Abstract
Our goal was to evaluate the therapeutic potential of a novel antibody to the insulin growth factor-1 receptor (IGF-1-R; AMG 479) in endometrial cancer cells. The endometrial cancer cell lines, ECC-1/PRAB72 and RL-95-2, were used. Treatment with AMG 479 (0.02-200 nmol/L) resulted in inhibition of cell proliferation at 72 to 120 hours. Insulin growth factor-1 (0.15-7.5 nmol/L) stimulated growth in both cell lines (range of 15%-42%, P = .0025-.0445), which could be blocked by pretreatment with AMG 479 (mean of 29% for ECC-1/PRAB72, P = .006-.007; mean of 36% for RL-95-2, P = .0002-.0045). AMG 479 suppressed IGF-1-R kinase activity in a dose-dependent manner. Cells treated with AMG 479 underwent either G1 (ECC-1/PRAB72) or G2 (RL-95-2) arrest. AMG 479 decreased human telomerase reverse transcriptase (hTERT) mRNA expression in both endometrial cancer cell lines. Treatment with AMG 479 rapidly blocked IGF-1-induced phosphorylation of IFG-1-R, Akt, and p44/42. Thus, manipulation of the IGF-1-R pathway may serve as a promising therapeutic strategy for the treatment of endometrial cancer.
Keywords: endometrial cancer, insulin growth factor-1, insulin growth factor-1 receptor, phosphatidylinositol 3-kinase/Akt pathway, mitogen-activated protein kinase pathway, telomerase
Introduction
Endometrial cancer is the 4th most common cancer among women in the United States, and the death rate from this disease has alarmingly increased by 227% over the past 10 years. Type I or those tumors of endometrioid histology comprise 70% to 80% of cases and are thought to arise from persistent unopposed estrogen stimulation, either endogenous or exogenous. Women who develop these tumors are typically perimenopausal or postmenopausal and often have risk factors such as obesity, hyperlipidemia, nulliparity, diabetes mellitus and insulin resistance, polycystic ovarian syndrome (PCOS), hypertension, and late-onset menopause. Obesity, which increases bioavailable estrogen levels by enhancing the conversion of androstenedione to estrone in peripheral adipose tissue, has been linked to a 5-fold increased risk of endometrial cancer. Diabetes and insulin resistance have been linked to a 2- to 3-fold increased risk of developing this disease.1–4
Prolonged exposure to unopposed estrogen secondary to obesity results in excess proliferation and ultimately the development of endometrial cancer. Estrogen acts through its nuclear receptor and subsequently regulates transcription of a variety of genes, including IGF-1. Cyclic changes in IGF-1 expression and signaling have been implicated in the regulation of the menstrual cycle, with increased transcription of IGF-1 in the proliferative phase as compared to the secretory phase. Insulin growth factor-1 mediates its proliferative effects on the endometrium through binding and activation of the insulin growth factor-1 receptor (IGF-1-R), which subsequently leads to activation of the phosphatidylinositol 3-kinase (PI3K)/Akt pathway and mitogen-activated protein kinase (MAPK) pathways.
The PI3K/Akt pathway is negatively regulated by phosphatase and tensin homolog (PTEN), and loss of PTEN expression is one of the most common molecular abnormalities associated with endometrial cancers. Loss of PTEN results in phosphorylation of Akt and ultimately, stimulation of cell growth and promotion of cell survival. Increased expression of the IGF-1-R has been found in endometrial hyperplasia and endometrial cancer specimens as compared to proliferative endometrium.5 Insulin growth factor-1 receptor was shown to be highly phosphorylated in cases of endometrial hyperplasia and cancer but not in proliferative endometrium, indicating increased activation of this receptor.5 Activated IGF-1-R was associated with increased Akt phosphorylation.5 In addition, higher fasting insulin levels have been positively associated with type I endometrial cancers in a prospective case-cohort study.6 Thus, given the potential interplay between the IGF-1 signaling and the PI3K/Akt and MAPK pathways, the IGF-1-R is a logical therapeutic target for type I endometrial cancers.
AMG 479 (Amgen, Thousand Oaks, California) is a fully human monoclonal antibody isolated against the human IFG-1R and is currently being evaluated in phase II trials.7–10 The IGF-1-R is a transmembrane receptor tyrosine kinase. Autophosphorylation of the IGF-1-R occurs after binding of the IGF-1 or IGF-2 ligands. In total, 3 tyrosine residues (Tyr1131, Tyr 1135, and Tyr1136) within the kinase domain are the earliest major autophosphorylation sites, and phosphorylation of these 3 sites is required for kinase activation.11–13 Preclinical work in human pancreatic cell lines has shown that AMG 479 effectively blocks binding of IGF-1 and IGF-2 to the IGF-1-R, without cross-reacting to the insulin receptor (IR), resulting in decreased cell proliferation.14 In addition, AMG 479 has been found to inhibit IGF-1-R activity in pancreatic carcinoma xenograft models, with a corresponding inhibition of tumor growth.14 Given these promising results, our goal was to evaluate the effect of AMG 479 on cell proliferation and expression of key targets involved in IGF-1-R signaling in endometrial cancer cell lines.
Methods
Cell Culture and Reagents
Two type I endometrial cancer cell lines, ECC-1/PRAB72 and RL-95-2, were used in these experiments. The ECC-1/PRAB72 cell line was generously provided by Dr Leen J. Blok (Erasmus Medical Center Rotterdam, The Netherlands). The ECC-1/PRAB72 cell line is both estrogen- and progesterone-sensitive and was derived after transfection with the human progesterone receptor A (hPRA) and B (hPRB).15 The RL-95-2 cell line expresses both the estrogen and the progesterone receptors. Both cell lines were maintained in Dulbecco’s-Modified Eagle’s Medium (DMEM) supplemented with 5% fetal bovine serum (FBS), 300 mmol/L l-glutamine, 5 μg/mL bovine insulin, 10 000 units/mL penicillin, and 10 000 μg/mL streptomycin under 5% CO2. AMG 479 was provided as a stock solution by Amgen Pharmaceuticals. Methyl thiazolyl-diphenyl-tetrazolium (MTT) dye and anti-β-actin antibody were purchased from Sigma-Aldrich (St Louis, Missouri). The anti-phosphorylated-IGF-1-R, anti-total-IGF-1-R, anti-phophorylated-AKT, anti-total-AKT, anti-phosphorylated-p42/44, anti-total-p42/44 antibodies, the IGF-1-R activity enzyme-linked immunosorbent assay (ELISA) kit, apoptosis screening ELISA kit, and the caspase-3 ELISA kit were purchased from Cell Signaling Technology (Beverly, Massachusetts). Enhanced chemiluminescence Western blotting detection reagents were purchased from Amersham Biosciences (Arlington Heights, Illinois). All other chemicals were purchased from Sigma-Aldrich.
