Abstract
Women with endometriosis have significant emotional distress; however, the contribution of stress to the pathophysiology of this disease is unclear. We used a rat model of endometriosis to examine the effects of stress on the development of this condition and its influence on inflammatory parameters. Female Sprague-Dawley rats were subjected to swim stress for 10 consecutive days prior to the surgical induction of endometriosis by suturing uterine horn implants next to the intestinal mesentery (endo-stress). Sham-stress animals had sutures only, and an endo-no stress group was not subjected to the stress protocol. At the time of sacrifice on day 60, endometriotic vesicles were measured and colons assessed for macroscopic and microscopic damage. Colonic tissue and peritoneal fluid were collected for inflammatory cell analysis. Endometriosis, regardless of stress, produced a decrease in central corticotropin-releasing factor immunoreactivity, specifically in the CA3 subregion of the hippocampus. Prior exposure to stress increased both the number and severity of vesicles found in animals with endometriosis. Stress also increased colonic inflammation, motility, myeloperoxidase levels, and numbers of mast cells. In summary, prior stress may contribute to the development and severity of endometriosis in this animal model through mechanisms involving cell recruitment (eg, mast cells), release of inflammatory mediators, and deregulation of hypothalamic–pituitary axis responses in the hippocampus.
Keywords: corticotropin releasing factor, endometriosis, inflammation, mast cells, rat, stress
Introduction
Endometriosis, a disease defined as the presence of endometrial glands and stroma outside the endometrial cavity, is characterized by chronic, severe pain, and infertility.1 The symptoms of this disease, which occur throughout the reproductive life of patients, are considered significant sources of stress.2–5 In fact, patients with endometriosis have reported to have high levels of stress due to the negative impact of the symptoms in all aspects of life, including work, relationships, and fertility.6–11 Moreover, women with endometriosis report more stress and a higher negative impact on daily activities than women with other pain syndromes.12,13Given that women are more prone to undergo stress possibly in part due to fluctuations in hormone levels that occur during puberty, pregnancy, and menopause14and that exposure to stress can cause and/or exacerbate numerous diseases, we hypothesize that exposure to stress can worsen endometriosis as well.
In the past, gynecologists often described the typical endometriosis patient as being white, upper middle class, and career oriented.15 These patients were described as obsessive, overanxious, and ambitious. Many had stressful careers and had perhaps deliberately delayed having children in order to pursue their professional ambitions.15 With improved diagnosis since the introduction of the laparoscopy and more awareness, it is apparent that endometriosis is found in many different types of women, regardless of race or socioeconomic status. However, it is still unknown whether there are psychological traits that may influence or predispose a woman to endometriosis. Interestingly, Low and colleagues reported that women with endometriosis scored higher on psychoticism, introversion, and both state and trait anxiety.16 Since the psychometric analyses were done prior to surgical diagnosis, such that the women presenting with chronic pelvic pain did not know the cause of their symptoms until after the surgery, the authors argue that they did not expect to see differences between the 2 groups (both groups scored equally on pain measures, both groups could have had high anxiety scores due to their upcoming surgery), yet patients that eventually were diagnosed with endometriosis were significantly more anxious.16 Thus, they concluded that a certain psychological profile, characterized by high anxiety levels, could make women more vulnerable to endometriosis. High levels of stress due to endometriosis have also been documented using physiologic and neural reactivity studies, including electroencephalography, digital skin temperature, electrodermal response, and electromyography.17 This case report showed that endometriosis symptoms were associated with parameters consistent with prolonged stress reactions and concluded that patients with endometriosis may benefit from self-regulation training and psychotherapy.
When the body is placed under stress, it secretes cortisol (corticosterone in the case of rodents). Chronic activation of stress responses, including the hypothalamic–pituitary–adrenal (HPA) axis and the sympathetic–adrenal–medullary axis, can lead to deregulation of corticotropin releasing factor (CRF) in hypothalamic and other limbic brain areas such as the hippocampus. Consequently, this can result in increased expression of glucocorticoid receptors by a variety of immune cells, which can bind to and become activated by systemic cortisol/corticosterone.18Therefore, it has been suggested that markers of HPA axis functioning might serve as an indicator of chronic overreaction of the body’s stress system.19 The stress caused by endometriosis symptoms has been hypothesized to cause a deregulation in the neuroendocrine–immune axis leading to disease.3,20 Hypocortisolism has been reported for a number of pain conditions that cause chronic stress including endometriosis.21,22 Over the long term, this could result in a number of negative health outcomes such as several disease conditions (eg, diabetes, hypertension, cancer, and cardiovascular disease).23,24 Abnormal HPA responses have also been documented in patients with chronic pelvic pain and mood disorders (eg, anxiety, depression, posttraumatic stress syndrome).25
The stress–illness relationship is now viewed as a complex process encompassing predisposing stressors and moderating factors, such as social factors and coping.26 However, whether stress affects the prevalence or disease severity of endometriosis has not been systematically studied before. The aim of these studies was to assess whether prior stress increases the likelihood of developing endometriosis and can exacerbate the severity of the disease and to identify the neuroimmune mechanisms involved.
Materials and Methods
The experiments reported herein were performed in accordance with the principles described in the “Guide for the Care and Use of Laboratory Animals,” Publication No. DHMS (NIH) 86-23.
Animal Model
Studies were performed with female Sprague-Dawley rats weighing 200 to 250 g (Southern Veterinary Service, PSM, Puerto Rico); with 9 to 10 animals per treatment group (N = 9-10). All animals were maintained in a restricted-access room with controlled temperature (23°C) and light–dark cycle. Standard laboratory chow and tap water were provided ad libitum. All experimental procedures involving animals were approved by the Animal Care and Use Committee at Ponce School of Medicine. Animals were handled for 7 days (5 min/d per rat) prior to beginning the experiment in order to reduce experimenter-induced stress on the animal, and daily vaginal cytological smears were carried out for all rats to check their reproductive cyclicity (Figure 1). The experiments were carried out at the same time of day (early afternoon) to minimize the influence of circadian rhythms.
Figure 1.
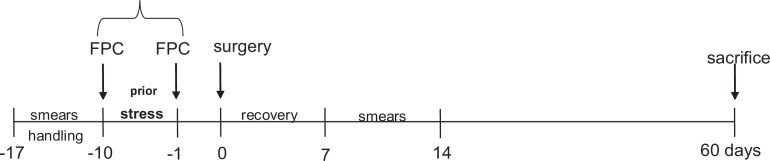
Experimental design. Animals were subjected to swim stress for 10 consecutive days prior to the surgical induction of endometriosis. All animals were checked for regular cycling by analysis of smears during the protocol and sacrificed 60 days after surgery. FPC indicates fecal pellet count.
Stress Protocol
To induce stress animals were exposed to a swim stress protocol using a water maze protocol for 10 days prior to the surgical induction of endometriosis or sham surgery (Figure 1).27 The water maze apparatus consisted of a plastic pool (150 cm diameter and 60 cm deep) filled with water (37°C ± 1°C) and made opaque with nontoxic water-soluble paint. The position of the animals during the task was monitored and recorded using a ceiling-mounted video camera connected to a computerized tracking/imaging analyzer system (HVS-Image, Hampton, UK; Watermaze Software, Edinburgh, UK). Each animal received 10 trials of training per day, for 10 days. The duration of each trial was 60 seconds, separated by a 1 minute intertrial interval, to allow the animal to rest. The water temperature in the pool was closely monitored and maintained at 37°C. For each trial, the animals were released into the water facing the edge of the pool from 1 of the 4 equally spaced locations in a pseudorandom order and allowed to swim for 60 seconds. The colonic propulsive activity was assessed by counting the number of fecal pellets expelled during each stress trial.28,29 The average from the 10 trials was calculated for each day of the stress protocol and expressed as the number of fecal pellets/minute. The endo-no stress animals were maintained undisturbed in their respective home cages and fecal pellets expelled during the stress protocol were counted. Stressed animals were then randomly assigned to 1 of the 2 experimental groups: sham-stress or endo-stress.
