Abstract
Endometriosis is an often painful disorder in which the endometrial glands and stroma grow outside the uterus. The disease affects women’s quality of life and is a common cause of infertility. In this review, we describe promising new developments in the field based on in vitro assays and rodent models, each of which has the potential to be beneficial in the treatment of this disease. We will specifically describe the role of anti-inflammatory drugs, selective estrogen, or progesterone modulators, statins, antiangiogenic agents, and the potential for targeting stem cells as likely methods to hone in and eliminate endometriosis. The most promising of these potential therapies are currently slated for further testing in both rodent and nonhuman primate trials.
Keywords: Endometriosis, ICON, tissue factor, statins, stem cells, SERM, SPRM
Introduction
Endometriosis is a gynecological disorder characterized by the presence of endometrial tissue outside the uterus.1 This disease affects approximately 10% of reproductive-aged women and 20% to 50% of infertile women.2,3 Despite its frequency and its impact on quality of life, our understanding of the pathogenesis of endometriosis remains incomplete.4 Endometrial lesions are primarily located on the pelvic peritoneum and ovaries but can also be found in the pericardium, pleura, lung parenchyma, and even the brain.5 Implants can result in substantial morbidity, including pelvic adhesions, pain, fatigue, bowel disorders, and infertility requiring extensive and sometimes ineffective medical and surgical treatments.5 This disease is not only costly but also physically and psychologically debilitating.
The etiology of the disease likely reflects retrograde menstruation, coelomic metaplasia, or both.1,4,5. However, it also involves a complex interplay of genetic, anatomic, environmental, and immunologic factors.6–9 Intense macrophage infiltration and excess cytokine expression play a critical role in the development of the endometriosis-related chronic inflammatory processes.9–12 Endometriotic implant nidation also requires remodeling of the local peritoneal environment mediated by extracellular matrix (ECM)-degrading proteases.13–15 Matrix metalloproteinases (MMPs) play the dominant role in such tissue remodeling. Endometriotic lesions display enhanced expression of MMP-1, -3, and -7.16,17 A similar overexpression of these MMPs has been observed in a baboon model.18.
Stem cells also contribute to endometriosis and may be particularly relevant to disease occurring outside of the peritoneal cavity, in locations such as the lung or ureter.19,20 Recent functional analyses provide evidence of isolation and characterization of human endometrial stem/progenitor cells.21 Indeed, several lines of experimental evidence suggest that endometrial stem/progenitor cells function in the development of endometriosis21 (for review, see Sasson and Taylor21). There is a major public health need for more effective endometriosis therapies producing fewer and less severe side effects. For example, it is well known that estrogen is a strong mitogen for endometriotic cells; however, therapies such as gonadotropin-releasing hormone (GnRH) agonists, which dramatically reduce estradiol production, are associated with numerous side effects and can only be used for a limited period of time. Recent studies demonstrating that estradiol is not only secreted by the ovary but has been found to be locally produced by endometriotic tissue22 has suggested the therapeutic use of aromatase inhibitors and selective estrogen receptor modulators (SERMs).23 Although the molecular mechanisms by which endometriosis persists in locations outside the uterus are not clear, the establishment and progression of endometriosis require angiogenesis providing another therapeutic target.13–15
In this review, we will summarize the recent work of our collaborative studies and others on novel treatments for endometriosis including tissue factor (TF) targets, SERMs, and statins and how they have been tested in a mouse model of endometriosis.
Experimental Endometriosis in a Chimeric Mouse Model
To conduct preclinical evaluation of potential new therapies, we and others have developed chimeric experimental endometriosis models in which human endometrial tissues are established at ectopic sites in immunocompromised mice.24 Athymic nude mice, which have a spontaneous mutation in the forkhead box N1 (Foxn1) gene, are an immunocompromised strain of mice that are particularly well suited for conducting preclinical testing of novel agents that target human endometrial cells. For our studies, we have established experimental disease in nude mice using proliferative phase human endometrial tissue obtained by biopsy from women with or without endometriosis and injected into mice either intraperitonally or subcutaneously along the ventral midline.
In our hands, we have found that lesions established in nude mice are similar to the spontaneous disease in women, exhibiting the classic characteristics of endometriosis both grossly and microscopically.25 Experimental endometriosis can also be established using tissues obtained via collection of menstrual effluent or from surgical specimens obtained from women with endometriosis.26–29 Studies such as these have revealed important differences between eutopic endometrial tissues obtained from women with endometriosis compared to eutopic or ectopic tissues obtained from disease-free women. For example, we have demonstrated that endometrial tissues obtained from women with endometriosis exhibit a reduced response to progesterone which promotes the development of experimental endometriosis.29,30 To target this mechanism, we used both progesterone therapy and Tanaproget, a selective progesterone receptor modulator described below.31,32 Thus, in our initial report describing this model, we examined the impact of progesterone therapy on establishment of experimental endometriosis by normal human endometrium.24 In these studies, we found that progesterone treatment greatly reduced the extent of experimental disease in mice bearing endometrial xenografts from women without endometriosis.24 Since progesterone is a potent differentiation agent, we have found that short-term treatment of mice bearing normal endometrial xenografts with the synthetic progestin medroxyprogesterone acetate led to stromal cell decidualization within human tissues.33 However, longer-term treatment with this compound eliminates disease established by normal tissues.29 These studies suggest that endometrial tissues, growing at an ectopic site in the peritoneum of nude mice, largely retain a similar level of responsiveness to progesterone that would be observed within the normal eutopic endometrium. In contrast, progesterone therapy fails to prevent the establishment and progression of experimental endometriosis when xenografts are established with eutopic endometrial tissue obtained from women with endometriosis.29,30
Therefore, we additionally examined whether a potent, nonsteroidal progesterone receptor agonist would have a better therapeutic profile compared to natural progesterone. In this study, Tanaproget, a selective progesterone receptor modulator developed by Wyeth Research (Collegeville, Pennsylvania), was found to be more effective than either progesterone or medroxyprogesterone acetate in reducing experimental endometriosis in our model when ectopic disease was established by eutopic endometrial tissues acquired from women with endometriosis.29
A second series of studies in the mouse model of endometriosis was conducted in collaboration with Wyeth Research examining the efficacy of ERB-041, a selective estrogen receptor-β (ER-β) agonist with anti-inflammatory activity, and is believed to induce an increase in normal immune surveillance.34 It is also a highly selective ER-β agonist that is >200-fold selective for ER-β based on a competitive radioligand-binding assay. ERB-041 has been extensively characterized in various models of estrogen action and found to be inactive on classic estrogenic targets such as the uterus, mammary gland, and bone. However, it has potent anti-inflammatory effects and we previously reported that this compound causes lesion regression in an experimentally induced model of endometriosis using human tissue and therefore may have utility in treating this disease.35