Cell Proliferation Assay
Cell proliferation was determined by a MTT assay. Briefly, ECC-1/PRAB72 and RL-95-2cells (8 × 103) were plated onto 96-well culture plates for 24 hours. Cells were then treated with varying doses of AMG 479 or IGF-1 alone and in combination for 48, 72, 96, and 120 hours, using 0.5% stripped serum media. For the combination experiments, cells were pretreated with AMG 479 for 6 hours followed by the addition of IGF-1. Viable cell densities were determined by metabolic conversion of the dye MTT. Subsequently, the MTT assay results were read by measuring the optical density at 595 nm. The effects on cell proliferation of AMG 479, IFG-I, or both in combination were calculated as a percentage of control cell growth obtained from PBS (1%) treated cells grown in the same 96-well plates.
Insulin Growth Factor-1 Receptor Activity
Insulin growth factor-1 receptor activity was determined using the PathScan® Phospho-IGF-1-R β (Tyr1131) Sandwich ELISA Antibody Pair Protocol (Cell Signaling Technology, Danvers, Massachusetts) according to the manufacturer’s published protocol. Briefly, ECC-1/PRAB72 and RL-95-2 cells were cultured in 6-well plates for 24 hours and then starved overnight. Cells were treated with AMG 479 at varying concentrations in 5% fetal calf serum supplemented media for 60 minutes or exposed to AMG 479 (0.3 μg/mL), IGF-1 or both in combination in 0.5% stripped serum media for 15 minutes. For the combination experiments, cells were pretreated with AMG 479 for 3 or 6 hours followed by the addition of IGF-1. Cells were then washed with cold PBS and lysed with cell lysis buffer. The bicinchoninic acid (BCA) Protein Assay Kit (Pierce, Rockford, Illinois) was used to determine the protein concentration from the supernatant for each cell sample. A total of 100 μL of sample diluent plus 30 μg of protein was added to the ELISA plates and then incubated overnight at 4°C. The ELISA plates were washed with wash buffer followed by incubation with the detection antibody, TMB substrate, and stop solution, according to the manufacturer’s protocol. The ELISA plates were read by measuring absorption at 450 nm within 30 minutes after adding the STOP solution.
Flow Cytometry
ECC-1/PRAB72 and RL-95-2 cell lines were plated at 2.5 × 105 cells/well in 6-well plates in their corresponding media for 24 hours prior to treatment with AMG 479. Subsequently, the cells were either treated with different concentrations of AMG 479 for 36 hours or starved overnight and then treated with 15% serum and AMG 479 at varying concentrations for 36 hours. Cells were collected, washed twice with PBS, fixed in a 90% methanol solution, and then stored at −20°C until flow cytometric analysis was performed. On the day of analysis, the cells were washed and centrifuged twice using cold PBS to remove all traces of methanol. The cells were resuspended in 100 μL PBS and 10 μL of RNase A solution (D-PBS, 250 μg/mL DNase-free RNase A [Sigma-Aldrich], 10 mmol/L EDTA) followed by incubation for 30 minutes at 37°C. After incubation, 110 μL of propidium ioidide (PI; 100 μg/mL) stain was added to each tube and allowed to incubate at 4°C for at least 30 minutes prior to the analysis. Florescence was determined by flow cytometry on a CyAn (Beckman Coulter) fluorescence-activated cell sorter. ModFit (Verity Software House) was utilized for the analysis that controlled for dead cells and debris.
Apoptosis Assay
Apoptosis was measured by caspase-3 activity using the PathScan Apoptosis Multi-Target Sandwich ELISA Kit (Cell Signaling Technology, Danvers, Massachusetts) according to the manufacturer’s published protocol. Both cell lines were cultured in 6-well plates with the concentrations of 2 to 4 × 105 cells/well for 24 hours and then treated with AMG 479 at varying doses in 0.5% stripped serum for an additional 24 hours. The cells were lysed and the protein concentrations were measured to confirm equal loading onto an ELISA plate. Reagents were added as described by the manufacturer, and the ELISA plates were read by measuring absorption at 450 nm.
Real-Time Reverse Transcription Polymerase Chain Reaction
Cells were cultured for 24 hours and then treated with AMG 479 at varying doses in 0.5% stripped serum media for an additional 48 hours. Total RNA was isolated using the RNeasy kit (Qiagen Corporation, Germantown, Maryland). The reverse transcription and PCR reactions were performed using the TaqMan Gold 1-step reverse transcription polymerase chain reaction (RT-PCR) kit in the ABI Prism 7700 Sequence Detection System (Applied Biosystems, Foster City, California). Reverse transcription was carried out at 48°C for 30 minutes. The PCR condition consisted of a 10-minute step at 95°C and 40 cycles at 95°C for 15 seconds and 65°C for 1 minute. A housekeeping control gene acidic ribosomal phosphoprotein P0 (RPLP0, also known as 36B4) was used as an internal control to correct for the differences in the amount of RNA in each sample. The standard curve for human telomerase reverse transcriptase (hTERT) was generated by using dilutions of a known amount of cRNA synthesized by in vitro transcription of a cloned fragment. The normalized level of hTERT in each sample was estimated by a ratio of the hTERT level to the RPLP0 levelas described previously.16
Western Blot Analysis
The RL-95-2 and ECC-1/PRAB72 cells were plated at 2.5 × 105 cells/well in 6-well plates for 24 hours. Cells were treated with AMG 479, IFG-1, or both in combination at various time points. For the combination experiments, cells were pretreated with AMG 479 for 3 or 6 hours followed by the addition of IGF-1. Equal amounts of protein were separated by gel electrophoresis and transferred onto a High Hybond Hydrophobic Plyvinylidene Difluoride (PVDF) membrane (GE Healthcare Life Sciences, Piscataway, New Jersey). The membranes were blocked with 5% nonfat dry milk, and then incubated with a 1:1000 dilution of primary antibody overnight at 4°C. The membrane was then washed and incubated with a secondary peroxidase-conjugated antibody for 1 hour after washing. Antibody binding was detected using an enhanced chemiluminescence detection system (GE Healthcare Life Sciences, Piscataway, New Jersey). Western blot films were digitized, and band net intensities were quantified by a densitometer using the Genegynome Image System (Sygene, Maryland).
