Abstract
Rates of contamination of blood cultures obtained when skin was prepared with iodine tincture versus chlorhexidine were compared. For iodine tincture, the contamination rate was 2.7%; for chlorhexidine, it was 3.1%. The 0.41% difference is not statistically significant. Chlorhexidine has comparable effectiveness and is safer, cheaper, and preferred by staff, so it is an alternative to iodine tincture.
Contaminated blood cultures cause unnecessary costs and poor patient care and promote the use of unnecessary antibiotics. The current “gold standard” skin preparation is iodine tincture. A new less toxic product ChloraPrep, a one-step application of 2% chlorhexidine gluconate and 70% isopropyl alcohol, is now available. Several studies have established that for preparing the skin for insertion of intravenous lines, chlorhexidine is superior to povidone-iodine or alcohol alone (1, 4-6, 9; N. Chaiyakunapruk, D. L. Veenstra, S. Saint, and B. A. Lipsky, Abstr. 28th Annu. Meet. Assoc. Prof. Infect. Control Epidemiol., abstr. 99, 2001; D. G. Maki, V. Knasinski, L. L. Narans, and B. J. Gordon, Program Abstr. 11th Soc. Healthcare Epidemiol. Am., abstr. 142, 2001; D. G. Maki, V. Knasinski, L. L. Narans, and B. J. Gordon, Program Abstr. 11th Soc. Healthcare Epidemiol. Am., abstr. 142, 2001; G. Sheehan, K. Leicht, M. O'Brien, G. Taylor, and R. Rennie, Program Abstr. 33rd Intersci. Conf. Antimicrob. Agents Chemother. abstr. 1616, 1993) and that for preparing the skin prior to obtaining blood for culture, chlorhexidine is superior to povidone-iodine (8). However, only one smaller study compared the rates of blood culture contamination obtained with chlorhexidine versus those obtained with iodine tincture (10). To further document the efficacy of chlorhexidine, we compared the rates of blood culture contamination when iodine tincture was used to prepare the skin versus those obtained when chlorhexidine was used.
(This work was previously presented in part [103rd Gen. Meet. Am. Soc. Microbiol., abstr. C-095, p. 135, 2003].)
At Memorial Medical Center in Springfield, Ill., 11,738 blood cultures over two time periods (January 2002 to June 2002 and August 2002 to February 2003) were studied. The cultures were processed identically during each time period in an automated blood culturing instrument (BacT/Alert; Organon Teknika, Durham, N.C.). Both chlorhexidine and iodine tincture are manufactured by Medi-Flex Hospital Products, Inc. (Overland Park, Kans.). From January to June 2002, iodine tincture was used for antiseptic skin preparation. From August 2002 to February 2003, chlorhexidine was used. Since contiguous time periods were used to evaluate the products, a potential bias of seasonality was not accounted for. Therefore, to determine any effect of different time periods, we included contamination rates for the months of January to June 2003, during which time chlorhexidine alone was used. The procedures used for skin preparation with iodine tincture and chlorhexidine were in accordance with the package inserts.
Blood cultures were collected by a variety of hospital staff, including phlebotomists from the laboratory (who collect about one-third of the samples), staff in the Emergency Department, and nurses. Generally, the rates of contamination were lowest when the phlebotomy was done by the phlebotomy team and highest when it was done by the staff in the Emergency Department (1.5 to 3% higher). During each time period, two in-services about the importance of skin antisepsis were given. For adults, a blood culture consisted of a set of two (FAN Aerobic and FAN Anaerobic) bottles. A pediatric bottle could be used for children. Generally, the pediatric population (<18 years of age) was <5% of the patients. A blood culture was considered positive if either one or both bottles grew organisms. If a patient had more than two cultures taken and only one was positive for coagulase-negative staphylococci, viridans group streptococci, nutritionally deficient streptococci, Peptostreptococcus spp., diphtheroids, or Propionibacterium, Bacillus, or Micrococcus spp., then that culture was considered contaminated. If a patient had only one culture taken and one of the organisms named above was present in that single culture, then data from that culture were discarded. Data were retrieved by the Cerner laboratory information system. Questionnaires were distributed to assess phlebotomists' opinions of the two products.