Hence, following establishment of xenograft lesions for 11 to 14 days, nude mice were treated with ERB-041 for up to 17 days. This treatment was associated with complete lesion regression in the majority of mice; however, the effectiveness of ERB-041 in this study appeared to be dependent upon tissue localization. Specifically, although intraperitoneal lesions were frequently found within the control treatment group, intraperitoneal lesions were never found following ERB-041 treatment. In contrast, subcutaneous lesions were present in half of the mice receiving this agent. Although the reasons for such differential treatment effects based on lesion location were not fully explored, additional studies by our collaborators suggested that ERB-041 may be inducing an increase in normal immune surveillance within the peritoneal cavity.34
Selective ER modulators
While nonhormonal approaches are needed, hormonal therapies are often effective and will still represent important therapeutic tools in the treatment of endometriosis. The SERMs have actions that are tissue-selective, often acting as predominantly ER agonists in the skeleton, on serum lipid metabolism, and on a number of coagulation factors, but as ER antagonists in the breast and uterus.36 For example, raloxifene, a SERM currently used to prevent osteoporosis in postmenopausal women, does not induce endometrial proliferation in ovariectomized rats.37 However, a clinical trial evaluated treatment with raloxifene in women with chronic pelvic pain due to endometriosis. Patients with biopsy-proven endometriosis were randomly allocated to 6 months of daily treatment with raloxifene (180 mg) or placebo. This study was terminated when the raloxifene group experienced greater pain and had a second surgery significantly sooner than the placebo group.38
Another SERM, bazedoxifene (BZA), is currently under evaluation for the treatment of osteoporosis and, in combination with conjugated estrogens (CE), for menopausal symptoms. The BZA molecule does not stimulate the endometrium in postmenopausal women38 and also effectively antagonizes CE-induced uterine stimulation.39 A comparison of the effect ofBZA, raloxifene, and lasofoxifene, alone or in combination with estradiol in the murine uterus, demonstrated that BZA has the greatest antagonistic and the least agonistic effect.40 These data suggest that BZA may be superior to other SERMs in antagonizing the effect of 17β-estradiol or other ER agonists on endometriosis as well.
Studies from our laboratory have now determined the efficacy of BZA in the mouse model of endometriosis. Treatment was not initiated until the establishment of lesions (8 weeks). The total number of identifiable lesions was not different between groups, consistent with the generation of a similar number of initial lesions. After 8 weeks of BZA or vehicle-control treatment, the BZA-treated animals consistently demonstrated decreased lesion size compared to vehicle-treated animals. Macroscopic analysis showed that there were no signs of active endometriotic implants in BZA-treated mice. Endometriotic lesions appeared smaller, blanched, and inactive in the BZA-treated group, while those in the control group typically appeared red and friable. Histology demonstrates diminished glands and a decrease in luminal cell height as a consequence of BZA treatment. Bazedoxifene is a promising therapy for endometriosis (2011, epub ahead of print).
Angiogenic Blockers
Studies described above suggest targeting steroid action may be beneficial to the treatment of endometriosis; however, the steroid responsiveness of endometriotic tissues can vary markedly between patients.41,42 Thus, examination of nonsteroidal agents which may act to impede lesion survival is also warranted. Angiogenesis is a critical mechanism that allows the establishment and growth of endometriotic lesions. For example, survival of endometriotic lesions requires the development of a vascular supply; therefore, inhibitors of angiogenesis would be expected to inhibit development of the disease. Blood vessels which are immature and have limited associations with pericyte cells can be most easily targeted by angiogenic inhibitors. Thus, in a collaborative study43 with our laboratory, we demonstrated that pericyte-free vessels supplied the human endometrial lesions in the nude mouse model of experimental endometriosis. Moreover, this study also demonstrated that treatments with soluble vascular endothelial growth factor receptor 1, a vascular endothelial growth factor receptor antagonist, prevented the formation of ectopic human lesions in mice, suggesting that the use of angiogenic inhibitors may be a useful therapeutic strategy following surgical removal of ectopic disease. However, therapeutic regression of established lesions would be of far greater utility to most women with endometriosis and such a therapy would potentially decrease the need for surgical intervention. We recently demonstrated the therapeutic utility of such an agent, immunoconjugate (ICON), a novel compound which targets TF that is aberrantly expressed on endometriotic endothelium, promotes devascularization, and prompts regression of well-established disease in our experimental model.25 The studies with ICON will be described in further detail later in this article.
Tissue Factor as a Target of Aberrant Angiogenesis
While angiogenesis occurs throughout fetal growth and development, in adults it is limited to the endometrium during the menstrual cycle, the ovary during formation of the corpus luteum, and in pathological states including wound healing, diabetic retinopathy, tumor growth, and endometriosis.44,45 Several factors are involved in physiologic and pathologic angiogenesis in the human endometrium. Vascular endothelial growth factor is the primary vasculogenic and angiogenic factors.46,47 However, other factors such as angiopoietins, fibroblast growth factors, platelet-derived growth factors, and interleukin 8 (IL-8) exert more complex effects and can either stimulate or inhibit the process of angiogenesis.48–50 Interestingly, peritoneal fluid from women with endometriosis is highly angiogenic.51–53
In addition to these classic angiogenic agents, TF, the key initiator of the hemostatic cascade,54 mediates angiogenesis using a variety of distinct intracellular signaling pathways.55 Tissue factor is aberrantly expressed in pathologically growing endothelium.56,57 The full-length TF molecule exists on the cell surface as a cryptic form that is inert, a procoagulant form that rapidly binds factor VIIa to initiate coagulation, and a signaling form that binds VIIa and cleaves protease-activated receptor 2 (PAR-2), to initiate biochemical pathways that promote inflammation, tumor progression, and angiogenesis.58 Thus, detection of immunoreactive TF does not necessarily correspond to clotting activity. The angiogenic function of TF is now known to be mediated through a complex series of intracellular-signaling pathways57,59 and its absolute requirement has been demonstrated by the embryonic lethality observed in knockout mice.60,61 Thus, TF–/– embryos die at embryonic day E10.5 and display disorganization of the yolk sac vasculature.60–62
Initial experiments to target aberrantly expressed endothelial TF were conducted in a xenograft mouse model of human malignant melanoma utilizing an immunoconjugate molecule consisting of 2 noncoagulation-inducing mutated factor VIII (fVII) molecules and an immunoglobulin Fc domain.63 The results demonstrated that the molecule, ICON, bound to TF both on tumor cells and in rapidly growing vascular tumor endothelial cells.63 The Fc domain activates a natural killer (NK) cell cytolytic response against the TF-bearing cells and causes the C1q protein to initiate the complement pathway.64,65
Recent studies from our group have demonstrated that TF is overexpressed in eutopic and ectopic endometrium from women with endometriosis.25. Strong immunostaining for TF was observed in the endothelium of ectopic endometrial vessels derived from affected women, whereas no TF immunostaining was observed in the endothelium of control eutopic endometria. We posited that TF may be vital for the growth and survival of endometriotic lesions and could serve as a target for therapy. To this end, we studied the effects of ICON, and using the mouse model of endometriosis described above, we demonstrated that ICON destroyed pre-established endometriotic implants by vascular disruption without apparent toxicity, reduced fertility, or subsequent teratogenic effects.25
Given these results, we conclude that ICON could serve as a novel, nontoxic, and effective treatment for women with endometriosis. Immunoconjugate is a novel nonhormonal potential therapy that may eliminate some of the side effects of hormone-altering therapy.