Statistical Analysis
The results for experiments were normalized to the mean of the control and analyzed using the Student’s t test. STATA software (StataCorp, College Station, Texas) was used to perform the statistical analyses.
Results
Sensitivity of Endometrial Cancer Cells to AMG 479
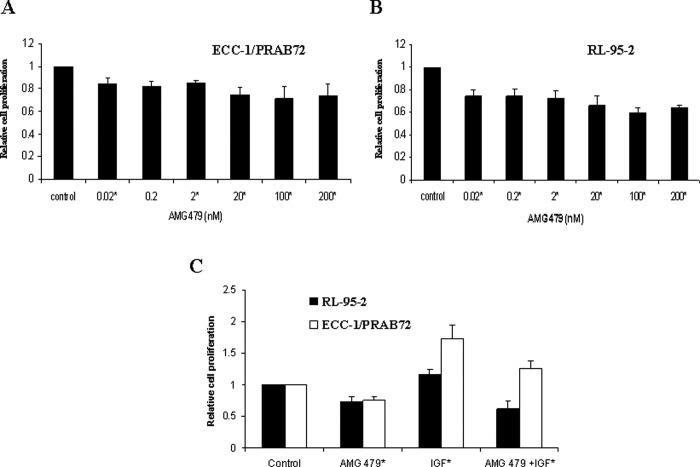
We examined the effect of AMG 479 on proliferation in 2 endometrial cancer cell lines. Treatment with AMG 479 (0.02-200 nmol/L) alone vs control (1% PBS) resulted in inhibition of cell proliferation at 72 to 120 hours (mean of 21% for ECC-1/PRAB72, P = .0005-.0123; mean of 31% for RL-95-2, P = .0001-.0030) (Figure 1A and B). Treatment with IGF-1 (0.15-7.5 nmol/L) stimulated growth in both of these cell lines (range of 15%-42%, P = .0025-.0445) as compared to PBS-treated controls. On the contrary, IGF-1-induced growth could be effectively blocked by pretreatment with AMG 479 for 6 hours (mean of 29% for ECC-1/PRAB72, P = .006-.007; mean of 36% for RL-95-2, P = .0002-.0045; Figure 1C). The Student’s t test was used to assess differences between groups. Thus, AMG 479 can effectively suppress IGF-induced endometrial cancer cell growth.
Figure 1.
Effect of AMG 479 on proliferation of endometrial cancer cells. The ECC-1/PRAB72 (A) and RL-95-2 (B) cell lines were cultured in the presence of varying concentrations of AMG 479 for 5 days. AMG 479 inhibited proliferation in both of these cell lines. Subsequently, both endometrial cancer cell lines were pretreated with AMG 479 (6.6 nmol/L) for 6 hours and then exposed to insulin growth factor-1 (IGF-1; 3.7 nmol/L) for 72 hours. AMG 479 blocked IGF-1-induced cell proliferation in both of these cell lines (C). Relative growth of cells was determined by MTT assay. The results are shown as the mean ± SE of triplicate samples and are representative of 3 independent experiments (* Indicates statistically significant difference.).
Effect of AMG 479 on IGF-1-R Activity
To assess the effect of AMG 479 on the activity of the IGF-1-R, the phosphotyrosine levels (Tyr 1131) of the activated IGF-1-R were measured by ELISA. Treatment with AMG 479 alone (0.02-200 nmol/L) for 1 hour significantly reduced IGF-1-R activity in a dose-dependent manner in both of the endometrial cancer cell lines (P = .0030-.0377 for ECC-1/PRAB72, P = .0059-.0437 for RL-95-2) as compared to PBS-treated controls (Figure 2A). As expected, the cells treated with IGF-1 alone (3.7 nmol/L) for 15 minutes demonstrated a dramatic increase in IGF-1-R kinase activity (P = .0040 for ECC-1/PRAB72, P = 0.0060 for RL-95-2; Figure 2B). However, pretreatment with AMG 479 (2 nmol/L) was able to potently block IGF-1 (0.15-7.5 nmol/L) stimulated autophosphorylation of the IGF-1-R in both endometrial cancer cell lines (P = .0050-.0327 for ECC-1/PRAB72, P = .0062-.0197 for RL-95-2; Figure 2B). The comparison group was IGF-1 (3.7 nmol/L) stimulated IGF-1-R kinase activity. The Student t test was used to assess differences between groups. Similar results were found after 3 and 6 hours of pretreatment with AMG 479 prior to exposure to IGF-1. This indicates that AMG 479 can successfully inhibit the kinase activity of the IGF-1-R, even in the presence of increasing concentrations of IGF-1.
Figure 2.
