. 38: 1818-1822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ceri, H., M. E. Olson, C. Stremick, R. R. Read, D. Morck, and A. Buret. 1999. The Calgary biofilm device: new technology for rapid determination of antibiotic susceptibilities of bacterial biofilms. J. Clin. Microbiol. 37: 1771-1776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Cystic Fibrosis Foundation. 2002. Cystic Fibrosis Foundation patient registry 2001 annual data report to the center directors. Cystic Fibrosis Foundation, Bethesda, Md.
- 11.Drenkard, E., and F. M. Ausubel. 2002. Pseudomonas biofilm formation and antibiotic resistance are linked to phenotype variation. Nature 416: 740-743. [DOI] [PubMed] [Google Scholar]
- 12.Equi, A., I. M. Balfour-Lynn, A. Bush, and M. Rosenthal. 2002. Long-term azithromycin in children with cystic fibrosis: a randomized, placebo-controlled crossover trial. Lancet 360: 978-984. [DOI] [PubMed] [Google Scholar]
- 13.Frederiksen, B., C. Koch, and N. Hoiby. 1997. Antibiotic treatment of initial colonization with Pseudomonas aeruginosa postpones chronic infection and prevents deterioration of pulmonary function in cystic fibrosis. Pediatr. Pulmonol. 23: 330-335. [DOI] [PubMed] [Google Scholar]
- 14.Gibson, R. L., J. Emerson, S. McNamara, J. L. Burns, M. Rosenfeld, A. Yunker, N. Hamblett, F. Accurso, M. Dovey, P. Hiatt, M. W. Konstan, R. Moss, G. Retsch-Bogart, J. Wagener, D. Waltz, R. Wilmott, P. L. Zeitlin, B. Ramsey, et al. 2003. Significant microbiologic effect of inhaled tobramycin in young children with cystic fibrosis. Am. J. Respir. Crit. Care Med. 167: 841-849. [DOI] [PubMed] [Google Scholar]
- 15.Hentzer, M., G. M. Teitzel, G. J. Balzer, A. Heydorn, S. Molin, M. Givskov, and M. R. Parsek. 2001. Alginate overproduction affects Pseudomonas aeruginosa biofilm structure and function. J. Bacteriol. 183: 5395-5401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ichimiya, T., K. Takeoka, K. Hiramatsu, K. Hirai, T. Yamasaki, and M. Nasu. 1996. The influence of azithromycin on the biofilm formation of Pseudomonas aeruginosa in vitro. Chemotherapy 42: 186-191. [DOI] [PubMed] [Google Scholar]
- 17.Lam, J., R. Chan, K. Lam, and J. W. Costerton. 1980. Production of mucoid microcolonies by Pseudomonas aeruginosa within infected lungs in cystic fibrosis. Infect. Immun. 28: 546-556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Mah, T.-F. B. Pitts, B. Pellock, G. C. Walker, P. S. Stewart, and G. A. O'Toole. 2003. A genetic basis for Pseudomonas aeruginosa biofilm antibiotic resistance. Nature 426: 306-310. [DOI] [PubMed] [Google Scholar]
- 19.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial susceptibility testing. Approved standard M100-S13. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 20.Nickel, J. C., I. Ruseska, J. B. Wright, and J. W. Costerton. 1985. Tobramycin resistance of Pseudomonas aeruginosa cells growing as a biofilm on urinary catheter material. Antimicrob. Agents Chemother. 27: 619-624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Prince, A. S. 2002. Biofilms, antimicrobial resistance, and airway infection. N. Engl. J. Med. 347: 1110-1111. [DOI] [PubMed] [Google Scholar]
- 22.Ramsey, B. W. 1996. Management of pulmonary disease in patients with cystic fibrosis. N. Engl. J. Med. 335: 179-188. [DOI] [PubMed] [Google Scholar]
- 23.Ramsey, B. W., M. S. Pepe, J. M. Quan, K. L. Otto, A. B. Montgomery, J. Williams-Warren, M. Vasiljev-K, D. Borowitz, C. M. Bowman, B. C. Marshall, S. Marshall, and A. L. Smith. 1999. Intermittent administration of inhaled tobramycin in patients with cystic fibrosis. N. Engl. J. Med. 340: 23-30. [DOI] [PubMed] [Google Scholar]
- 24.Ratjen, F., G. Doring, and W. H. Nikolaizik. 2001. Effect of inhaled tobramycin on early Pseudomonas aeruginosa colonization in patients with cystic fibrosis. Lancet 358: 983-984. [DOI] [PubMed] [Google Scholar]
- 25.Regelmann, W. E., G. R. Elliott, W. J. Warwick, and C. C. Clawson. 1990. Reduction of sputum Pseudomonas aeruginosa density by antibiotics improves lung function in cystic fibrosis more than do bronchodilators and chest physiotherapy alone. Am. Rev. Respir. Dis. 141: 914-921. [DOI] [PubMed] [Google Scholar]
- 26.Rosenfeld, M., R. L. Gibson, S. McNamara, J. Emerson, J. L. Burns, R. Castile, P. Hiatt, K. McCoy, C. B. Wilson, A. Inglis, A. Smith, T. R. Martin, and B. W. Ramsey. 2001. Early pulmonary infection, inflammation, and clinical outcomes in infants with cystic fibrosis. Pediatr. Pulmonol. 32: 356-366. [DOI] [PubMed] [Google Scholar]
- 27.Saiman, L., B. C. Marshall, N. Mayer-Hamblett, J. L. Burns, A. L. Quittner, D. A. Cibene, S. Coquillette, A. Y. Fieberg, F. J. Accurso, P. Campbell III, et al. 2003. A multicenter, randomized placebo controlled, double-blind trial of azithromycin in patients with cystic fibrosis chronically infected with Pseudomonas aeruginosa. JAMA 290: 1749-1756. [DOI] [PubMed] [Google Scholar]
- 28.Saiman, L. F. Mehar, W. W. Niu, H. C. Neu, K. J. Shaw, G. Miller, and A. Prince. 1996. Antibiotic susceptibility of multiply resistant Pseudomonas aeruginosa isolated from patients with cystic fibrosis, including candidates for transplantation. Clin. Infect. Dis. 23: 532-537. [DOI] [PubMed] [Google Scholar]
- 29.Saiman, L., D. Schidlow, and A. Smith (ed.). 1994. Concepts in care: microbiology and infectious disease in cystic fibrosis, vol. V. Cystic Fibrosis Foundation, Bethesda, Md.