Induction of Endometriosis
Endometriosis was induced surgically during estrus under pentobarbital anesthesia, based on the method of Vernon and colleagues.30,31 Briefly, the distal 2 cm of the right uterine horn were removed and immersed in warm (37°C) sterile culture medium. The endometrium was exposed by opening the uterine horn lengthwise with a pair of sterile scissors. Four pieces of uterine horn measuring 2 mm × 2 mm were cut. These implants were sutured with the endometrial surface next to the mesenteric vessels of the small intestine. In the sham operated group, 4 sutures were attached to the mesentery of the intestine without uterine implants, and the right uterine horn was massaged with fingertips for 2 minutes to minimize any effects resulting from the mechanical handling of the uterine horn (that might have occurred in the endometriosis animals). The peritoneal cavity was kept moist with copious amounts of saline solution throughout the surgery to reduce adhesions. Based on prior studies,30,31we allowed endometriosis to progress for 60 days following the induction surgery before sacrificing.
Collection of Tissues
At the time of sacrifice, all animals had a cytological smear taken to allow for interpretation of any effects of the stage of estrus cycle on the experimental results. Peritoneal fluid was aseptically aspirated using a sterile micropipette, taking care not to contaminate with blood, and stored at −20°C for future analyses. A smear was also prepared on a microscope slide for assessment of peritoneal inflammation (see below). A laparotomy was performed to allow for assessment of disease severity as described below and to collect tissues. The whole colon was removed and tissue segments were fixed in 10% formalin, or weighed, frozen on dry ice, and then stored at −20°C until assayed.
Assessment of Endometriosis Severity
The peritoneal cavity was systematically examined for the presence of the implants and the original sutures. The classifications of vesicles in terms of grades of growth were carried out as previously described.31,32 In brief, the site of the implants was examined for the presence/development of vesicles or cysts and their diameter measured. Vesicles <2 mm received a grade of 2, vesicles with fluid ≥ 2 mm <4.5 mm received a grade of 3, and vesicles ≥4.5 mm received a grade of 4. If the implant had disappeared, it received a grade of 1.
Assessment of Peritoneal Inflammation
A Wright stained smear was prepared from a drop of peritoneal fluid to enable the quantification of inflammatory cells. The number of white blood cells (WBCs), and a WBC differential in the peritoneal fluid was determined microscopically by examining 5 high-power fields (×400) and obtaining an average number.
Colonic Microscopic Damage
After routine processing, colon tissue segments of 4 µm were stained with hematoxylin–eosin to determine the extent of inflammatory infiltration and the appearance of the underlying muscle layers. Histological assessment of damage was performed using previously published criteria.33 Briefly, the loss of mucosal architecture (0-3: absent, mild, and severe), muscle thickness (0 = muscle is less than 1/2 of mucosal thickness; 1 = muscle is 1/2 to 3/4 of mucosal thickness; 2 = muscle is equal to mucosal thickness; and 3 = all muscles) neutrophil infiltration (0 = none; 1= in muscularis mucosae; 2 = in lamina propria/villi; 3 = in serosa), crypt abscess formation (0 = absent; 1 = present), and goblet cell depletion (0 = absent; 1 = present) were evaluated. The score of each variable was added to give a total microscopic damage score (maximum of 11).
Microscopic Analysis for Mast Cells
Segments of rat colon were fixed in 10% formalin, processed by routine histological techniques, and mounted on glass slides. The sections were stained with Toluidine blue to permit the visualization of the mast cells. The numbers of mast cells in 10 randomly selected high-power fields were counted and the mean number of mast cells per field calculated.
Measurement of Neutrophil Infiltration
Tissue myeloperoxidase (MPO) activity was determined in colonic tissues as an index of granulocyte infiltration. Myeloperoxidase is an enzyme found within the azurophilic granules of neutrophils and other cells of myeloid origin. It has been demonstrated previously that these levels reflect the state of inflammation in the mucosa of the intestine.34 Approximately 100 mg of flash-frozen tissues that were collected from both the distal region and most proximal region of the colon were analyzed. A modification of the technique described by Bradley et al was used.35 In this assay, hydrogen peroxide is broken down by myeloperoxidase released from tissue samples by the addition of a detergent. The resultant oxygen radical combines with a hydrogen donor and the colorimetric change is measured on a plate reader.
Brain CRF Immunohistochemistry and Quantification
After tissues were collected, rat brains were fixed by aortic arch perfusion with 4% paraformaldehyde. The brains were removed and post-fixed for 24 hours in 30% sucrose/10% buffered formalin. Coronal sections were obtained on a Leica Vibratome (40 µm) and stored in cryoprotectant until immunocytochemical processing. Prior to immunocytochemistry, coronal sections of each treatment groups were coded with hole punches and pooled into single crucibles. Tissue sections were rinsed in phosphate buffer (PB) followed by Tris-buffered saline (TBS; pH 7.6) and incubated in 0.5% bovine serum albumin (BSA) in TBS for 30 minutes. Tissue sections were incubated for 24 hours at room temperature and for 24 hours at 4°C in guinea pig polyclonal antiserum raised against human/rat CRF (1:7000; Bachem Penninsula, San Carlos, California) in 0.1% BSA in TBS with 0.25% Triton X-100. This antibody has been previously characterized for specificity.36 Sections were then rinsed in TBS and incubated with peroxidase–avidin complex (at twice the recommended dilution; Vector, Burlingame, California) for 30 minutes followed by development in 3,3’-diaminobenzidine (DAB) and H2O2 in TBS for 6 minutes. Sections were mounted on slides, dehydrated, and cover slipped. For quantitative densitometry, images of regions of interest (eg, parvocellular and magnocellular subdivisions from the paraventricular nucleus of the hypothalamus [PVA]; in the hippocampus, the CA3 subregions) were captured using a CCD Camera on a Nikon 200 microscope at the same illumination level for all images within a comparison group.37 The average pixel density (of 256 gray levels) for each selected region was determined. It has been previously shown that light microscopic pixel density linearly correlated with the density of dynorphin-labeled dense-core vesicles (average r = .92).37 Most neuropeptides such as dynorphin and CRF are stored within dense-core vesicles.38 In addition, pixel density obtained at the light microscopy level from linearly correlated with the transmittance obtained from neutral density filters with defined transmittance (Pearson correlation, r = .998).37 Regions of interest were outlined to determine the mean gray density. To compensate for background staining and control for variations in illumination level between images, the average pixel density for 3 regions that lack labeling was subtracted. Average values for each animal from 2 separate experimental runs were used to determine the mean group average. Values from control and experimental tissue processed together were statistically compared to determine the differences in immunohistochemical-labeling density.
Statistical Analysis
Data were analysed by using GraphPad Instat version 3.0 (GraphPad Software, San Diego, California) and SPSS 19. A P < .05 was considered to represent a statistically significant difference. Numerical variables such as weight, optical density units, CRF immunoreactivity, fecal pellet counts, MPO levels, and mast cell numbers were evaluated for normality using the Shapiro-Wilk test. The mean difference ± the standard error of the mean (SEM) was used to assess the differences before and after exposure to stress and among treatment groups. In order to assess the statistical significance of the mean differences, a parametric 1-way analysis of variance (ANOVA) was used for normally distributed variables, using the Bonferroni correction test for the post hoc pairwise contrasts. A nonparametric Kruskal-Wallis H test was used for not normally distributed variables, and the Mann-Whitney U test was used for the post hoc pairwise contrasts after taking into account the accumulation of the type I error.