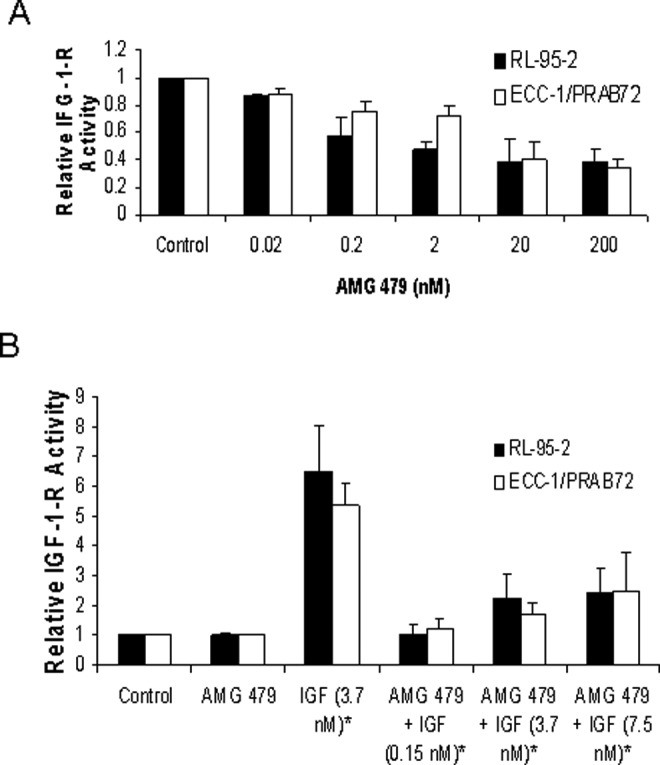
The effects of AMG 479 on insulin growth factor-1 receptor (IGF-1-R) activity. RL-95-2 and ECC-1/PRAB72 cells were starved overnight and then treated with 5% fetal bovine serum (FBS) and varying concentrations of AMG 479 alone for 60 minutes (A), or treated with AMG (2 nmol/L) in combination with IGF-1 for 15 minutes (B). The phosphotyrosine levels (Tyr 1131) of the activated IGF-1-R were measured by enzyme-linked immunosorbent assay (ELISA). Treatment with AMG 479 alone significantly reduced IGF-1-R activity in a dose-dependent manner in both of the endometrial cancer cell lines (A). As expected, cells treated with IGF-1 alone (3.7 nmol/L) for 15 minutes demonstrated a dramatic increase in IGF-1-R kinase activity (B). AMG 479 (2 nmol/L) was able to potently block IGF-1 (0.15-7.5 nmol/L) stimulated autophosphorylation of the IGF-1-R in both endometrial cancer cell lines (B). These results are representative of two2 independent experiments (* Indicates statistically significant difference.).
Effect of AMG 479 on Cell Cycle and Apoptosis
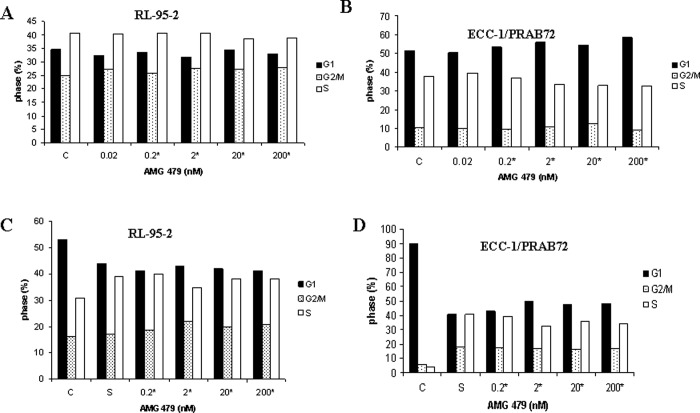
To characterize the mechanism of growth inhibition by AMG 479, the cell-cycle profile and induction of apoptosis was analyzed after treatment with AMG 479. The ECC-1/PRAB72 cells treated with AMG 479 underwent increased G1 arrest as demonstrated by flow cytometric analysis (Figure 3B ). In contrast, the RL-95-2 cells treated with AMG 479 underwent increased G2 arrest (Figure 3A). The percentage change ranged from 9% to 13% for the ECC-1/PRAB72 cell line and 11% to 13% for the RL-95-2 cell line as compared to PBS-treated controls (Student t test, P = .008-.0090). In order to investigate the impact of growth factors on control of the cell cycle by AMG 479, the cells were starved overnight and then treated with 15% serum alone or in combination with AMG 479. As expected, serum stimulation resulted in transition of cells from G1 to S phase by 24 hours, with a concomitant decrease in G1 phase (Figure 3C and D). AMG 479 significantly blocked serum-induced entry to S phase, resulting in G1 cell-cycle arrest in the ECC-1/PRAB72 cell line and G2 arrest in the RL-95-2 cell line (Figure 3C and D). The percentage change ranged from 13% to 19% for the ECC-1/PRAB72 cell line and 15% to 28% for the RL-95-2 cell line as compared to the serum-stimulated control (Student t test, P = .0028-.0377). The effect of AMG 479 on either G1 or G2 arrest appeared to be dose-dependent.
Figure 3.
Induction of cell-cycle arrest by AMG 479. RL-95-2 and ECC-1/PRAB72 cells were cultured for 24 hours and treated with AMG 479 at varying concentrations in 5% fetal bovine serum (FBS) for 36 hours (A and B) or starved overnight and then stimulated with 15% serum and AMG 479 at the noted concentrations for 36 hours (C and D). Cell-cycle analysis was performed by flow cytometry. AMG 479 inhibited cell-cycle progression by arrest in G1 phase in the ECC-1/PRAB72 cells, and G2/M phase in the RL-95-2 cells. Results shown are representative of 1 of the 3 independent experiments. Statistical significant results are indicated by a *, as determined using the Student t test.
An apoptosis assay using an antibody to caspase-3 was performed after exposure to AMG 479. Caspases are a family of cysteine proteases that act in a cascade to trigger apoptosis. Caspase-9 is an initiator caspase that is thought to activate the effector caspases (caspase-3 and -6) involved in actual cell disassembly. Caspase-3 is considered to be a specific marker for epithelial apoptosis. AMG 479 increased apoptosis by almost 3-fold but only at higher doses of treatment (200 nmol/L) as demonstrated by increased caspase-3 activity (P = .0017; Figure 4 ). Lower doses of AMG 479 had little effect on caspase-3 activity in this cell line. For the RL-95-2 cell line, there was no significant increase in caspase-3 activity at any dose of AMG 479 (Figure 4). In order to exclude other apoptotic targets involved, similar experiments were performed to assess the activity of p53, Bcl-2, Poly(ADP-ribose) Polymerase (PARP), and Bcl-2-Associated Death (BAD) in the RL-95-2 cell line. Even at higher doses (200 nmol/L), AMG 479 had little effect on the activity of any of these markers of apoptosis (data not shown).