Targeting Stem Cells for the Treatment of Endometriosis
Stem cells are relatively undifferentiated cells that give rise to more fully differentiated functional adult cells, and also replicate themselves, allowing for continued cycles of cellular regeneration. Many tissues contain progenitor stem cells that give rise to a limited repertoire of cells found in the tissue in which the cells reside. As the endometrium is rapidly regenerated in each menstrual cycle or estrus cycle, it should not be surprising that progenitor stem cells have been identified in the uterus.66 Recent studies have demonstrated that the endometrium contains endometrial/stem progenitor cells.21 These cells repopulate the endometrium in each cycle and may explain the vast regenerative capacity of this tissue. However, the existence of a large number of progenitor stem cells in the endometrium may also be detrimental as endometrial stem cells shed through the fallopian tube and into the peritoneal cavity during menses.21 Thus, a price of the impressive regenerative capacity of the endometrium is the potential for these stem cells to give rise to excess endometrial cells in the form of endometriosis or cancer. Indeed, based on the animal studies in primates and rodent models described above, it is clear that normal endometrial cell samples contain these progenitor cells and can give rise to endometriosis.5,21,67–70
It remains to be determined whether women with endometriosis have simply an increased flux of endometrial progenitor stem cells to the peritoneal cavity or, alternatively, have an increase in stem cell survival and/or proliferative capacity. Additionally, they may have a peritoneal environment that fosters their growth along with genetic, immunologic, and environmental factors that contribute to progenitor stem cell survival and propagation. A better understanding of these factors will likely lead to the potential for novel treatment of endometriosis.
In addition to the progenitor cells within the uterus, we have shown that stem cells from other organs can also contribute to the regeneration or repair of the uterus. These multipotent stem cells can differentiate into multiple cell types, including those from organs distinct from those in which they reside. Bone marrow is a well-characterized source of multipotent mesenchymal stem cells.66 We have previously demonstrated that bone marrow-derived mesenchymal stem cells can give rise to endometrial cells.71 To investigate the possibility that cells of extrauterine origin could repopulate the endometrium, we examined Human Leukocyte Antigen (HLA)-mismatched bone marrow transplant recipients for donor HLA expression in endometrial cells.72 Each recipient had a bone marrow donor with an HLA type that enabled the determination of the origin of any cell. An HLA type was determined by immunohistochemistry, and donor-derived cells were distinguished from CD45+ leukocytes in the endometrium.72 Donor-derived endometrial cells were detected in endometrial biopsy samples from all bone marrow recipients and none of the controls. These findings demonstrate that endometrial cells can originate from donor-derived bone marrow cells and suggest that nonuterine stem cells contribute to the regeneration of endometrial tissue.72 The ability of bone marrow-derived cells to repopulate the endometrium was confirmed in an animal model. After male to female bone marrow transplantation, male donor-derived bone marrow cells were found in the uterine endometrium of female mice.73 Although uncommon, these cells can differentiate into stromal and epithelial cells. The examination of a sexually dimorphic organ, such as the uterus, demonstrates the ability of male bone marrow, which cannot harbor circulating endometrial cells, to generate endometrium de novo and proves their mesenchymal stem cell origin. Finding Y chromosome-bearing endometrial cells demonstrates the potential to recapitulate embryonic developmental pathways that were never activated in males; bone marrow-derived stem cells (BMDCs) may have vast regenerative capacity.
In addition to the regenerating normal endometrium, we have shown that mesenchymal stem cells can also give rise to endometriosis using an autologous murine model. After generation of experimental endometriosis by ectopic endometrial implantation in the peritoneal cavity, bone marrow from LacZ transgenic mice was used for stem cell transplantation. These LacZ-expressing cells were found in the wild-type ectopic endometrium implanted in the peritoneal cavity of hysterectomized LacZ transgenic mice.71 The ability of stem cells to engraft endometriosis has implications for the origin and progression of this disease. It also establishes a novel theory for the origin of endometriosis. In addition to the classic theories, stem cells are a novel source of endometriosis. This mechanism may explain endometriosis in areas remote from the peritoneal cavity or in men given estrogens. Ectopic differentiation of stem cells is a novel mechanism of disease.
Current research in our group centers on elucidating the factors that enable these cells to engraft an ectopic site and drive them toward endometrial differentiation. Preliminary data are providing several pathways to target in order to prevent stem cell flux and the initiation and progression of endometriosis. For example, tobacco use inhibits stem cell flux to endometrium and endometriosis.74 While we would not advocate tobacco use in the treatment of endometriosis, it may explain the lower incidence of this disease in smokers. Individual components of tobacco smoke may prove useful in the treatment of this disease. Of therapeutic interest, the molecular targeting of deregulated signaling pathways by these cells and their local microenvironment, blocking key self-renewal pathways and prosurvival-signaling pathways, preventing cell recruitment, flux and adhesion through interference with chemokines, and adhesion molecules that regulate these processes, as well as inhibiting abnormally activated pathways within the cells or surrounding niche cells, represent new potential strategies for the development of more effective clinical treatments.75
Statins in Treatment of Endometriosis
There is growing evidence supporting the concept that statins may provide a novel and effective treatment of endometriosis ameliorating a broad range of its aspects. Here, we outline the mechanisms of action of statins in relation to key pathophysiological features of endometriosis and present the findings of in vitro and in vivo studies evaluating the effects of statins on endometrial and endometriotic tissues and on animal models of endometriosis.