Results
Anxiety
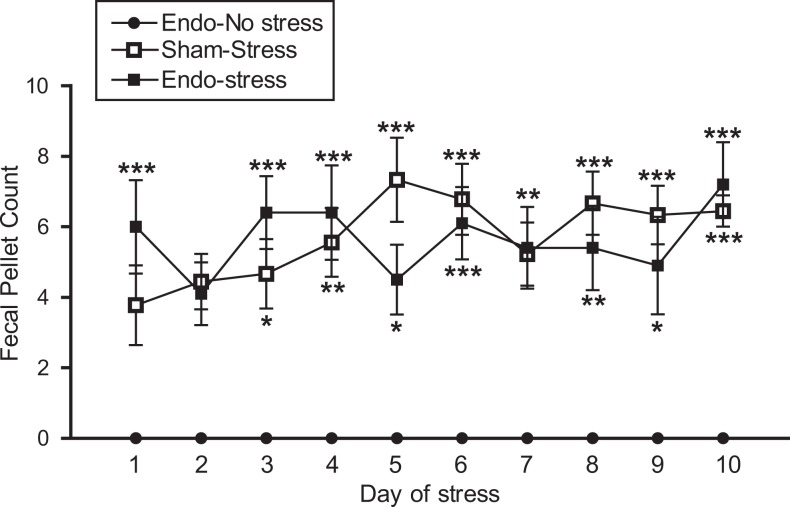
The number of fecal pellets produced during the stress protocol was used as an indirect index of anxiety related to stress.28,39–41 During the 10 days of the stress protocol, the fecal output of the endo-no stress animals (placed in a new clean cage) remained at about the level of zero (Figure 2). All animals receiving stress had significantly higher defecation than those not receiving stress throughout the 10-day period, indicating higher levels of anxiety regardless of whether the animals had endometriosis or not. There appeared to be no difference in fecal output between the 2 groups receiving stress (endo-stress and sham-stress), indicating that both groups were equally anxious.
Figure 2.
Stress increases anxiety levels. Both the sham-stress and endo-stress groups had increased fecal pellet counts compared to endo-no stress, indicating increased anxiety levels *P < .05,**P < .01,***P < .001 versus endo-no stress (9-10 animals/group).
Vesicle Size
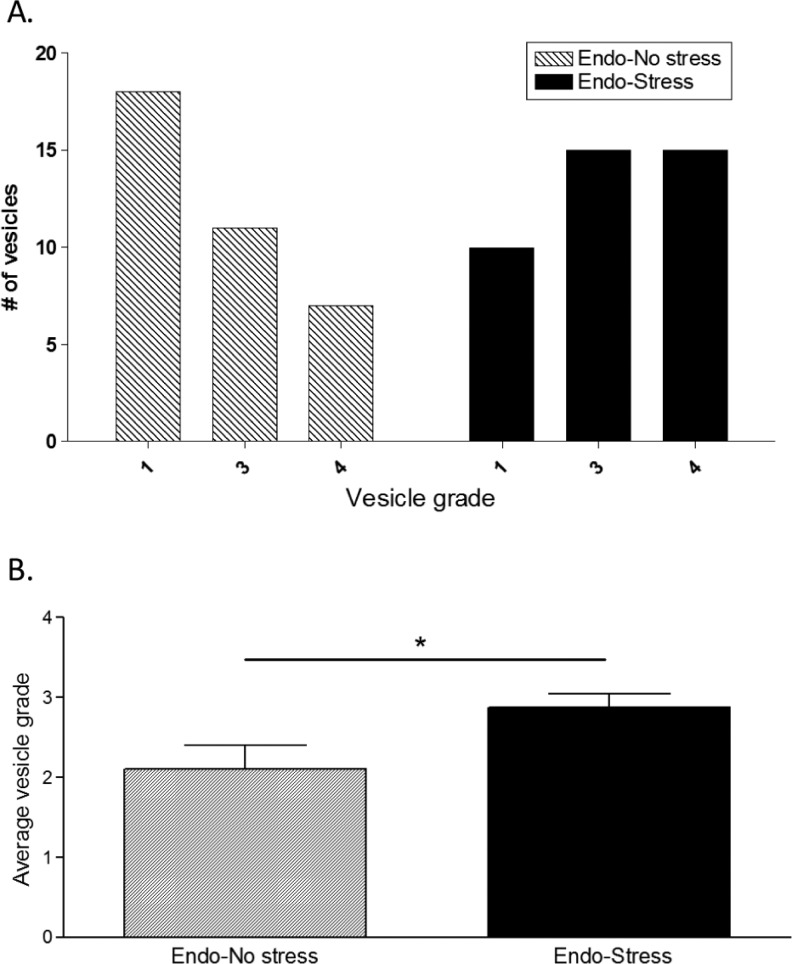
There was no difference in the body weight between the groups at the time of sacrifice. After the animals were sacrificed, classification of the vesicles was performed as described.31 As expected, none of the sham-stress animals developed vesicles at the suture sites. In contrast, all of the experimental (implanted) rats developed vesicles in at least one of the implant sites. The endo-no stress animals developed a vesicle in 50% of their sutures. This was increased to 75% in the endo-stress group (Figure 3A). In addition, the frequency of vesicles with a higher grade of severity per animal was significantly increased in those animals that were subjected to stress, with 50% of the vesicles having a diameter larger than 4.5 mm (grade 4; Figure 3A and B).
Figure 3.
Prior stress increases implant frequency and severity. None of the sham-stress animals developed vesicles. The endo-no stress group developed a vesicle in 50% of their sutures (grades 2, 3, or 4). Prior exposure to stress increased both, (A) the number of vehicles developed and (B) the average vesicle grade (9-10 animals/group; *P < .05 vs endo-no stress).
Although most of the rats were surgically induced when in estrus phase (sometimes in proestrus), at the time of sacrifice there was a spread among the different phases, with the majority (about 80%) being in either proestrus or estrus, since all animals were sacrificed exactly 60 days after surgery (Table 1). Notably, there was a difference in the estrus phase at sacrifice between the groups: in the endo-no stress group, 67% of animals were in proestrus; while in the group receiving stress, only 30% were in that phase.
Table 1.