Figure 4.
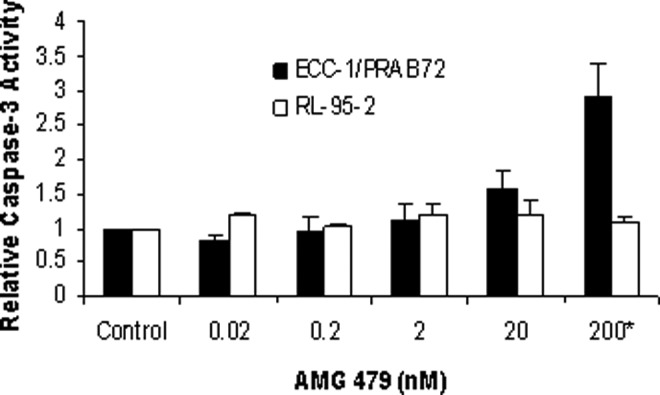
The effects of AMG 479 on apoptosis. The RL-95-2 and ECC-1/PRAB72 cells were cultured for 24 hours and then treated with AMG 479 at the indicated concentrations for an additional 24 hours. Apoptosis was assessed using an antibody to caspase-3. AMG 479 increased apoptosis in the ECC-1/PRAB72 cell line but only at high doses of treatment (200 nmol/L). Lower doses of AMG 479 had little effect on caspase-3 activity in this cell line. For the RL-95-2 cell line, there was no significant increase in caspase-3 activity at any dose of AMG 479. The results are shown as the mean ± SD and are representative of 2 independent experiments (* indicates statistically significant difference.).
These results suggest that AMG 479 predominantly inhibits cell growth via cell-cycle arrest in these endometrial cancer cell lines. This effect may involve either the G1 or the G2 checkpoint, depending on the endometrial cancer cell line. Induction of apoptosis may contribute to the anti-proliferative effect of AMG 479 in some endometrial cancer cells, especially at higher but yet still clinically relevant doses of treatment (200 nmol).17
Effect of AMG 479 on hTERT mRNA Level
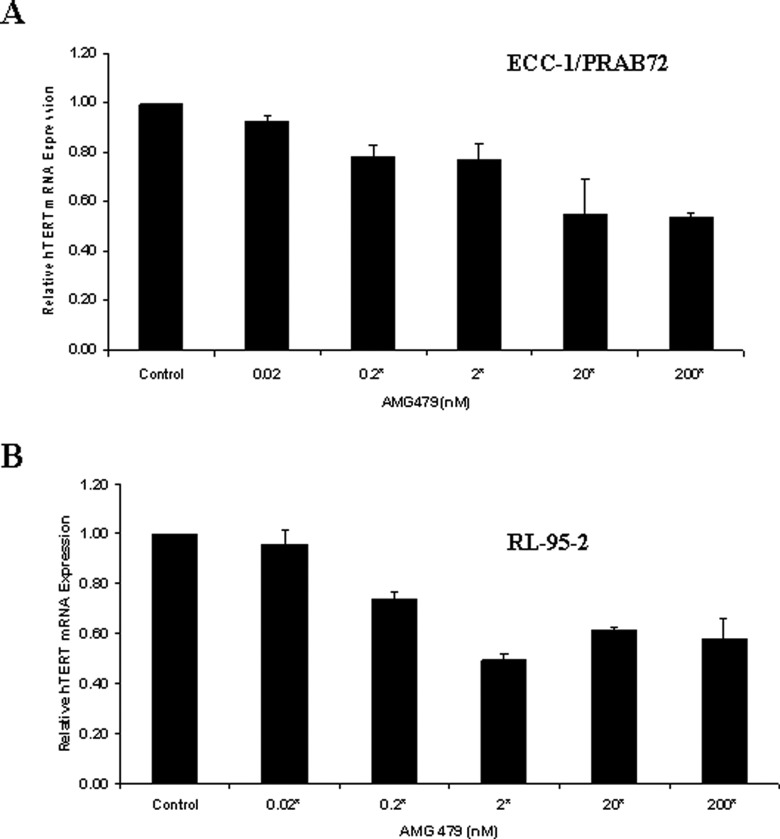
The maintenance of telomere length via the expression of telomerase is vital to the ability of cancer cells to remain proliferative. The hTERT gene encodes the catalytic subunit of telomerase. Human telomerase reverse transcriptase expression is the rate-limiting determinant of the enzymatic activity of human telomerase and is thought to be a sensitive marker of telomerase function and cell proliferation. Real-time RT-PCR was used to quantify hTERT mRNA expression in the endometrial cancer cell lines. Treatment with AMG decreased hTERT mRNA expression in a dose-dependent manner in both cell lines at 24 hours (data not shown) and 48 hours (Figure 5 ) (P = .0040-.0377 for ECC-1/PRAB72, P = .0175-.0481 for RL-95-2). These data suggest that AMG 479 may inhibit telomerase activity by rapidly decreasing hTERT mRNA levels. The measurement of hTERT mRNA expression may be implicated as a biomarker for inhibition of cell proliferation with AMG 479.
Figure 5.
AMG 479 decreased human telomerase reverse transcriptase (hTERT) mRNA expression in a dose-dependent manner in (A) ECC-1/PRAB72 and (B) RL-95-2 cells. Both cells were cultured for 24 hours and then treated with the indicated concentrations of AMG 479 for an additional 48 hours. Human telomerase reverse transcriptase expression was determined by real-time reverse transcription polymerase chain reaction (RT-PCR). The results are shown as the mean ± SE of 2 independent experiments (* indicates statistically significant difference.).