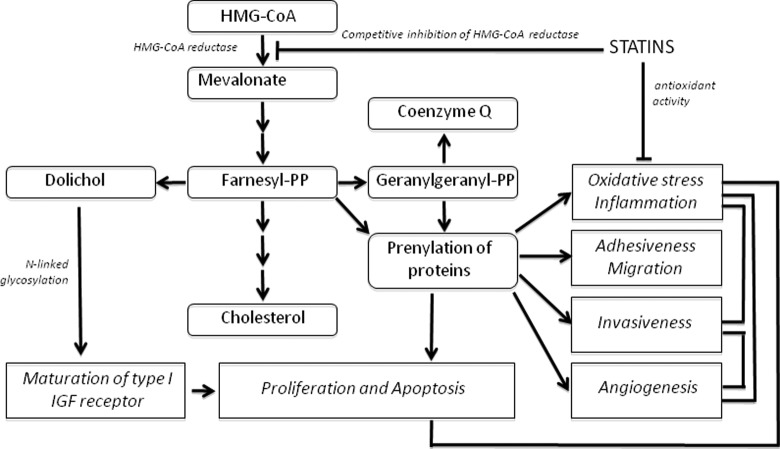
Statins are competitive inhibitors of 3-hydroxy-3-methylglutaryl-coenzyme A reductase (HMG-CoAR; Figure 1 ), a rate-limiting step of the mevalonate pathway. This pathway consists of a series of reactions leading to the formation of a key product: farnesyl pyrophosphate (FPP), which in turn, may be converted into important molecules including cholesterol, dolichol, coenzyme Q (ubiquinone), and geranylgeranyl pyrophosphate (GGPP).76 The FPP and GGPP molecules are, respectively, substrates for farnesylation and geranylgeranylation, a process collectively known as isoprenylation. Isoprenylation is essential for membrane attachment and the function of several families of proteins, especially Ras and Ras-related GTP-binding proteins, subunits of trimeric G proteins, and protein kinases.77
Figure 1.
Inhibition of HMG-CoRA by statins. Statins are competitive inhibitors of HMG-CoAR, a rate-limiting step of the mevalonate pathway. By competing with the reductase, statins are believed to decrease oxidative stress, apoptosis, invasiveness, and angiogenesis. 3- HMG-CoAR indicates hydroxy-3-methylglutaryl-coenzyme A reductase.
Statin-induced inhibition of the mevalonate pathway has complex and profound effects on cell function including proliferation, apoptosis, cell morphology, motility, and gene expression. Statins also possess intrinsic antioxidant activity and antagonize oxidation by hydroxyl as well as peroxyl radicals.78 This activity is relevant to endometriosis, which is characterized by inflammation and increased oxidative stress.79
Effects of statins on adhesion of endometrial tissues to peritoneum may be related to inhibition of isoprenylation and consequent alteration of the cytoskeleton due to reduced activity of key small GTPases.80,81 Statins may reduce the expression of integrins and/or affect their activation.82,83 In cultures of endometrial stromal cells in a 3-dimensional (3-D) matrix, lovastatin alters cellular morphology and decreases cell adhesiveness to collagen fibers.84 Our recent study has demonstrated that simvastatin profoundly alters endometrial stromal cell and cytoskeleton morphology, disrupting F-actin fibers.85
Another likely target of action of statins pertains to their effects on invasiveness of endometriotic cells. Invasiveness may be affected by MMPs leading to local destruction of the ECM and consequent establishment of the disease.16 Indeed, in women with endometriosis, the expression of several MMPs is increased, while the expression of the tissue inhibitor metalloproteinase 2 (TIMP-2) is reduced.86,87 In several biological systems, statins have been shown to inhibit MMPs and increase TIMP-1.88–90 These effects are likely induced by the inhibition of isoprenylation. Recently, we have shown that simvastatin inhibits both basal and IL-1α-induced expression of MMP-3 in human endometrial stromal cells.91
Thus, statins may inhibit excessive growth of endometrial cells by several mechanisms including inhibition of isoprenylation of proteins involved in the stimulation of proliferation (eg Ras), reduction of oxidative stress, and possibly, inhibition of dolichol synthesis.
As mentioned above, proliferation of endometrial stroma is stimulated by moderate oxidative stress and inhibited by antioxidants.92 In this context, direct antioxidant activity of statins may contribute to a decrease in cell proliferation. Finally, inhibition of HMG-CoA reductase by statins may reduce one of the downstream products of the mevalonate pathway, dolichol, which is required for maturation of type I insulin-like growth factor 1(IGF-1) receptors and hence may decrease the mitogenic effect of IGF-1 on endometrial stromal cells.
Several studies have demonstrated that statins reduce proliferation and induce apoptosis of endometrial stromal cells. A report by Piotrowski et al demonstrated that mevastatin and simvastatin exert a concentration-dependent inhibition of DNA synthesis in endometrial stromal cells in parallel with the reduction of the number of viable cells and inhibition of the Mitogen-Activated Protein Kinases/Extracellular Signal-Regulated Kinases (MAPK/ERK) 1/2 pathway.93 These effects of statins were related to the inhibition of the mevalonate pathway but independent of the supply of cholesterol. Comparable effects were reported by Esfandiari et al94 who showed that lovastatin induced a concentration-dependent inhibitory effect on cell growth in an in vitro model of endometriosis-like tissue. The above studies were carried out using cells derived from eutopic endometrium. More recently, these findings were also confirmed in the studies demonstrating inhibitory effects of simvastatin on proliferation of cells collected from endometriomas.84 Another study performed on ectopic endometrial tissues has shown that atorvastatin increased the level of insulin-like growth factor-binding protein 1 (IGFBP-1) in cultures treated with lipopolysaccharide.95 Since the growth of endometrial tissues is affected by IGFs, increased IGFBP-1 may contribute to the inhibition of cell proliferation.
We have shown that simvastatin induces apoptosis of endometrial stromal cells in a time- and concentration-dependent fashion, as determined by morphological changes, increased activity of executioner caspases, and DNA fragmentation.85 All these effects were abrogated by GGPP, indicating that simvastatin induced apoptosis by inhibition of geranylgeranylation.
In vascular tissues, both in vitro and in vivo studies have demonstrated that atorvastatin decreases reactive oxygen species (ROS) production; the effects may be related to reduced expression of some of the components of nicotinamide adenine dinucleotide phosphate (NADPH) oxidase.96 Comparative in vitro studies have indicated that simvastatin was particularly effective in quenching hydroxyl radicals and fluvastatin most effectively scavenged peroxyl radicals.78 Immunomodulatory actions of statins have been demonstrated in clinical and animal studies; these actions may be beneficial in the treatment of autoimmune diseases.97 Most evidence supports the concept that these effects are related to the inhibition of isoprenylation of proteins rather than lowering the cholesterol level.98
The above properties of statins are likely to be relevant to the treatment of endometriosis; however, to date, we are not aware of studies addressing this important issue.
Naturally Occurring Products to Treat Endometriosis
Importantly, in addition to the agents described above, mouse models of human endometriosis have also been used to examine a number of naturally occurring compounds for potential therapeutic benefits. Within our group, we recently described the treatment of nude mice with resveratrol, a compound with anti-inflammatory effects which occurs naturally in berries, nuts, and red wine. Ovariectomized nude mice were provided a slow-release estrogen capsule 24 hours prior to intraperitoneal injection of normal proliferative human endometrial tissues. Treatment with resveratrol (6 mg/mouse) was initiated 24 hours after tissue injection and continued for up to 20 days. We found that mice receiving resveratrol exhibited significantly fewer lesions, while lesions that were present were smaller and apoptotic.33 Importantly, resveratrol did not appear to impact apoptotic activity within the uteri of treated mice. Although it is important to note that the doses used were pharmacologic and could not be obtained from normal consumption of resveratrol-containing foods, these data are promising and may suggest a potential role for resveratrol supplementation in the treatment of endometriosis in women.