Stage of Cycle at the Time of Sacrifice
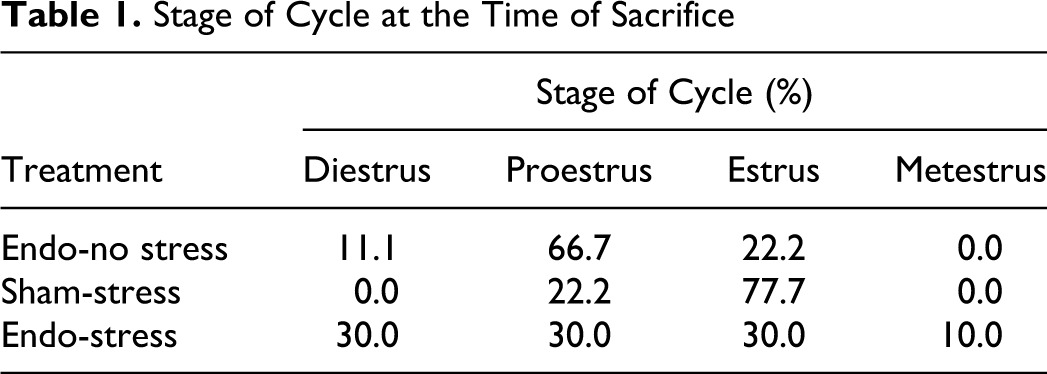
| Stage of Cycle (%) | ||||
|---|---|---|---|---|
| Treatment | Diestrus | Proestrus | Estrus | Metestrus |
| Endo-no stress | 11.1 | 66.7 | 22.2 | 0.0 |
| Sham-stress | 0.0 | 22.2 | 77.7 | 0.0 |
| Endo-stress | 30.0 | 30.0 | 30.0 | 10.0 |
Colonic Damage and Cell Infiltration
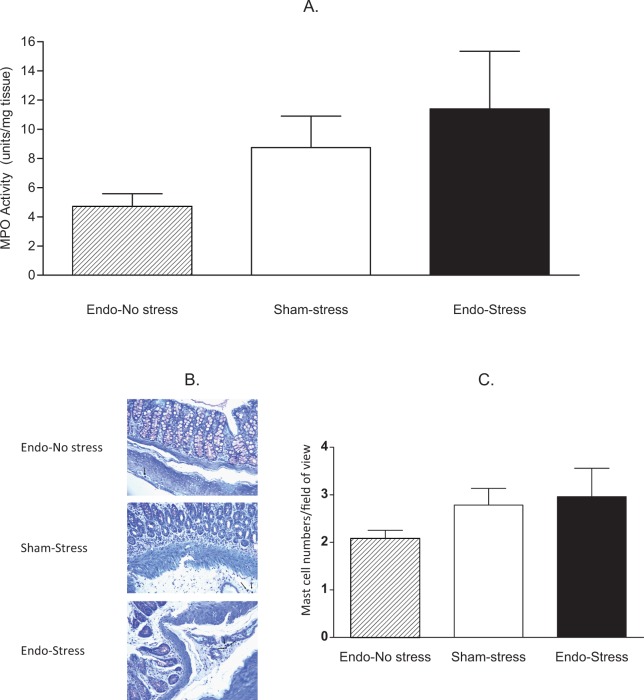
Segments of colons were analyzed for measurement of MPO activity, to give an indication of neutrophil infiltration. The levels of MPO were higher in the colon of all animals exposed to stress. Endo-stress animals had MPO levels ∼3-fold higher than endo-no stress, but this difference did not reach statistical significance (11.40 ± 3.94 vs 4.71 ± 0.87 units/mg of tissue; n = 8-10; Figure 4A).
Figure 4.
Effect of prior stress on myeloperoxidase (MPO) activity and mast cell numbers. A, The endo-stress animals had the highest MPO levels in colonic tissue (9-10 animals/group), but this difference did not reach statistical significance. B, Representative tissue sections of colon; ×400; C, Average mast cell numbers per field of view in the colon (5-6 animals/group).
Segments of colons were stained with hematoxylin and eosin for microscopic analysis, and the parameters taken into consideration included epithelium integrity, cellular infiltration, goblet cell depletion, crypts assess formation, and muscle thickness. No differences were found in the histology scores for the colon, being equally high across all groups (6.75 ± 0.56 for endo-no stress, 7.29 ± 0.18 for sham-stress, and 7.25 ± 0.49 for endo-stress).
Colonic tissue from endo-no stress rats had few mast cells (2.08 ± 0.17 mast cells/high-power field of view). The numbers of mast cells increased in endo-stress (2.96 ± 0.60 mast cells/high-power field of view) and sham-stress animals (2.78 ± 0.350 mast cells/high-power field of view; Figure 4B and C), but this difference did not reach statistical significance.
Peritoneal Fluid Cell Analysis
The number of WBCs in the peritoneal fluid was measured as a marker of peritoneal inflammation in the endo-stress and endo-no stress rats. Although not significantly different, the endo-stress rats had greater inflammatory cell infiltration in the peritoneal fluid (12.00 ± 3.51 WBC/high-power field of view) compared to endo no-stress rats (7.25 ± 1.15 WBC/high-power field of view). A differential analysis revealed that more than 50% of the cells in the peritoneal fluid were monocytes, and the rest were neutrophils and lymphocytes. Very few basophils or eosinophils were found, and the cellular distribution was similar between the groups.
Corticosterone
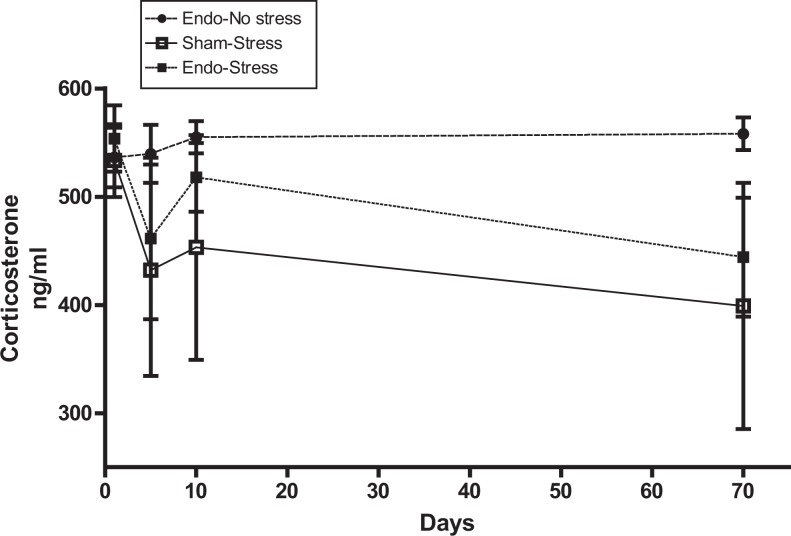
Levels of corticosterone were measured during the stress protocol on 3 different days (days 1, 5, and 10) and on the day of sacrifice. Endo-no stress rats surprisingly showed the highest corticosterone levels in comparison with the rats that received the stress, although this did not reach significance (Figure 5).
Figure 5.
Corticosterone levels in serum. Endo-no stress rats have the highest corticosterone levels. Endo-stress and sham-stress animals have a similar pattern of corticosterone levels (4-6 animals per group).
Central CRF Changes
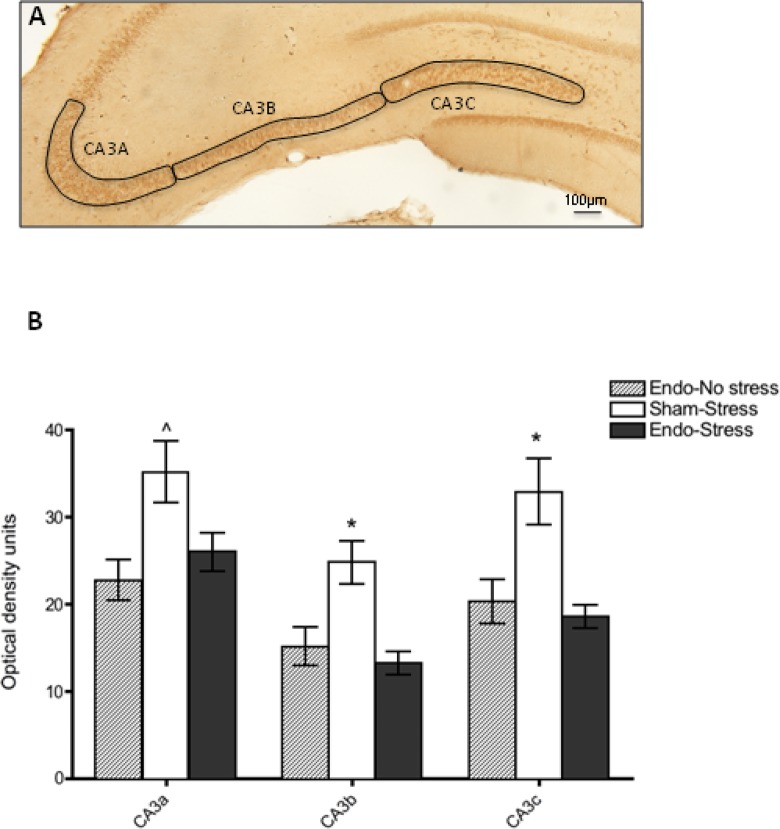
Coronal brain sections through the hypothalamus and hippocampus were processed for CRF-like immunohistochemistry. Quantitative light densitometry analysis of the parvocellular and magnocellular subdivisions of the PVA (Figure 6A) revealed a higher CRF immunoreactivity in the parvocellular compared to the magnocellular subdivision, further supporting prior reports42; however, no significant differences were observed between sham-stress, endometriosis-stress, or endometriosis-no stress groups (Figure 6B). Interestingly, analysis of the hippocampal subregions (Figure 7A) showed that in the CA3 subregions, sham-stress animals showed significantly different CRF immunoreactivity compared to both the endo-stress and endo-no stress groups. Analysis of varaince revealed a significant main effect of group for CA3a (F (2,17) = 6.22, P < 0.05), CA3b (F (2,17) = 9.5, P < .01), and CA3c (F (2,17) = 8.21, P < .01). Post hoc analysis revealed that sham-stress animals were significantly higher than the other groups (see Figure 7B). Control sections omitting primary antibody during the incubation showed no specific labeling in any of the CA3 areas and optical density was lower than background labeling on experimental sections.