Effect of AMG 479 on IGF-1-R and its Downstream Pathways
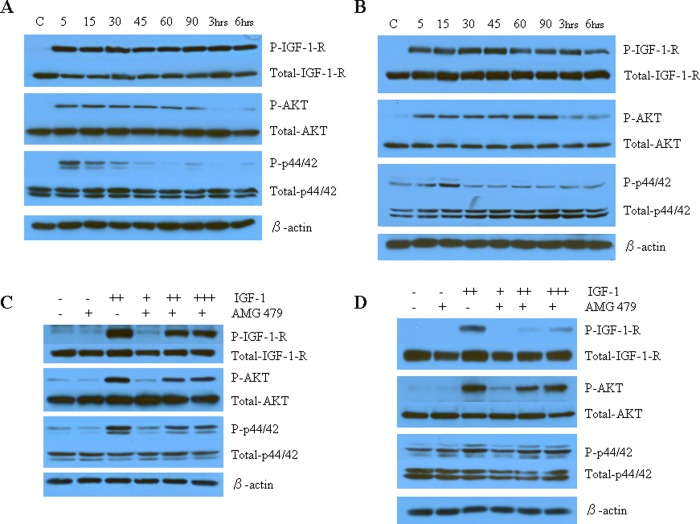
To investigate the mechanisms underlying the antiproliferative activity of AMG 479, we characterized the effect of AMG 479 on IGF-1-R and its relevant downstream cell signaling targets. Ligand-dependent signaling through the IGF-1-R leads to the phosphorylation and activation of PI3K/Akt and MAPK, both of which have been implicated in tumor cell proliferation and survival. As would be expected, IGF-1 alone rapidly induced phosphorylation of IGF-1-R within 5 minutes of exposure in both of the endometrial cancer cell lines (Figure 6A and B). This effect persisted for at least 6 hours after treatment. Similarly, treatment with IGF-1 resulted in phosphorylation of both Akt and MAPK (p42/44) after only 5 minutes of exposure. There was little effect on total-IGF-1-R, total-Akt or total-p42/p44 expression. To determine whether AMG 479 was capable of blocking signaling by IGF-1, Western blots were performed on cells treated with ligand in the presence or absence of AMG 479 pretreatment. AMG 479 blocked the phosphorylation of IGF-1-R, Akt, and p42/44 induced by IGF-1 (Figure 6C and D). AMG 479 decreased phosphorylation of Akt by 1.5-fold in the ECC-1/PRAB72 and by 2.5-fold in the RL-95-2 cell line, as determined by densitometric quantitation. In parallel, phosphorylation of p42/44 was decreased by 1.4-fold in the ECC-1/PRAB72 and by 1.3-fold in the RL-95-2 cell line. Similar results were found after 3 and 6 hours of pretreatment with AMG 479 prior to exposure to IGF-1. Once again, there was little effect on total-IGF-1-R, total-Akt, or total-p42/p44 expression. Thus, antibody-mediated blockade of ligand binding to the IGF-1-R by AMG 479 suppressed downstream signaling of the 2 principal IGF-1-mediated pathways, the PI3K/Akt and MAPK pathways.
Figure 6.
The effect of AMG 479 on IGF-1-R and its downstream cell signaling targets as assessed by Western immunoblotting. The ECC-1/PRAB72 (A and C) and RL-95-2 (B and D) cell lines were cultured for 24 hours followed by starving for 12 hours. The cell media was replaced with fresh media containing 0.5% stripped fetal bovine serum (FBS) and AMG 479 or IGF-1 as indicated. (A and B) IGF-1 activated IGF-1-R and its signaling pathway. Insulin growth factor-1 (7.5 nmol/L) alone rapidly induced phosphorylation of IGF-1-R in both of the endometrial cancer cell lines. Similarly, treatment with IGF-1 resulted in phosphorylation of both Akt and MAPK (p42/44) after only 5 minutes of exposure. (C and D) AMG 479 blocked the phosphorylation of IGF-1-R, AKT, and p42/44 induced by IGF-1. Serum-starved ECC-1/PRAB72 and RL-95-2 cells were pretreated with AMG 479 (6.6 nmol/L) for 3 hours and subsequently stimulated with different concentrations of IGF-1 for 15 minutes (+, 0.15 nmol/L; ++, 3.7 nmol/L; +++, 7.5 nmol/L). These results are representative of 2 independent experiments. There was little effect on total-Akt, total-p42/p44, or total IGF-1-R expression in either of these experiments.
Discussion
AMG 479 is a fully monoclonal antibody against the IGF-1-R that is under investigation in Phase II trials for colorectal, small cell lung, breast and pancreatic cancer as well as Ewing’s sarcoma.7–10 Our findings suggest that AMG 479 may also have anti-tumor activity for endometrial cancer. We found that AMG 479 was a potent inhibitor of IGF-1-stimulated cell proliferation in 2 endometrial cancer cell lines, predominantly through G1 and G2 cell-cycle arrest. In addition, AMG 479 suppressed hTERT mRNA expression in both of these cell lines. Finally, AMG 479 was able to potently block IGF-1-stimulated autophosphorylation and kinase activity of the IGF-1-R, resulting in subsequent inhibition of phosphorylation of the 2 major downstream signaling targets of the IGF-1-R pathway, Akt, and MAPK (p42/p44).
We have demonstrated that AMG 479 suppressed proliferation via G1 or G2 cell-cycle arrest, depending on the endometrial cancer cell line being evaluated (Figure 3). AMG 479 was also able to induce apoptosis in 1 of the 2 endometrial cancer cell lines. This effect was only seen at higher doses of treatment (200 nmol/L); however, this dose is still in the clinically therapeutic range (Figure 4).17 This suggests cell-cycle arrest as opposed to cell death is most likely the predominant contributor to the anti-proliferative effect of AMG 479 in endometrial cancer cells. Similar to our findings, AMG 479 as well as other blocking antibodies to the IGF-1-R appears to have varying effects on cell-cycle control and apoptosis even among cell lines derived from the same type of tumor. In human pancreatic cell lines, the efficacy of AMG 479 was the consequence of increased apoptosis in 1 cell line and decreased proliferation in the other.14 Differential effects of the fully human anti-IGF-1-R antibody, A12, were also found in human prostate cancer cell lines grown as xenografts.18 A12 induced G1 cell-cycle arrest in the androgen-dependent prostate cancer cell line as opposed to G2 arrest in the androgen-independent cell line.18 In addition, apoptosis was induced only in tumors derived from the androgen-dependent prostate cancer cell line.18 We confirm that induction of G2 arrest is an alternative mechanism of action of blocking antibodies to the IGF-1-R. Despite the varying effects of IGF-1-R blocking antibodies on cell-cycle control and apoptosis among cancer cells, this had little bearing on overall tumor regression in in vivo models14,18 and therefore will most likely not be important in regard to clinical response and outcomes to these agents.