Finally, perhaps as a consequence of both the disease and associated multiple pelvic surgeries, patients with endometriosis frequently exhibit severe adhesive disease. Thus, we recently developed a model in which we can examine the inflammation-associated development and therapeutic inhibition of adhesions in our experimental endometriosis model. Specifically, we demonstrated that a recent surgery promoted both the development of experimental endometriosis and endometriosis-related adhesions. Additionally, thiazolidinediones, a class of pharmacologic agents, which activate the peroxisome proliferator-activated receptor-γ (PPAR-γ) receptor family, have potent anti-angiogenic and anti-inflammatory effects,99,100 and members of this family have been found to be effective in preventing experimental endometriosis (ie pioglitazone) as well as adhesive disease in rats (rosiglitazone). Therefore, we additionally examined whether pioglitazone treatment would also be beneficial in the prevention of surgical adhesions associated with endometriosis. Our experimental model results suggest that pioglitazone was highly effective in reducing the development of inflammation-associated postsurgical adhesions.101
Conclusions
Endometriosis is a disease that is still poorly understood. Through elucidation of the molecular and cellular regulation of endometrial growth and endometriosis, we have devised several novel investigational tools and therapies. It should be pointed out, however, that endometriosis is a complicated multifactorial syndrome which in fact may represent several diseases. Genetic and immunologic factors play a key role in its pathophysiology.102 We describe here our recent work suggesting that ICON, statins, SERMs, and selective progesterone receptor modulators (SPRMs) may offer new alternatives in the treatment of this syndrome although further study is needed to explore how these agents will fare in cases where endometrial lesions are located in different areas of the body. Figure 2 shows these findings. Several of these therapies are currently undergoing testing in murine and nonhuman primate models. Some may evolve into effective new therapies necessary for the treatment of endometriosis.
Figure 2.
Novel therapies targeting endometriosis. The inflammation caused by endometriosis is shown by red arrows, indicating stem cells, estrogens, and/or low progesterone response, and as yet, unknown causes; whereas the novel therapies are shown by blue arrows and their potential mechanism of action described. SERM indicates selective estrogen receptor modulator; SPRM, selective progesterone receptor modulator; P, progesterone; ICON, immunoconjugate molecule.
Footnotes
The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: sponsored by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the Specialized Cooperative Centers Program in Reproduction and Infertility Research.
References
- 1. Giudice LC, Kao LC. Endometriosis. Lancet. 2004;364(9447):1789–1799 [DOI] [PubMed] [Google Scholar]
- 2. Taylor RN, Lebovic DI, Mueller MD. Angiogenic factors in endometriosis. Ann NY Acad Sci. 2002;955:89–100; discussion 118,, 396–406 [DOI] [PubMed] [Google Scholar]
- 3. Krikun G, Lockwood CJ, Paidas MJ. Tissue factor and the endometrium: from physiology to pathology. Thromb Res. 2009;124(4):393–396 [DOI] [PubMed] [Google Scholar]
- 4. Sharpe-Timms KL, Young SL. Understanding endometriosis is the key to successful therapeutic management. Fertil Steril. 2004;81(5):1201–1203 [DOI] [PubMed] [Google Scholar]
- 5. Rogers PA, D’Hooghe TM, Fazleabas A, et al. Priorities for endometriosis research: recommendations from an international consensus workshop. Reprod Sci. 2009;16(4):335–346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schindler AE. Pathophysiology, diagnosis and treatment of endometriosis. Minerva Ginecol. 2004;56(5):419–435 [PubMed] [Google Scholar]
- 7. Taylor RN, Lundeen SG, Giudice LC. Emerging role of genomics in endometriosis research. Fertil Steril. 2002;78(4):694–698 [DOI] [PubMed] [Google Scholar]
- 8. Wenzl R, Kiesel L, Huber JC, Wieser F. Endometriosis: a genetic disease. Drugs Today (Barc). 2003;39(12):961–972 [DOI] [PubMed] [Google Scholar]
- 9. Kyama CM, Debrock S, Mwenda JM, D’Hooghe TM. Potential involvement of the immune system in the development of endometriosis. Reprod Biol Endocrinol. 2003;1:123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Chegini N. Peritoneal molecular environment, adhesion formation and clinical implication. Front Biosci. 2002;7:e91–e115 [DOI] [PubMed] [Google Scholar]
- 11. Gazvani R, Templeton A. Peritoneal environment, cytokines and angiogenesis in the pathophysiology of endometriosis. Reproduction. 2002;123(2):217–226 [DOI] [PubMed] [Google Scholar]
- 12. Lin YJ, Lai MD, Lei HY, Wing LY. Neutrophils and macrophages promote angiogenesis in the early stage of endometriosis in a mouse model. Endocrinology. 2006;147(3):1278–1286 [DOI] [PubMed] [Google Scholar]
- 13. Barcz E, Rozewska ES, Kaminski P, Demkow U, Bobrowska K, Marianowski L. Angiogenic activity and IL-8 concentrations in peritoneal fluid and sera in endometriosis. Int J Gynaecol Obstet. 2002;79(3):229–235 [DOI] [PubMed] [Google Scholar]
- 14. Drenkhahn M, Gescher DM, Wolber EM, Meyhoefer-Malik A, Malik E. Expression of angiopoietin 1 and 2 in ectopic endometrium on the chicken chorioallantoic membrane. Fertil Steril. 2004;81(suppl 1):869–875 [DOI] [PubMed] [Google Scholar]
- 15. Ferrero S, Ragni N, Remorgida V. Antiangiogenic therapies in endometriosis. Br J Pharmacol. 2006;149(2):133–135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Osteen KG, Yeaman GR, Bruner-Tran KL. Matrix metalloproteinases and endometriosis. Semin Reprod Med. 2003;21(2):155–164 [DOI] [PubMed] [Google Scholar]
- 17. Osteen KG, Bruner-Tran KL, Ong D, Eisenberg E. Paracrine mediators of endometrial matrix metalloproteinase expression: potential targets for progestin-based treatment of endometriosis. Ann NY Acad Sci. 2002;955:139–146; discussion 157,–138, 396–406 [DOI] [PubMed] [Google Scholar]