Figure 6.
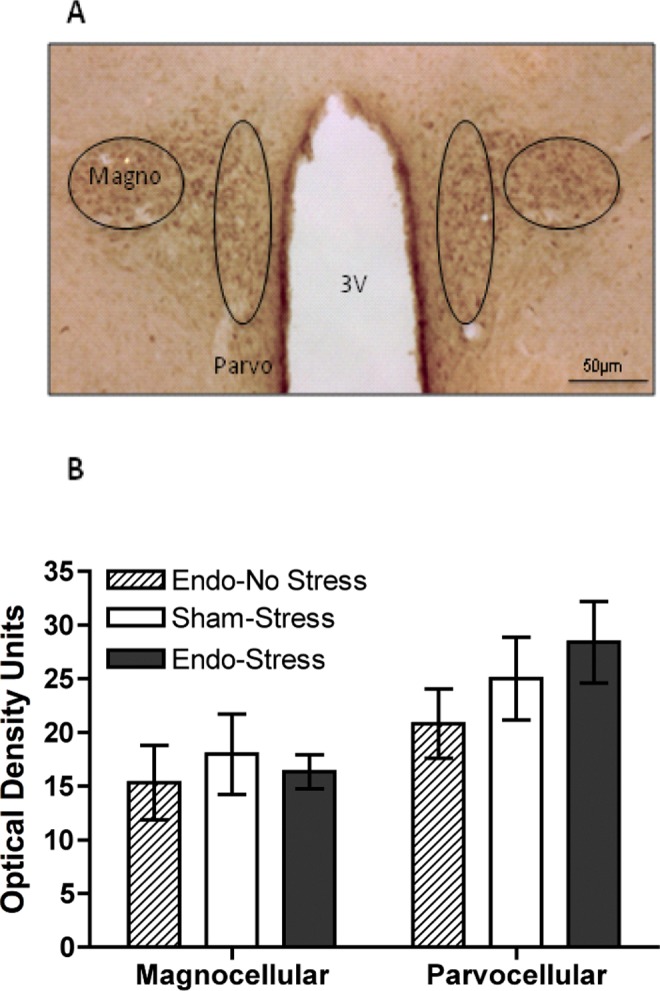
Hypothalamic CRF immunoreactivity. A, Representative tissue section of the parvocellular and magnocellular subdivisions of the paraventricular nucleus of the hypothalamus. B, Quantitative light densitometry analysis showed no significant differences in CRF immunoreactivity between endo-no stress, sham-stress and endo-stress groups. CRF indicates corticotropin releasing factor.
Figure 7.
Hippocampal CRF immunoreactivity. A, Representative tissue section of the CA3 regions of the hippocampus from a sham-stress animal. B, Quantitative light densitometry analysis revealed higher levels of CRF in CA3 regions of sham-stress animals compared to both the endo-stress and endo-no stress. *represents P < .05 compared to the other 2 groups; ^represents P < .05 from endo-no-stress group only.
Discussion
This is the first study presenting evidence for the deleterious effects of stress in the development of endometriosis and its associated inflammatory parameters. We showed that rats with surgically induced endometriosis receiving prior stress had vesicles of a greater severity and number, higher levels of MPO, and greater mast cell infiltration in colonic tissues. This suggests that stress prior to endometriosis most likely contributed to the development of vesicles in this animal model through mechanisms involving cell recruitment (eg, mast cells and neutrophils) and release of cell mediators.
At the time of sacrifice, animals were found to be distributed between the different stages of the cycle, with the majority being in either proestrus or estrus phase. Due to normal variations in the thickness of both eutopic and ectopic endometrium with cycle phase, it is expected that this tissue would be thicker in proestrus and estrus compared to the other phases. Notably, even with this variable, vesicles in the endo-no stress group (where close to 70% of animals were in proestrus) were still significantly smaller than those in the group receiving stress (only 30% in proestrus), pointing to a possible underestimation of the stress effect.
Stress stimulates the hypothalamus to manufacture and release CRF, causing the pituitary gland to produce and release corticosteroids (primarily cortisol in humans and corticosterone in rodents) into the body. In addition to its effects on the brain, cortisol/corticosterone also affects the immune system.19 Acute stressors are associated with an upregulation of the immune system, while prolonged increase in cortisol levels has been shown to depress immunologic function.43 The physiological mechanisms activated by stress are likely an adaptation to help cope with the threat at hand; the transformation of these stress responses into physiological illness depends upon the severity and persistence of the stress response.44
A diurnal rhythm in plasma corticosterone in both male and female rats is known to occur.45These authors found that there was an increase in corticosterone levels in the afternoon, thus we carried out our stress protocol in the early afternoon. Regardless, since the measurements were taken in all animals at the same time of the day, any variations due to timing are accounted for in our results, which showed no significant differences in the levels of systemic cortisol in the rats with endometriosis with or without prior stress. Others have reported that women with endometriosis have lower salivary and follicular fluid cortisol levels46,47 but higher serum cortisol levels in infertile women with advanced endometriosis.48 In other chronic diseases that can cause pain, such as gastrointestinal (GI) disorders, chronic fatigue syndrome, and women with dysmenorrhea, the cortisol levels have been shown to be decreased.22,49,50Evidently, more studies are necessary to elucidate the role of altered cortisol levels in endometriosis. Moreover, it is possible that other neuroendocrine factors, including central CRF levels, could be more accurate and informative markers of HPA axis deregulation than systemic cortisol levels.
It is well known that in chronic diseases, there are elevated levels of inflammatory cytokines and a disrupted HPA axis activity, producing changes in central CRF levels.51–53 Here we showed no changes in CRF levels in the hypothalamic PVA. This suggests that the main hypothalamic-CRF secreting nucleus is not affected by prior stress in either control or endometriosis animals. Since the stress occurred 60 days prior to sacrifice, sufficient time has lapsed after the stress occurred to normalize the CRF levels in the PVA. We decided to focus on the hippocampal formation because this structure has been demonstrated to be sensitive to chronic stressors54 as well as to cytokine effects.55 Our results showed a decrease in hippocampal CRF immunoreactivity in animals with endometriosis compared to sham-operated animals, independent of stress. A reasonable explanation for decreased CRF in the endometriosis rats is that the condition by itself might produce immunological changes similar to a chronic stressor. Alternatively, decreased central CRF might reflect an adaptive response to the chronic disease state. Interestingly, our results are in accord with a recent report by Vincent and colleagues, showing that mean cortisol levels were lower in women with dysmenorrhea and that levels were negatively correlated with duration of pain.21 The authors conclude that patients with endometriosis have evidence of a suppressed HPA axis response and alterations in the central processing of stressful factors. The CRF levels and number of mast cells have been shown to be elevated in endometriosis biopsies,56 while no differences were observed in peritoneal fluid levels of CRF and CRF-binding protein between controls and women with endometriosis.57 Thus, the endometriosis-specific changes in CRF expression appear to be different at the central versus local levels. Our observations provide further evidence that HPA axis deregulation might also be occurring in endometriosis, and open the possibility of further exploring the role of the associated neuroimmune mechanisms in this disease.