In this study, AMG 479 was found to decrease hTERT expression in the endometrial cancer cell lines, which, to our knowledge, is the first time AMG 479 has been linked to the regulation of telomerase activity (Figure 5). Although we did not assess telomerase activity directly, hTERT expression is the rate-limiting determinant of the enzymatic activity of human telomerase and is thought to accurately correspond to telomerase function. Insulin growth factor-1 has been found to stimulate telomerase activity in prostate cancer cell lines, potentially through phosphorylation of hTERT by Akt and subsequent upregulation of hTERT mRNA expression.19 Increased expression of IGF-1 and IGF-1-R has also been positively correlated with increased telomerase activity and a higher cell proliferation index in endometrial cancer tumors.20 It is certainly reasonable to hypothesize that AMG 479 inhibits the effects of IGF-1 on telomerase activity by blocking binding of this ligand to the IGF-1-R. However, our experiments were performed in the absence of exogenous IGF-1, suggesting that AMG 479 may have a more direct effect on hTERT mRNA transcription.
Despite the well-established link between obesity, insulin resistance, and endometrial cancer, there have been few studies in regards to the IGF-1-R pathway and the pathogenesis of this disease. As previously described, IGF-1 binds to the extracellular domains of the IGF-1-R, resulting in activation of its intracellular tyrosine kinase domain and autophosphorylation of the receptor. This effect was validated in the endometrial cancer cell lines (Figure 2). In addition, AMG 479 was able to potently block IGF-1-induced autophosphorylation of the IGF-1-R in both of these cell lines (Figure 2). Subsequently, activated IGF-1-R phosphorylates its substrates, culminating in the initiation of the proliferative and anti-apoptotic signal transduction pathways, PI3K/Akt and MAPK. We confirmed that IGF-1 rapidly induced phosphorylation of IGF-1-R followed by phosphorylation of both Akt and MAPK (p42/p44) in the endometrial cancer cell lines. Furthermore, AMG 479 was successful in blocking ligand-stimulated phosphorylation of the IGF-1-R, Akt, and MAPK (p42/p44). Thus, we clearly demonstrate that AMG 479 is capable of preventing autophosphorylation of the IGF-1-R by IGF-1 in endometrial cancer cells and more importantly that this leads to inhibition of critical downstream signaling targets involved in cell growth and survival.
We postulate that increased circulating estrogen levels secondary to obesity may result in increased signaling through the IGF-1-R pathway in the endometrium of these high-risk women, leading to type I endometrial cancers. Estrogen itself does not increase IGF-1-R expression but progesterone has been shown to downregulate IGF-1-R expression. In the normal endometrium, progesterone antagonizes the actions of estrogen and inhibits estrogen-induced cell proliferation. Therefore, it may be the imbalance of estrogen and progesterone in the endometrium that results in overexpression of the IGF-1-R and stimulation of this pathway in endometrial cancers. Unfortunately, body mass index (BMI) or diabetic history has not been correlated with alterations in IGF-1-R expression and activation. Thus, it remains to be determined whether women who are obese and have evidence of insulin resistance will be more susceptible to pharmacologic suppression of IGF-1 signaling by AMG 479 than their normal weight, nondiabetic counterparts.
While most women with endometrial cancer will be diagnosed with early-stage disease and have a high cure rate with surgery alone, up to 25% of women will have advanced stage disease and require additional treatment. Advanced or recurrent endometrial cancer is notoriously difficult to treat with poor response rates and dismal overall survival. Given the limited success of traditional therapies with cytotoxic or hormonal regimens, the search has been for novel agents that target specific cellular signaling pathways thought to be essential in endometrial cancer progression and metastasis. Based on our study, blockade of the IGF-1-R seems to be a viable chemotherapeutic strategy for endometrial cancer. In addition, increased efficacy has been shown in vitro and in vivo for AMG 479 as well as other IGF-1-R-blocking antibodies when used in combination with other cytotoxic agents, suggesting that AMG 479 may also serve as a chemosensitizer.14,21,22 This may invariably hold true for endometrial cancer and is worthy of future evaluation for this disease.
Of note, two other endometrial cancer cell lines were tested with AMG 479 and were minimally affected by this treatment (data not shown). The 2 cell lines were (1) Ishikawa which is an ER-positive type I endometrial cancer cell line and (2) SPEC-2 which is an ER-negative type II endometrial cancer cell line. ECC-1/PRAB72 and RL-95-2, the cell lines used in this study, are both ER-positive. Given these findings, ER status does not seem to be a predictor of responsiveness to AMG 479 and more work needs to be done to elicit other potential biomarkers for sensitivity to this agent. In this pursuit, priority should be given to the identification of biomarkers both within the tumor and in the metabolic composition of the host that would stratify women to who would achieve the best clinical response to IGF-1-R inhibition and potentially individualize care for this disease.
Authors' Note:
This work was presented at the 2009 Annual Meeting of the American Association for Cancer Research, Denver, Colorado.
Footnotes
The authors declared no conflicts of interest with respect to the authorship and/or publication of this article.
The authors disclosed receipt of the following financial support for the research and/or authorship of this article: The V Foundation for Cancer Research and the Steelman Fund (Bae-Jump VL and Gehrig PA), The project described was also supported by Award Number KL2RR025746 (UNC Clinical Translational Science Award-K12 Scholars Program) from the National Center for Research Resources (Bae-Jump VL).