- 18. Fazleabas AT, Brudney A, Gurates B, Chai D, Bulun S. A modified baboon model for endometriosis. Ann NY Acad Sci. 2002;955:308–317; discussion 340,–302, 396–406 [DOI] [PubMed] [Google Scholar]
- 19. Augoulea A, Lambrinoudaki I, Christodoulakos G. Thoracic endometriosis syndrome. Respiration. 2008;75(1):113–119 [DOI] [PubMed] [Google Scholar]
- 20. Ludwig M, Bauer O, Wiedemann GJ, Diedrich K. Ureteric and pulmonary endometriosis. Arch Gynecol Obstet. 2001;265(3):158–161 [DOI] [PubMed] [Google Scholar]
- 21. Sasson IE, Taylor HS. Stem cells and the pathogenesis of endometriosis. Ann NY Acad Sci. 2008;1127:106–115 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Bulun SE, Cheng YH, Pavone ME, et al. Estrogen receptor-beta, estrogen receptor-alpha, and progesterone resistance in endometriosis. Semin Reprod Med. 2010;28(1):36–43 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Simsa P, Mihalyi A, Kyama CM, Mwenda JM, Fulop V, D’Hooghe TM. Selective estrogen-receptor modulators and aromatase inhibitors: promising new medical therapies for endometriosis?. Womens Health (Lond Engl). 2007;3(5):617–628 [DOI] [PubMed] [Google Scholar]
- 24. Bruner KL, Matrisian LM, Rodgers WH, Gorstein F, Osteen KG. Suppression of matrix metalloproteinases inhibits establishment of ectopic lesions by human endometrium in nude mice. J Clin Invest. 1997;99(12):2851–2857 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Krikun G, Hu Z, Osteen K, et al. The immunoconjugate “icon” targets aberrantly expressed endothelial tissue factor causing regression of endometriosis. Am J Pathol. 2010;176(2):1050–1056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Zamah NM, Dodson MG, Stephens LC, Buttram VC, Jr, Besch PK, Kaufman RH. Transplantation of normal and ectopic human endometrial tissue into athymic nude mice. Am J Obstet Gynecol. 1984;149(6):591–597 [DOI] [PubMed] [Google Scholar]
- 27. Bergqvist A, Jeppsson S, Kullander S, Ljungberg O. Human uterine endometrium and endometriotic tissue transplanted into nude mice. Morphologic effects of various steroid hormones. Am J Pathol. 1985;121(2):337–341 [PMC free article] [PubMed] [Google Scholar]
- 28. Nisolle M, Casanas-Roux F, Donnez J. Early-stage endometriosis: adhesion and growth of human menstrual endometrium in nude mice. Fertil Steril. 2000;74(2):306–312 [DOI] [PubMed] [Google Scholar]
- 29. Bruner-Tran KL, Zhang Z, Eisenberg E, Winneker RC, Osteen KG. Down-regulation of endometrial matrix metalloproteinase-3 and -7 expression in vitro and therapeutic regression of experimental endometriosis in vivo by a novel nonsteroidal progesterone receptor agonist, tanaproget. J Clin Endocrinol Metab. 2006;91(4):1554–1560 [DOI] [PubMed] [Google Scholar]
- 30. Bruner-Tran KL, Eisenberg E, Yeaman GR, Anderson TA, McBean J, Osteen KG. Steroid and cytokine regulation of matrix metalloproteinase expression in endometriosis and the establishment of experimental endometriosis in nude mice. J Clin Endocrinol Metab. 2002;87(10):4782–4791 [DOI] [PubMed] [Google Scholar]
- 31. Olive DL. Role of progesterone antagonists and new selective progesterone receptor modulators in reproductive health. Obstet Gynecol Surv. 2002;57(11 suppl 4):S55–S63 [DOI] [PubMed] [Google Scholar]
- 32. Hemmings R, Rivard M, Olive DL, et al. Evaluation of risk factors associated with endometriosis. Fertil Steril. 2004;81(6):1513–1521 [DOI] [PubMed] [Google Scholar]
- 33. Bruner-Tran KL, ME M, Osteen KG. Chimeric Models of Experimental Endometriosis (in press) Giudice LC. (editor). 2010. [Google Scholar]
- 34. Hammond MG, Oh ST, Anners J, Surrey ES, Halme J. The effect of growth factors on the proliferation of human endometrial stromal cells in culture. Am J Obstet Gynecol. 1993;168(4):1131–1136; discussion 1136–1138 [DOI] [PubMed] [Google Scholar]
- 35. Harris HA, Bruner-Tran KL, Zhang X, Osteen KG, Lyttle CR. A selective estrogen receptor-beta agonist causes lesion regression in an experimentally induced model of endometriosis. Hum Reprod. 2005;20(4):936–941 [DOI] [PubMed] [Google Scholar]
- 36. Peng J, Sengupta S, Jordan VC. Potential of selective estrogen receptor modulators as treatments and preventives of breast cancer. Anticancer Agents Med Chem. 2009;9(5):481–499 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Stygar D, Muravitskaya N, Eriksson B, Eriksson H, Sahlin L. Effects of SERM (selective estrogen receptor modulator) treatment on growth and proliferation in the rat uterus. Reprod Biol Endocrinol. 2003;1:40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Stratton P, Sinaii N, Segars J, et al. Return of chronic pelvic pain from endometriosis after raloxifene treatment: a randomized controlled trial. Obstet Gynecol. 2008;111(1):88–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Pickar JH, Yeh IT, Bachmann G, Speroff L. Endometrial effects of a tissue selective estrogen complex containing bazedoxifene/conjugated estrogens as a menopausal therapy. Fertil Steril. 2009;92(3):1018–1024 [DOI] [PubMed] [Google Scholar]
- 40. Peano BJ, Crabtree JS, Komm BS, Winneker RC, Harris HA. Effects of various selective estrogen receptor modulators with or without conjugated estrogens on mouse mammary gland. Endocrinology. 2009;150(4):1897–1903 [DOI] [PubMed] [Google Scholar]
- 41. Vercellini P, De Giorgi O, Oldani S, Cortesi I, Panazza S, Crosignani PG. Depot medroxyprogesterone acetate versus an oral contraceptive combined with very-low-dose danazol for long-term treatment of pelvic pain associated with endometriosis. Am J Obstet Gynecol. 1996;175(2):396–401 [DOI] [PubMed] [Google Scholar]
- 42. Yap C, Furness S, Farquhar C. Pre and post operative medical therapy for endometriosis surgery. Cochrane Database Syst Rev. 2004;3:CD003678 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Hull ML, Charnock-Jones DS, Chan CL, et al. Antiangiogenic agents are effective inhibitors of endometriosis. J Clin Endocrinol Metab. 2003;88(6):2889–2899 [DOI] [PubMed] [Google Scholar]