It is now widely accepted that stressful life events can impact the health of an individual, including immunological health. Endometriosis is associated with increased secretion of proinflammatory cytokines and impaired function of cell-mediated natural immunity.58,59 This network of locally produced cytokines modulates the growth of ectopic endometrial implants, and involvement of these inflammatory mechanisms has been demonstrated previously in the rat model.60–63 In humans, ectopic endometrial tissue is surrounded by abundant fibrotic tissue and inflammatory infiltrate, including mast cells, but the triggering factors for these processes are not yet understood.64 These infiltrating mast cells exhibit degranulation and scattered granules, suggesting that an abnormal immune response, specifically a hypersensitivity reaction, is strongly related to endometriosis.65 Anaf et al demonstrated that in deeply infiltrating endometriosis lesions (the most painful type), there was an increase in both activated and degranulating mast cells, and a close histological relationship with nerves possibly contributing to the associated pain.66 Specific nerve fibers have been found in endometriotic tissues, which correlate with the density and severity of pain in patients. Our results provide further support for the role of mast cells, and perhaps their mediators in this condition, similar to other inflammatory diseases of the intestine where the involvement of stress and the immune system appears crucial to disease progression.67,68 This suggests that manipulation of the nerve/mast cell interactions could represent a novel therapeutic target for pain and GI-related symptoms caused by endometriosis.69
To our knowledge, very few studies have investigated the involvement of stress on the development of endometriosis. Based on the observation that the prevalence of spontaneous endometriosis in captive baboons increased with time spent in captivity, D’Hooghe et al concluded that psychological stress may affect the prevalence of endometriosis.70 While other factors may play a role (such as age, more interrupted menstrual cycle, captive diet), this study presented the first indirect evidence of the relationship between a chronic stressor and endometriosis. In patients, further indirect evidence includes a doubled risk for shorter cycle length in women with stressful jobs (a known risk factor for endometriosis71) compared with those who did not consider their jobs stressful,72 and dysmenorrhea is twice as common in women reporting high levels of stress in the preceding menstrual cycle.73 In addition, there is ample evidence from qualitative and quantitative studies that document the significant negative impact on quality of life in patients with endometriosis.10,11 Taken together, data from both animal models and human studies strongly suggest that stress does play a role in endometriosis. Whether stress is a causal or exacerbating factor in disease is still unknown. Our results fill an important gap in knowledge of the endometriosis field, by providing evidence supporting the involvement of stress-activated neuroinflammatory mechanisms in this chronic, painful disease.
The studies described herein are limited in that, as with all animal models, some conclusions cannot be directly generalized to the human scenario. Limitations of our study include the fact that our measurement of fecal pellet count, while considered an indirect measure of increased intestinal motility that is often linked with a high anxiety state, is not directly comparable to a similar measurement in patients. Also, in this series of experiments we are examining one type of stressor during a defined time period. In the human condition, patients are subjected to many different types of stress, which can be present in a chronic or acute fashion or may actually result from dealing with the symptoms of the disease. In this set of experiments we also did not include a sham-no stress group since we have previously shown that rats with experimental endometriosis have higher levels of inflammatory factors (such as inflammatory cells in the peritoneal fluid) than rats with a sham-surgery (equivalent to sham-no stress).60 The present studies were therefore designed to test the hypothesis that stress will make these parameters even worse. Our data support this hypothesis and provide evidence for the role of neuroinflammatory mechanisms in this disease. Other strengths of this model include the fact that the mechanisms underlying activation of the HPA axis by stress and immune mechanisms are highly similar between rodents and primates. Endometriosis has been identified as a heterogeneous, complex disease, and it is highly plausible that the existence of ongoing stress may contribute to the development of this disease through disturbances created in the immune and neuroendocrine systems. As such, our results point to the importance of future research in this area to lead to a better understanding of this disease and the importance of the “brain–body–brain cross talk.” This may help to establish psychological, behavioral, and stress-reduction interventions as part of multidisciplinary preventive and clinical management to better treat patients with this condition.
Acknowledgments
The authors would like to acknowledge the technical support of Myrella L. Cruz and Perla Baez during surgeries, Alcira Benitez for histological preparation and analysis, and helpful feedback from discussions with Dr Michael W. Vernon. In addition, the authors acknowledge the epidemiological and statistical support to this project by the Epidemiology and Biostatistics CORE Program of Ponce School of Medicine, especially Dr Manuel Bayona. This CORE Program is federally funded by NIH/NCRR/RCMI Grant Number 2G12RR03050.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: These studies were supported in part by F31 GM082281 (MC), R01HD050559 (IF), S06-GM08239 (KJT and IF) and R15AT006373 (CBA) from the National Institutes of Health (NIH), by G12-RR03050 (CBA) from the National Center of Research Resources (NCRR), a component of the NIH and by a Health Professions Division Research Grant from Nova Southeastern University (ATR). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of the NCRR or NIH.