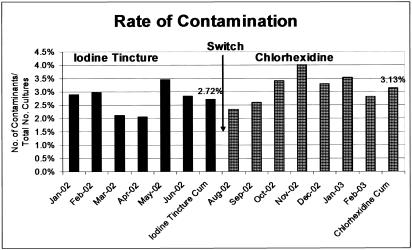
From January 2002 to June 2002 (when iodine tincture was used), 32 positive blood cultures with potential contaminants were discarded; from August 2002 to February 2003 (when chlorhexidine was used), 30 were discarded. During the time when iodine tincture was used, the average contamination rate was 2.72% (158 contaminants in 5,802 cultures); during the time when chlorhexidine was used, the average contamination rate was 3.13% (186 contaminants in 5,936 cultures) (Fig. 1). The 0.41% (3.13% − 2.72%) difference was not statistically significant (P = 0.188, chi-square analysis). The contaminating organisms found with both iodine tincture and chlorhexidine were similar in distribution (Table 1). The most common organisms causing contamination were coagulase-negative staphylococci (125 of 158 or 79.1% with iodine tincture and 137 of 186 or 73.7% with chlorhexidine), followed by diphtheroids (12 of 158 or 7.6% with iodine tincture and 22 of 186 or 11.8% with chlorhexidine) and viridans group streptococci (9 of 158 or 5.7% with iodine tincture and 11 of 186 or 5.9% with chlorhexidine). Despite the use of the different products, there was minimal variation in the rates of contamination from month to month during different years (Table 2).
FIG. 1.
Graph of contamination rates obtained with the two different skin preparations compared in this study. Cum, cumulative.
TABLE 1.
Distribution of organisms causing contamination in the two time periods studied
| Organisms | No. (%) of organisms
|
|
|---|---|---|
| Iodine tincture | Chlorhexidine | |
| Bacillus spp. | 5 (3.2) | 8 (4.3) |
| Coagulase-negative staphylococci | 125 (79.1) | 137 (73.7) |
| Diphtheroids | 12 (7.6) | 22 (11.8) |
| Micrococci | 4 (2.5) | 3 (1.6) |
| Nutritionally deficient streptococci | 1 (0.6) | 0 |
| Propionibacteria | 1 (0.6) | 5 (2.7) |
| Peptostreptococci | 1 (0.6) | 0 |
| Viridans group streptococci | 9 (5.7) | 11 (5.9) |
| All contaminant organisms | 158 (100) | 186 (100) |
TABLE 2.
Contamination rates during the same months in different years
| Mo | Contamination rate with Iodine tincture in 2002 (%) | Contamination rate with chlorhexidine in 2003 (%) |
|---|---|---|
| January | 2.9 | 3.0 |
| February | 3.0 | 2.6 |
| March | 2.1 | 3.5 |
| April | 2.1 | 2.7 |
| May | 3.5 | 2.1 |
| June | 2.8 | 3.5 |
| Cumulative | 2.7 | 2.9 |
The cost of chlorhexidine in this hospital was 16 cents less per kit. The time required for skin antisepsis by chlorhexidine was 40 s less than that required for skin antisepsis by iodine tincture. Fifteen phlebotomists and nurses answered questionnaires about their subjective impressions. All but one preferred chlorhexidine. The reasons cited were that chlorhexidine was easier, faster, and less messy.
The study that directly compared iodine tincture to chlorhexidine for skin preparation for blood cultures had results similar to but slightly different from ours (10). The study by Trautner et al. of 430 blood cultures found a slightly higher contamination rate with iodine tincture, but the difference was not statistically significant (P = 0.62) (10).