- 44. Torry DS, Torry RJ. Angiogenesis and the expression of vascular endothelial growth factor in endometrium and placenta. Am J Reprod Immunol. 1997;37(1):21–29 [DOI] [PubMed] [Google Scholar]
- 45. Gargett CE, Rogers PA. Human endometrial angiogenesis. Reproduction. 2001;121(2):181–186 [DOI] [PubMed] [Google Scholar]
- 46. Smith SK. Angiogenesis, vascular endothelial growth factor and the endometrium. Hum Reprod Update. 1998;4(5):509–519 [DOI] [PubMed] [Google Scholar]
- 47. Smith SK. Regulation of angiogenesis in the endometrium. Trends Endocrinol Metab. 2001;12(4):147–151 [DOI] [PubMed] [Google Scholar]
- 48. Ferrara N, Frantz G, LeCouter J, et al. Differential expression of the angiogenic factor genes vascular endothelial growth factor (VEGF) and endocrine gland-derived VEGF in normal and polycystic human ovaries. Am J Pathol. 2003;162(6):1881–1893 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Ferrara N, Alitalo K. Clinical applications of angiogenic growth factors and their inhibitors. Nat Med. 1999;5(12):1359–1364 [DOI] [PubMed] [Google Scholar]
- 50. Laschke MW, Elitzsch A, Vollmar B, Vajkoczy P, Menger MD. Combined inhibition of vascular endothelial growth factor (VEGF), fibroblast growth factor and platelet-derived growth factor, but not inhibition of VEGF alone, effectively suppresses angiogenesis and vessel maturation in endometriotic lesions. Hum Reprod. 2006;21(1):262–268 [DOI] [PubMed] [Google Scholar]
- 51. Gescher DM, Haensel A, Meyhofer-Malik A, Malik E. [The importance of angiogenesis for the pathogenesis of endometriosis]. Zentralbl Gynakol. 2003;125(7-8):243–246 [DOI] [PubMed] [Google Scholar]
- 52. Oosterlynck DJ, Meuleman C, Sobis H, Vandeputte M, Koninckx PR. Angiogenic activity of peritoneal fluid from women with endometriosis. Fertil Steril. 1993;59(4):778–782 [DOI] [PubMed] [Google Scholar]
- 53. Sokolov DI, Solodovnikova NG, Pavlov OV, Niauri DA, Volkov NN, Sel’kov SA. Study of cytokine profile and angiogenic potential of peritoneal fluid in patients with external genital endometriosis. Bull Exp Biol Med. 2005;140(5):541–544 [DOI] [PubMed] [Google Scholar]
- 54. Nemerson Y. Tissue factor and hemostasis. Blood. 1988;71(1):1–8 [PubMed] [Google Scholar]
- 55. Belting M, Dorrell MI, Sandgren S, et al. Regulation of angiogenesis by tissue factor cytoplasmic domain signaling. Nat Med. 2004;10(5):502–509 [DOI] [PubMed] [Google Scholar]
- 56. Versteeg HH, Peppelenbosch MP, Spek CA. Tissue factor signal transduction in angiogenesis. Carcinogenesis. 2003;24(6):1009–1013 [DOI] [PubMed] [Google Scholar]
- 57. Versteeg HH, Ruf W. Emerging insights in tissue factor-dependent signaling events. Semin Thromb Hemost. 2006;32(1):24–32 [DOI] [PubMed] [Google Scholar]
- 58. Wolberg AS, Monroe DM, Roberts HR, Hoffman MR. Tissue factor de-encryption: ionophore treatment induces changes in tissue factor activity by phosphatidylserine-dependent and -independent mechanisms. Blood Coagul Fibrinolysis. 1999;10(4):201–210 [PubMed] [Google Scholar]
- 59. Versteeg HH, Ruf W. Tissue factor coagulant function is enhanced by protein-disulfide isomerase independent of oxidoreductase activity. J Biol Chem. 2007;282(35):25416–25424 [DOI] [PubMed] [Google Scholar]
- 60. Carmeliet P, Mackman N, Moons L, et al. Role of tissue factor in embryonic blood vessel development. Nature. 1996;383(6595):73–75 [DOI] [PubMed] [Google Scholar]
- 61. Toomey JR, Kratzer KE, Lasky NM, Stanton JJ, Broze GJ., Jr Targeted disruption of the murine tissue factor gene results in embryonic lethality. Blood. 1996;88(5):1583–1587 [PubMed] [Google Scholar]
- 62. Bugge TH, Xiao Q, Kombrinck KW, et al. Fatal embryonic bleeding events in mice lacking tissue factor, the cell-associated initiator of blood coagulation. Proc Natl Acad Sci USA. 1996;93(13):6258–6263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Hu Z, Sun Y, Garen A. Targeting tumor vasculature endothelial cells and tumor cells for immunotherapy of human melanoma in a mouse xenograft model. Proc Natl Acad Sci USA. 1999;96(14):8161–8166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Hu Z, Garen A. Intratumoral injection of adenoviral vectors encoding tumor-targeted immunoconjugates for cancer immunotherapy. Proc Natl Acad Sci USA. 2000;97(16):9221–9225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65. Hu Z, Garen A. Targeting tissue factor on tumor vascular endothelial cells and tumor cells for immunotherapy in mouse models of prostatic cancer. Proc Natl Acad Sci USA. 2001;98(21):12180–12185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Du H, Taylor HS. Stem cells and female reproduction. Reprod Sci. 2009;16(2):126–139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67. Kim JY, Tavare S, Shibata D. Counting human somatic cell replications: methylation mirrors endometrial stem cell divisions. Proc Natl Acad Sci USA. 2005;102(49):17739–17744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Braundmeier AG, Fazleabas AT. The non-human primate model of endometriosis: research and implications for fecundity. Mol Hum Reprod. 2009;15(10):577–586 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69. Kannan A, Fazleabas AT, Bagchi IC, Bagchi MK. The transcription factor C/EBPbeta is a marker of uterine receptivity and expressed at the implantation site in the primate. Reprod Sci. 2010;17(5):434–443 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. D'Hooghe TM, Kyama CM, Chai D, et al. Nonhuman primate models for translational research in endometriosis. Reprod Sci. 2009;16(2):152–161 [DOI] [PubMed] [Google Scholar]
- 71. Du H, Taylor HS. Contribution of bone marrow-derived stem cells to endometrium and endometriosis. Stem Cells. 2007;25(8):2082–2086 [DOI] [PubMed] [Google Scholar]
- 72. Taylor HS. Endometrial cells derived from donor stem cells in bone marrow transplant recipients. JAMA. 2004;292(1):81–85 [DOI] [PubMed] [Google Scholar]