References
- 1. Child TJ, Lin Tan S. Endometriosis: etiology, pathogenesis and treatment. Drugs. 2001;61(12):1735–1750 [DOI] [PubMed] [Google Scholar]
- 2. Cox H, Henderson L, Wood R, Cagliarini G. Learning to take charge: women's experiences of living with endometriosis. Complement Ther Nurs Midwifery. 2003;9(2):62–68 [DOI] [PubMed] [Google Scholar]
- 3. Tariverdian N, Rucke M, Szekeres-Bartho J, et al. Neuroendocrine circuitry and endometriosis: progesterone derivative dampens corticotropin-releasing hormone-induced inflammation by peritoneal cells in vitro. J Mol Med. 2010;88(3):267–278 [DOI] [PubMed] [Google Scholar]
- 4. Toth B. Stress, inflammation and endometriosis: are patients stuck between a rock and a hard place? J Mol Med. 2010;88(3):223–225 [DOI] [PubMed] [Google Scholar]
- 5. Huntington A, Gilmour JA. A life shaped by pain: women and endometriosis. J Clin Nurs. 2005;14(9):1124–1132 [DOI] [PubMed] [Google Scholar]
- 6. Cox H, Henderson L, Andersen N, Cagliarini G, Ski S. Focus group study of endometriosis: struggle, loss and the medical merry-go-round. Int J Nurs Pract. 2003;9 (1):2–9 [DOI] [PubMed] [Google Scholar]
- 7. Lemaire GS. More than just menstrual cramps: symptoms and uncertainty among women with endometriosis. J Obstet Gynecol Neonatal Nurs. 2004;33(1):71–79 [DOI] [PubMed] [Google Scholar]
- 8. O'Donnell K, Badrick E, Kumari M, Steptoe A. Psychological coping styles and cortisol over the day in healthy older adults. Psychoneuroendocrinology. 2008;33(5):601–611 [DOI] [PubMed] [Google Scholar]
- 9. Denny E. I never know from one day to another how I will feel: pain and uncertainty in women with endometriosis. Qual Health Res. 2009;19(7):985–995 [DOI] [PubMed] [Google Scholar]
- 10. Fourquet J, Gao X, Zavala D, et al. Patient’s report on how endometriosis affects health, work, and daily life. Fertil Steril. 2010;93(7):2424–2428 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Fourquet J, Báez Figueroa M, Iriarte RI, Flores I. Quantification of the impact of endometriosis symptoms on health related quality of life and work productivity. Fertil Steril. 2011;96(1):107–112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Barnack JL, Chrisler JC. The experience of chronic illness in women: a comparison between women with chronic migraine headaches. Women Health. 2007;46(1):115–133 [DOI] [PubMed] [Google Scholar]
- 13. Cousineau TM, Domar AD. Psychological impact of infertility. Best Pract Res Clin Obstet Gynaecol. 2007;21(2):293–308 [DOI] [PubMed] [Google Scholar]
- 14. Baum A, Grunberg NE. Gender, stress, and health. Health Psychol. 1991;10(2):80–85 [DOI] [PubMed] [Google Scholar]
- 15. Peveler R, Edwards J, Daddow J, Thomas E. Psychosocial factors and chronic pelvic pain: a comparison of women with endometriosis and with unexplained pain. J Psychosom Res. 1996;40(3):305–315 [DOI] [PubMed] [Google Scholar]
- 16. Low WY, Edelmann RJ, Sutton C. A psychological profile of endometriosis patients in comparison to patients with pelvic pain of other origins. J Psychosom Res. 1993;37(2):111–116 [DOI] [PubMed] [Google Scholar]
- 17. Harrison V, Rowan K, Mathias J. Stress reactivity and family relationships in the development and treatment of endometriosis. Fertil Steril. 2005;83(4):857–864 [DOI] [PubMed] [Google Scholar]
- 18. Rybakina EG, Shanin SN, Fomicheva EE, Korneva EA. Cellular and molecular mechanisms of interaction between the neuroendocrine and immune systems under chronic fatigue syndrome in experiment. Ross Fiziol Zh Im I M Sechenova. 2009;95(12):1324–1335 [PubMed] [Google Scholar]
- 19. Kudielka BM, Kirschbaum C. Sex differences in HPA axis responses to stress: a review. Biol Psychol. 2005;69(1):113–132 [DOI] [PubMed] [Google Scholar]
- 20. Tariverdian N, Theoharides TC, Siedentopf F, et al. Neuroendocrine-immune disequilibrium and endometriosis: an interdisciplinary approach. Semin Immunopathol. 2007;29(2):193–210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Vincent K, Warnaby C, Stagg CJ, et al. Dysmenorrhoea is associated with central changes in otherwise healthy women. Pain. 2011;152(9):1966–75 [DOI] [PubMed] [Google Scholar]
- 22. Gur A, Cevik R, Sarac AJ, Colpan L, Em S. Hypothalamic-pituitary-gonadal axis and cortisol in young women with primary fibromyalgia: the potential roles of depression, fatigue, and sleep disturbance in the occurrence of hypocortisolism. Ann Rheum Dis. 2004;63(11):1504–1506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. McEwen BS. Stress, adaptation, and disease. Allostasis and allostatic load. Ann N Y Acad Sci. 1998;840:33–44 [DOI] [PubMed] [Google Scholar]
- 24. McEwen BS, Stellar E. Stress and the individual. Mechanisms leading to disease. Arch Intern Med. 1993;153(18):2093–2101 [PubMed] [Google Scholar]
- 25. Reagan LP, Grillo CA, Piroli GG. The As and Ds of stress: metabolic, morphological and behavioral consequences. Eur J Pharmacol. 2008;585(1):64–75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Pearlin LI, Lieberman MA, Menaghan EG, Mullan JT. The stress process. J Health Soc Behav. 1981;22(4):337–356 [PubMed] [Google Scholar]
- 27. Engelmann M, Ebner K, Landgraf R, Wotjak CT. Effects of Morris water maze testing on the neuroendocrine stress response and intrahypothalamic release of vasopressin and oxytocin in the rat. Horm Behavior. 2006;50(3):496–501 [DOI] [PubMed] [Google Scholar]
- 28. Maillot C, Million M, Wei JY, Gauthier A, Taché Y. Peripheral corticotropin-releasing factor and stress-stimulated colonic motor activity involve type 1 receptor in rats. Gastroenterology. 2000;119(6):1569–1579 [DOI] [PubMed] [Google Scholar]
- 29. Walsh RN, Cummins RA. The Open-Field Test: a critical review. Psychol Bull. 1976;83(3):482–504 [PubMed] [Google Scholar]
- 30. Vernon MW, Wilson EA. Studies on the surgical induction of endometriosis in the rat. Fertil Steril. 1985;44:684–694 [PubMed] [Google Scholar]
- 31. Appleyard CB, Cruz ML, Rivera E, Hernandez GA, Flores I. Experimental endometriosis in the rat is correlated with colonic motor function alterations but not with bacterial load. Reprod Sci. 2007;14 (8):815–824 [DOI] [PubMed] [Google Scholar]
- 32. Ingelmo MR, Quereda F, Acién P. Intraperitoneal and subcutaneous treatment of experimental endometriosis with recombinant human interferon-α-2b in a murine model. Fertil Steril. 1999;71(5):907–911 [DOI] [PubMed] [Google Scholar]
- 33. Hernández GA, Appleyard CB. Bacterial load in animal models of acute and chronic ‘reactivated’ colitis. Digestion. 2003;67(3):161–169 [DOI] [PubMed] [Google Scholar]
- 34. Boughton-Smith NK, Wallace JL, Whittle BJ. Relationship between arachidonic acid metabolism, myeloperoxidase activity and leukocyte infiltration in a rat model of inflammatory bowel disease. Agents Actions. 1998;25(1-2):115–123 [DOI] [PubMed] [Google Scholar]
- 35. Bradley PP, Priebat DA, Christensen RD, Rothstein G. Measurement of cutaneous inflammation: estimation of neutrophil content with an enzyme marker. J. Invest. Derm. 1982;78(3):206–209 [DOI] [PubMed] [Google Scholar]
- 36. Treweek JB, Jaferi A, Colago EE, Zhou P, Pickel VM. Electron microscopy localization of corticotropin-releasing factor (CRF) and CRF-receptor in rat and mouse central nucleus of the amygdala. J Com Neurol. 2009;512(3):323–335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Pierce JP, Kurucz OS, Milner TA. Morphometry of a peptidergic transmitter system: dynorphin B-like immunoreactivity in the rat hippocampal mossy fiber pathway before and after seizures. Hippocampus. 1999;9(3):255–276 [DOI] [PubMed] [Google Scholar]