- 30.Shah, C. B., M. W. Mittelman, J. W. Costerton, S. Parenteau, M. Pelak, R. Arsenault, and L. A. Mermel. 2002. Antimicrobial activity of a novel catheter lock solution. Antimicrob. Agents Chemother. 46: 1674-1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Singh, P. K., A. L. Schaefer, M. R. Parsek, T. O. Moninger, M. J. Welsh, and E. P. Greenberg. 2000. Quorum sensing signals indicate that cystic fibrosis lungs are infected with bacterial biofilms. Nature 407: 762-764. [DOI] [PubMed] [Google Scholar]
- 32.Smith, A. L., S. B. Fiel, N. Mayer-Hamblett, B. Ramsey, and J. L. Burns. 2003. Lack of association between in vitro antibiotic susceptibility testing of Pseudomonas aeruginosa isolates and clinical response to parental antibiotic administration in cystic fibrosis. Chest 123: 1495-1502. [DOI] [PubMed] [Google Scholar]
- 33.Smith, A. L., G. Redding, C. Doershuk, D. Goldman, E. Gore, B. Hilman, M. Marks, R. Moss, B. Ramsey, and T. Rubio. 1988. Sputum changes associated with therapy for endobronchial exacerbation in cystic fibrosis. J. Pediatr. 112: 547-554. [DOI] [PubMed] [Google Scholar]
- 34.Spoering, A. L., and K. Lewis. 2001. Biofilms and planktonic cells of Pseudomonas aeruginosa have similar resistance to killing of antimicrobials. J. Bacteriol. 183: 6746-6751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.West, S. H. E., L. Zeng, B. L. Lee, M. R. Kosorok, A. Laxova, M. J. Rock, M. J. Splaingard, and P. M. Farrell. 2002. Respiratory infections with Pseudomonas aeruginosa in children with cystic fibrosis: early detection by serology and assessment of risk factors. JAMA 287: 2958-2967. [DOI] [PubMed] [Google Scholar]
- 36.Whitchurch, C. B., T. E. Erova, J. A. Emery, J. L. Sargent, J. M. Harris, A. B. Semmler, M. D. Young, J. S. Mattick, and D. J. Wozniak. 2002. Phosphorylation of the Pseudomonas aeruginosa response regulator AlgR is essential for type IV fimbria-mediated twitching motility. J. Bacteriol. 184: 4544-4554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wiesemann, H. G., G. Steinkamp, F. Ratjen, A. Bauernfeind, B. Przyklenk, G. Doring, and H. von der Hardt. 1998. Placebo-controlled, double-blind, randomized study of aerosolized tobramycin for early treatment of Pseudomonas aeruginosa colonization in cystic fibrosis. Pediatr. Pulmonol. 25: 88-92. [DOI] [PubMed] [Google Scholar]
- 38.Wolter, J., S. Seeney, S. Bell, S. Bowler, P. Masel, and J. McCormack. 2002. Effect of long-term treatment with azithromycin on disease parameters in cystic fibrosis: a randomized trial. Thorax 57: 212-216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Worlitzsch, D., R. Tarran, M. Ulrich, U. Schwab, A. Cekici, K. C. Meyer, P. Birrer, G. Bellon, J. Berger, T. Weiss, K. Botzenhart, J. R. Yankaskas, S. Randell, R. C. Boucher, and G. Doring. 2002. Effects of reduced mucus oxygen concentration in airway Pseudomonas infections of cystic fibrosis patients. J. Clin. Investig. 109: 317-325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wozniak, D. J., T. J. Wyckoff, M. Starkey, R. Keyser, P. Azadi, G. A. O'Toole, and M. R. Parsek. 2003. Alginate is not a significant component of the extracellular polysaccharide matrix of PA14 and PAO1 Pseudomonas aeruginosa biofilms. Proc. Natl. Acad. Sci. USA 100: 7907-7912. [DOI] [PMC free article] [PubMed] [Google Scholar]