- 73. Du H, Taylor HS. Stem cells and reproduction. Curr Opin Obstet Gynecol. 2010;22(3):235–241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Zhou Y, Gan Y, Taylor HS. Cigarette smoke inhibits recruitment of bone-marrow-derived stem cells to the uterus. Reprod Toxicol. 2011;31(2):123–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Taylor HS, Fei X. Emx2 regulates mammalian reproduction by altering endometrial cell proliferation. Mol Endocrinol. 2005;19(11):2839–2846 [DOI] [PubMed] [Google Scholar]
- 76. Goldstein JL, Brown MS. Regulation of the mevalonate pathway. Nature. 1990;343(6257):425–430 [DOI] [PubMed] [Google Scholar]
- 77. Zhang FL, Casey PJ. Protein prenylation: molecular mechanisms and functional consequences. Annu Rev Biochem. 1996;65:241–269 [DOI] [PubMed] [Google Scholar]
- 78. Franzoni F, Quinones-Galvan A, Regoli F, Ferrannini E, Galetta F. A comparative study of the in vitro antioxidant activity of statins. Int J Cardiol. 2003;90(2-3):317–321 [DOI] [PubMed] [Google Scholar]
- 79. Santanam N, Murphy AA, Parthasarathy S. Macrophages, oxidation, and endometriosis. Ann NY Acad Sci. 2002;955:183–198; discussion 119,–200, 396–406 [DOI] [PubMed] [Google Scholar]
- 80. Pellegrin S, Mellor H. Actin stress fibres. J Cell Sci. 2007;120(pt 20):3491–3499 [DOI] [PubMed] [Google Scholar]
- 81. Nobes CD, Hall A. Rho, rac, and cdc42 GTPases regulate the assembly of multimolecular focal complexes associated with actin stress fibers, lamellipodia, and filopodia. Cell. 1995;81(1):53–62 [DOI] [PubMed] [Google Scholar]
- 82. Dobreanu M, Dobreanu D, Fodor A, Bacarea A. Integrin expression on monocytes and lymphocytes in unstable angina short term effects of atorvastatin. Rom J Intern Med. 2007;45(2):193–199 [PubMed] [Google Scholar]
- 83. Xu H, Zeng L, Peng H, et al. HMG-CoA reductase inhibitor simvastatin mitigates VEGF-induced “inside-out” signaling to extracellular matrix by preventing RhoA activation. Am J Physiol Renal Physiol. 2006;291(5):F995–F1004 [DOI] [PubMed] [Google Scholar]
- 84. Nasu K, Yuge A, Tsuno A, Narahara H. Simvastatin inhibits the proliferation and the contractility of human endometriotic stromal cells: a promising agent for the treatment of endometriosis. Fertil Steril. 2009;92(6):2097–2099 [DOI] [PubMed] [Google Scholar]
- 85. Sokalska A, Wong DH, Cress A, et al. Simvastatin induces apoptosis and alters cytoskeleton in endometrial stromal cells. J Clin Endocrinol Metab. 2010;95(7):3453–3459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86. Kyama CM, Overbergh L, Debrock S, et al. Increased peritoneal and endometrial gene expression of biologically relevant cytokines and growth factors during the menstrual phase in women with endometriosis. Fertil Steril. 2006;85(6):1667–1675 [DOI] [PubMed] [Google Scholar]
- 87. Chung HW, Lee JY, Moon HS, et al. Matrix metalloproteinase-2, membranous type 1 matrix metalloproteinase, and tissue inhibitor of metalloproteinase-2 expression in ectopic and eutopic endometrium. Fertil Steril. 2002;78(4):787–795 [DOI] [PubMed] [Google Scholar]
- 88. Sluijter JP, de Kleijn DP, Pasterkamp G. Vascular remodeling and protease inhibition—bench to bedside. Cardiovasc Res. 2006;69(3):595–603 [DOI] [PubMed] [Google Scholar]
- 89. Schweitzer M, Mitmaker B, Obrand D, et al. Atorvastatin modulates matrix metalloproteinase expression, activity, and signaling in abdominal aortic aneurysms. Vasc Endovascular Surg. 2010;44(2):116–122 [DOI] [PubMed] [Google Scholar]
- 90. Porter KE, Turner NA. Statins for the prevention of vein graft stenosis: a role for inhibition of matrix metalloproteinase-9. Biochem Soc Trans. 2002;30(2):120–126 [DOI] [PubMed] [Google Scholar]
- 91. Bruner-Tran KL, Osteen KG, Duleba AJ. Simvastatin protects against the development of endometriosis in a nude mouse model. J Clin Endocrinol Metab. 2009;94(7):2489–2494 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Foyouzi N, Berkkanoglu M, Arici A, Kwintkiewicz J, Izquierdo D, Duleba AJ. Effects of oxidants and antioxidants on proliferation of endometrial stromal cells. Fertil Steril. 2004;82(suppl 3):1019–1022 [DOI] [PubMed] [Google Scholar]
- 93. Piotrowski PC, Kwintkiewicz J, Rzepczynska IJ, et al. Statins inhibit growth of human endometrial stromal cells independently of cholesterol availability. Biol Reprod. 2006;75:107–111 [DOI] [PubMed] [Google Scholar]
- 94. Esfandiari N, Khazaei M, Ai J, et al. Effect of a statin on an in vitro model of endometriosis. Fertil Steril. 2007;87(2):257–262 [DOI] [PubMed] [Google Scholar]
- 95. Sharma I, Dhawan V, Mahajan N, Chand Saha S, Dhaliwal LK. In vitro effects of atorvastatin on lipopolysaccharide-induced gene expression in endometriotic stromal cells. Fertil Steril. 2010. 94(5):1639-46.e1 [DOI] [PubMed] [Google Scholar]
- 96. Wassmann S, Laufs U, Muller K, et al. Cellular antioxidant effects of atorvastatin in vitro and in vivo. Arterioscler Thromb Vasc Biol. 2002;22(2):300–305 [DOI] [PubMed] [Google Scholar]
- 97. Weber MS, Stuve O, Neuhaus O, Hartung HP, Zamvil SS. Spotlight on statins. Int MS J. 2007;14(3):93–97 [PubMed] [Google Scholar]
- 98. Greenwood J, Steinman L, Zamvil SS. Statin therapy and autoimmune disease: from protein prenylation to immunomodulation. Nat Rev Immunol. 2006;6(5):358–370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Aljada A, O’Connor L, Fu YY, Mousa SA. PPAR gamma ligands, rosiglitazone and pioglitazone, inhibit bFGF- and VEGF-mediated angiogenesis. Angiogenesis. 2008;11(4):361–367 [DOI] [PubMed] [Google Scholar]
- 100. Imamoto E, Yoshida N, Uchiyama K, et al. Inhibitory effect of pioglitazone on expression of adhesion molecules on neutrophils and endothelial cells. Biofactors. 2004;20(1):37–47 [DOI] [PubMed] [Google Scholar]
- 101. Herington JL, Crispens MA, Carvalho-Macedo AC, et al. Development and prevention of postsurgical adhesions in a chimeric mouse model of experimental endometriosis. Fertil Steril. 2011;95(4):1295–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Mathur SP. Autoimmunity in endometriosis: relevance to infertility. Am J Reprod Immunol. 2000;44(2):89–95 [DOI] [PubMed] [Google Scholar]