- 38. Thureson-Klein AK, Klein RL. Exocytosis from neuronal large dense-cored vesicles. Int Rev Cytol. 1990;121:67–126 [DOI] [PubMed] [Google Scholar]
- 39. Venkova K, Johnson AC, Myers B, Greenwood-Van Meerveld B. Exposure of the amygdala to elevated levels of corticosterone alters colonic motility in response to acute psychological stress. Neuropharmacology. 2010;58(7):1161–1167 [DOI] [PubMed] [Google Scholar]
- 40. Bradesi S, Schwetz I, Ennes HS, et al. Repeated exposure to water avoidance stress in rats: a new model for sustained visceral hyperalgesia. Am J Physiol Gastrointest Liver Physiol. 2005;289(1):G42–G53 [DOI] [PubMed] [Google Scholar]
- 41. Taché Y, Kiank C, Stengel A. A role for corticotropin-releasing factor in functional gastrointestinal disorders. Curr Gastroenterol Rep. 2009;11(4):270–277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Bloom FE, Battenberg ELF, Rivier J, Vale W. Corticotropin releasing factor (CRF): immunoreactive neurones and fibers in rat hypothalamus. Regulatory peptides. 1982;4(1):43–48 [DOI] [PubMed] [Google Scholar]
- 43. DeLongis A, Folkman S, Lazarus RS. The impact of daily stress on health and mood: psychological and social resources as mediators. J Pers Soc Psychol. 1988;54(3):486–495 [DOI] [PubMed] [Google Scholar]
- 44. Williams K, Kurina LM. The social structure, stress, and women's health. Clin Obstet Gynecol. 2002;45(4):1099–1118 [DOI] [PubMed] [Google Scholar]
- 45. Atkinson HC, Waddell BJ. Circadian variation in basal plasma corticosterone and adrenocorticotropin in the rat: sexual dimorphism and changes across the estrous cycle. Endocrinology. 1997;138(9):3842–3848 [DOI] [PubMed] [Google Scholar]
- 46. Petrelluzzi KF, Garcia MC, Petta CA, Grassi-Kassisse DM, Spadari-Bratfisch RC. Salivary cortisol concentrations, stress and quality of life in women with endometriosis and chronic pelvic pain. Stress. 2008;11(5):390–397 [DOI] [PubMed] [Google Scholar]
- 47. Smith MP, Keay SD, Margo FC, et al. Total cortisol levels are reduced in the periovulatory follicle of infertile women with minimal-mild endometriosis. Am J Reprod Immunol. 2002;47(1):52–56 [DOI] [PubMed] [Google Scholar]
- 48. Lima AP, Moura MD, Rosa e Silva AAM. Prolactin and cortisol levels in women with endometriosis. Braz J Med Biol Res. 2006;39(8):1121–1127 [DOI] [PubMed] [Google Scholar]
- 49. Böhmelt AH, Nater UM, Franke S, Hellhammer DH, Ehlert U. Basal and stimulated hypothalamic-pituitary-adrenal axis activity in patients with functional gastrointestinal disorders and healthy controls. Psychosom Med. 2005;67(2):288–294 [DOI] [PubMed] [Google Scholar]
- 50. Cleare AJ, Blair D, Chambers S, Wessely S. Urinary free cortisol in chronic fatigue syndrome. Am J Psychiatry. 2001;158(4):641–643 [DOI] [PubMed] [Google Scholar]
- 51. McEwen BS, Kalia M. The role of corticosteroids and stress in chronic pain conditions. Metabolism. 2010;59(suppl 1):S9–S15 [DOI] [PubMed] [Google Scholar]
- 52. Neeck G, Crofford LJ. Neuroendocrine perturbations in fibromyalgia and chronic fatigue syndrome. Rheum Dis Clin North Am. 2000;26(4):989–1002 [DOI] [PubMed] [Google Scholar]
- 53. Leonard BE, Myint A. The psychoneuroimmunology of depression. Hum Psychopharmacol. 2009;24(3):165–175 [DOI] [PubMed] [Google Scholar]
- 54. McEwen BS, Milner TA. Hippocampal formation: shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55(2):343–55 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. You Z, Luo C, Zhang W, et al. Pro- and anti-inflammatory cytokines expression in rat's brain and spleen exposed to chronic mild stress: involvement in depression. Behav Brain Res. 2011;225(1):135–141 [DOI] [PubMed] [Google Scholar]
- 56. Kempuraj D, Papadopoulou N, Stanford EJ, et al. Increased numbers of activated mast cells in endometriosis lesions positive for corticotropin-releasing hormone and urocortin. Am J Reprod Immunol. 2004;52(4):267–275 [DOI] [PubMed] [Google Scholar]
- 57. Florio P, Luisi S, Viganò P, et al. Healthy women and patients with endometriosis show high concentrations of inhibin A, inhibin B, and activin A in peritoneal fluid throughout the menstrual cycle. Hum Reprod. 1998;13(9):2606–2611 [DOI] [PubMed] [Google Scholar]
- 58. Rier SE, Yeaman GR. Immune aspects of endometriosis: relevance of the uterine mucosal immune system. Semin Reprod Endocrinol. 1997;15 (3):209–220 [DOI] [PubMed] [Google Scholar]
- 59. Christodoulakos G, Augoulea A, Lambrinoudaki I, Sioulas V, Creatsas G. Pathogenesis of endometriosis: the role of defective ‘immunosurveillance'. Eur J Contracept Reprod Health Care. 2007;12(3):194–202 [DOI] [PubMed] [Google Scholar]
- 60. Rojas-Cartagena C, Appleyard CB, Santiago OI, Flores I. Experimental endometriosis is characterized by increased levels of soluble TNFRSF1B and downregulation of Tnfrsf1a and Tnfrsf1b gene expression. Biol Reprod. 2005;73(6):1211–1218 [DOI] [PubMed] [Google Scholar]
- 61. Flores I, Rivera E, Ruiz LA, Santiago OI, Vernon MW, Appleyard CB. Molecular profiling of experimental endometriosis identified gene expression patterns in common with human disease. Fert Steril. 2007;87(5):1180–1199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Sharpe-Timms KL. Using rats as a research model for the study of endometriosis. Ann NY Acad Sci. 2002;955:318–327 [DOI] [PubMed] [Google Scholar]
- 63. Konno R, Fujiwara H, Netsu S, et al. Gene expression profiling of the rat endometriosis model. Am J Reprod Immunol. 2007;58(4):330–343 [DOI] [PubMed] [Google Scholar]
- 64. Matsuzaki S, Canis M, Darcha C, Fukaya T, Yajima A, Bruhat MA. Increased mast cell density in peritoneal endometriosis compared with eutopic endometrium with endometriosis. Am J Reprod Immunol. 1998;40:291–294 [DOI] [PubMed] [Google Scholar]
- 65. Sugamata M, Ihara T, Uchiide I. Increase of activated mast cells in human endometriosis. Am J Reprod Immunol. 2005;53(3):120–125 [DOI] [PubMed] [Google Scholar]
- 66. Anaf V, Chapron C, Nakadi IE, De Moor V, Simonart T, Noel JC. Pain, mast cells, and nerves in peritoneal, ovarian and deep infiltrating endometriosis. Fertil Steril. 2006;86(5):1336–1343 [DOI] [PubMed] [Google Scholar]
- 67. O’Sullivan M, Clayton N, Breslin NP, et al. Increased mast cell in the irritable bowel syndrome. Neurogastroenterol Motil. 2000;12(5):449–457 [DOI] [PubMed] [Google Scholar]
- 68. Barbara G, Stanghellini V, De Giorgio R, et al. Activated mast cells in proximity to colonic nerves correlate with abdominal pain in irritable bowel syndrome. Gastroenterology. 2004;126(3):693–702 [DOI] [PubMed] [Google Scholar]
- 69. Medina MG, Lebovic DI. Endometriosis-associated nerve fibers and pain. Acta Obstetricia et Gynecologica. 2009;88(9):968–975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. D'Hooghe TM, Bambra CS, De Jonge I, Lauweryns JM, Koninckx PR. The prevalence of spontaneous endometriosis in the baboon (Papio anubis, Papio cynocephalus) increases with the duration of captivity. Acta Obstet Gynecol Scand. 1996;75(2):98–101 [DOI] [PubMed] [Google Scholar]
- 71. Cramer DW, Missmer SA. The epidemiology of endometriosis. Ann N Y Acad Sci. 2002;955:11–22 Discussion 34-36, 396-406 [DOI] [PubMed] [Google Scholar]
- 72. Fenster L, Waller K, Chen J, et al. Psychological stress in the workplace and menstrual function. Am J Epidemiol. 1999;149(2):127–134 [DOI] [PubMed] [Google Scholar]
- 73. Wang L, Wang X, Wang W, et al. Stress and dysmenorrhoea: a population based prospective study. Occup Environ Med. 2004;61(12):1021–1026 [DOI] [PMC free article] [PubMed] [Google Scholar]