Our findings are subject to at least two limitations. First, the use of different time frames is a theoretical problem. It is possible that the lack of a statistically significant difference observed was due to changes in patient population or seasonal trends. However, in subsequent months when chlorhexidine alone was used, the average contamination rate was 2.9% from January 2003 to June 2003, compared to the same time frame in the previous year in which iodine tincture was used with a contamination rate of 2.7% (Table 2). Second, a chart review was not done to determine if the isolate was really a true contaminant. However, we used the same widely used criteria in both arms of the study to determine contamination rates.
Iodine tincture has the disadvantage of being toxic when used repeatedly (4). Toxicity or sensitization due to chlorhexidine is very uncommon (2-4, 7). In contrast, iodinated antiseptics alter thyroid function in newborns of low birth weight because of systemic absorption of iodine (5). After discussions with infectious disease physicians, infection control staff, and committee, we will continue using chlorhexidine for antiseptic preparation of skin prior to obtaining blood for culture at this institution.
In conclusion, since chlorhexidine has comparable effectiveness to iodine tincture and is safer, cheaper, and preferred by users, it is an alternative to iodine tincture.
Acknowledgments
This study was supported in part by a grant from Medi-Flex Hospital Products, Inc.
REFERENCES
- 1.Cobett, S., and A. LeBlanc. 1999. IV site infection: a prospective, randomized clinical trial comparing the efficacy of three methods of skin antisepsis; CINA conference ′99. Off. J. Can. Intravenous Nurs. Assoc. 15:48-49. [Google Scholar]
- 2.Darmstadt, G. L. 2000. Neonatal skin care. Pediatr. Clin. N. Am. 47:757-782. [DOI] [PubMed] [Google Scholar]
- 3.Garland, J. S., C. P. Alex, C. D. Mueller, D. Otten, C. Shivpuri, et al. 2001. A randomized trial comparing povidone-iodine to a chlorhexidine gluconate-impregnated dressing for prevention of central venous catheter infections in neonates. Pediatrics 107:1431-1436. [DOI] [PubMed] [Google Scholar]
- 4.Garland, J. S., R. K. Buck, P. Maloney, D. M. Durkin, S. Toth-Lloyd, M. Duffy; P. Szocik, T. L. McAuliffe, and D. Goldmann. 1995. Comparison of 10% povidone-iodine and 0.5% chlorhexidine gluconate for the prevention of peripheral intravenous catheter colonization in neonates: a prospective trial. Pediatr. Infect. Dis. 14:510-516. [DOI] [PubMed] [Google Scholar]
- 5.Kinirons, B., O. Mimoz, L. Lafendi, T. Naas, et al. 2001. Chlorhexidine versus povidone iodine in preventing colonization of continuous epidural catheters in children. Anesthesiology 94:239-244. [DOI] [PubMed] [Google Scholar]
- 6.Maki, D. G., M. Ringer, and C. J. Alvarado. 1991. Prospective randomized trial of povidone-iodine, alcohol, and chlorhexidine for prevention of infection associated with central venous and arterial catheters. Lancet 338:339-343. [DOI] [PubMed] [Google Scholar]
- 7.Metry, D. W. 2000. Topical therapies and medications in the pediatric patient. Pediatr. Clin. N. Am. 47:867-876. [DOI] [PubMed] [Google Scholar]
- 8.Mimoz, O., A. Karim, A. Mercat, M. Cosserson, et. al. 1999. Chlorhexidine compared with povidone-iodine as skin preparation before blood culture. Ann. Int. Med. 131:834-837. [DOI] [PubMed] [Google Scholar]
- 9.Mimoz, O., L. Pieroni, C. Lawrence, A. Edouard, Y. Costa, K. Samii, and C. Brun-Buisson. 1996. Prospective, randomized trial of two antiseptic solutions for prevention of central venous or arterial catheter colonization and infection in intensive care unit patients. Crit. Care Med. 24:1818-1823. [DOI] [PubMed] [Google Scholar]
- 10.Trautner, B., J. Clarridge, and R. O. Darouche. 2002. Skin antisepsis kits containing alcohol and chlorhexidine gluconate or tincture of iodine are associated with low rates of blood culture contamination. Infect. Control Hosp. Epidemiol. 23:397-401. [DOI] [PubMed] [Google Scholar]