References
- 1. Chia VM, Newcomb PA, Trentham-Dietz A, Hampton JM. Obesity, diabetes, and other factors in relation to survival after endometrial cancer diagnosis. Int J Gynecol Cancer. 2007;17(2):441–446 [DOI] [PubMed] [Google Scholar]
- 2. Cust AE, Kaaks R, Friedenreich C, et al. Plasma adiponectin levels and endometrial cancer risk in pre- and postmenopausal women. J Clin Endocrinol Metab. 2007;92(1):255–263 [DOI] [PubMed] [Google Scholar]
- 3. Friberg E, Mantzoros CS, Wolk A. Diabetes and risk of endometrial cancer: a population-based prospective cohort study. Cancer Epidemiol Biomarkers Prev. 2007;16(2):276–280 [DOI] [PubMed] [Google Scholar]
- 4. Soliman PT, Wu D, Tortolero-Luna G, et al. Association between adiponectin, insulin resistance, and endometrial cancer. Cancer. 2006;106(11):2376–2381 [DOI] [PubMed] [Google Scholar]
- 5. McCampbell AS, Broaddus RR, Loose DS, Davies PJ. Overexpression of the insulin-like growth factor I receptor and activation of the AKT pathway in hyperplastic endometrium. Clin Cancer Res. 2006;12(21):6373–6378 [DOI] [PubMed] [Google Scholar]
- 6. Gunter MJ, Hoover DR, Yu H, et al. A prospective evaluation of insulin and insulin-like growth factor-i as risk factors for endometrial cancer. Cancer Epidemiol Biomarkers Prev. 2008;17(4):921–929 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Yuen JS, Macaulay VM. Targeting the type 1 insulin-like growth factor receptor as a treatment for cancer. Expert Opin Ther Targets. 2008;12(5):589–603 [DOI] [PubMed] [Google Scholar]
- 8. Chitnis MM, Yuen JS, Protheroe AS, Pollak M, Macaulay VM. The type 1 insulin-like growth factor receptor pathway. Clin Cancer Res. 2008;14(20):6364–6370 [DOI] [PubMed] [Google Scholar]
- 9. Hewish M, Chau I, Cunningham D. Insulin-like growth factor 1 receptor targeted therapeutics: novel compounds and novel treatment strategies for cancer medicine. Recent Pat Anticancer Drug Discov. 2009;4(1):54–72 [DOI] [PubMed] [Google Scholar]
- 10. Rodon J, DeSantos V, Ferry RJ, Jr, Kurzrock R. Early drug development of inhibitors of the insulin-like growth factor-I receptor pathway: lessons from the first clinical trials. Mol Cancer Ther. 2008;7(9):2575–2588 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hernandez-Sanchez C, Blakesley V, Kalebic T, Helman L, LeRoith D. The role of the tyrosine kinase domain of the insulin-like growth factor-I receptor in intracellular signaling, cellular proliferation, and tumorigenesis. J Biol Chem. 1995;270(49):29176–29181 [DOI] [PubMed] [Google Scholar]
- 12. Lopaczynski W, Terry C, Nissley P. Autophosphorylation of the insulin-like growth factor I receptor cytoplasmic domain. Biochem Biophys Res Commun. 2000;279(3):955–960 [DOI] [PubMed] [Google Scholar]
- 13. Baserga R. The IGF-I receptor in cancer research. Exp Cell Res. 1999;253(1):1–6 [DOI] [PubMed] [Google Scholar]
- 14. Beltran PJ, Mitchell P, Chung YA, et al. AMG 479, a fully human anti-insulin-like growth factor receptor type I monoclonal antibody, inhibits the growth and survival of pancreatic carcinoma cells. Mol Cancer Ther. 2009; 8(5):1095-1105. [DOI] [PubMed] [Google Scholar]
- 15. Hanifi-Moghaddam P, Sijmons B, Ott MC, et al. The hormone replacement therapy drug tibolone acts very similar to medroxyprogesterone acetate in an estrogen-and progesterone-responsive endometrial cancer cell line. J Mol Endocrinol. 2006;37(3):405–413 [DOI] [PubMed] [Google Scholar]
- 16. Bièche I, Nogues C, Paradis V, et al. Quantitation of hTERT gene expression in sporadic breast tumors with a real-time reverse transcription-polymerase chain reaction assay. Clin Cancer Res. 2000;6(2):452–459 [PubMed] [Google Scholar]
- 17. Tolcher AW, Sarantopoulos J, Patnaik A, et al. Phase I, pharmacokinetic, and pharmacodynamic study of AMG 479, a fully human monoclonal antibody to insulin-like growth factor receptor 1. J Clin Oncol. 2009;27(34):5800–5807 [DOI] [PubMed] [Google Scholar]
- 18. Wu JD, Odman A, Higgins LM, et al. In vivo effects of the human type I insulin-like growth factor receptor antibody A12 on androgen-dependent and androgen-independent xenograft human prostate tumors. Clin Cancer Res. 2005;11(8):3065–3074 [DOI] [PubMed] [Google Scholar]
- 19. Wetterau LA, Francis MJ, Ma L, Cohen P. Insulin-like growth factor I stimulates telomerase activity in prostate cancer cells. J Clin Endocrinol Metab. 2003;88(7):3354–9 [DOI] [PubMed] [Google Scholar]
- 20. Pavelić J, Radaković B, Pavelić K. Insulin-like growth factor 2 and its receptors (IGF 1R and IGF 2R/mannose 6-phosphate) in endometrial adenocarcinoma. Gynecol Oncol. 2007;105(3):727–735 [DOI] [PubMed] [Google Scholar]
- 21. Goetsch L, Gonzalez A, Leger O, et al. A recombinant humanized anti-insulin-like growth factor receptor type I antibody (h7C10) enhances the antitumor activity of vinorelbine and anti-epidermal growth factor receptor therapy against human cancer xenografts. Int J Cancer. 2005;113(2):316–328 [DOI] [PubMed] [Google Scholar]
- 22. Maloney EK, McLaughlin JL, Dagdigian NE, et al. An anti-insulin-like growth factor I receptor antibody that is a potent inhibitor of cancer cell proliferation. Cancer Res. 2003;63(16):5073–5083 [PubMed] [Google Scholar]