Abstract
Diabetes induces impairments in gene expression during embryonic development that leads to premature and improper tissue specialization. Retinoic acid receptors (RARs and retinoid X receptor [RXRs]) and mitogen-activated protein kinases (MAPKs) play crucial roles during embryonic development, and their suppression or activation has been shown as a determinant of the fate of embryonic organogenesis. We studied the activation of RARs and MAPKs in embryonic day 12 (E12) in embryos of rats under normal, diabetic, and diabetic treated with resveratrol ([RSV]; 100 mg/kg body weight) conditions. We found downregulation of RARs and RXRs expressions as well as their DNA-binding activities in the embryos exhibiting developmental delays due to diabetes. Furthermore, the phosphorylation of extracellular signal-regulated kinase (ERK) 1/2 was decreased and phosphorylation of c-Jun N-terminal kinase (JNK) 1/2 and p38 was increased. Interestingly, embryos of diabetic rats treated with RSV showed normalized patterns of RARs, RXRs, neuronal markers, and ERK, JNK and p38 phosphorylation.
Keywords: diabetic pregnancy, developmental delays, resveratrol, retinoic acid receptors, MAPKs
Introduction
Maternal diabetes is one of the leading causes of birth defects and perinatal mortality commonly referred to as diabetic embryopathy. Although the perinatal mortality rate has declined after the introduction of insulin, the frequency of birth defects is still higher in diabetic pregnancies. The development of almost all organs are affected by maternal diabetes; however, the central nervous system is comparatively more susceptible.1–3 The majority of the diabetic embryopathy studies address mostly visible physical defects, especially neural tube defects (NTDs) and cardiac defects, in developing fetuses which occur in ~11% of human cases and in ~25% of animals in studies.2,4 However, many studies have shown that some adult diseases might have their origin in fetal life due to the intrauterine environment during pregnancy. For example, children born to mothers with gestational diabetes are at higher risk of obesity and other metabolic disturbances.5 In addition, children of diabetic mothers have an increased predisposition to obesity and type 2 diabetes.6
Fetal exposure to a hyperglycemic environment alters embryonic transcriptional program affecting normal development.7 In addition, hyperglycemia-induced changes in the gene expression are associated with proliferation and cell-fate specification of embryonic neuronal stem cells.8 Data also indicate that preexisting tissue damage and aberrant cell specialization due to hyperglycemia cannot be reversed even after normoglycemic levels are achieved during pregnancy.9 Furthermore, exposure of the fetus to maternal hyperglycemia may have long-term impact on fetal cellular programming and therefore result in a greater predisposition to different diseases.10 Epidemiologic evidence indicates that mothers with diabetes mellitus during pregnancy increase the risk of their offspring developing schizophrenia in adulthood.11 Dysregulation of retinoid and thyroid hormone signaling is suggested to contribute to the development of schizophrenia.12
Retinoic acid receptors (RARα/β/γ) and retinoid X receptors (RXRα/β/γ) are nuclear transcription factors that mediate the effects of retinoic acid (RA) on gene expression.13 Studies have shown that RA (a physiological active metabolite of vitamin A) is necessary for the proper embryonic development and morphogenesis of neural tubes. However, it may not be involved in neural tube closure.14 Both RARs and RXRs form heterodimers that bind to RA-response elements (RAREs) in the promoters of target genes to regulate their transcription.13 Suppression of RARs due to vitamin A deficiency has been shown to contribute to embryonic skeleton hypoplasia.15 Selective activation of RXRs has been shown to exhibit antidiabetic effects and play a role in the activation of insulin-sensitizing genes.16 Mitogen-activated protein kinases (MAPKs) play a major role in growth, differentiation, and development, and their activation has been shown as a determinant of the fate of embryonic organogenesis when exposed to insult.17 Previous work from our laboratory has shown that RA affects MAPK signaling via PI3K (Phosphatidylinositol 3-kinases) and Rac1 during neuronal cell differentiation.18 In addition, diabetes inhibits the activation of Rac1 and reduces the expression of neuronal markers in rat embryonic day 16 (E16) cortical neurons.19
Resveratrol ([RSV]; 3,5,4′-trihydroxy-trans-stilbene) is a natural dietary compound. It has shown promising results for the treatment of many diseases in animal models.20 In a recent clinical trial, 30 days of RSV treatment induced metabolic changes in obese humans mimicking the effects of calorie restriction. These metabolic changes included a reduction in sleeping metabolic rate, blood pressure, and hepatic lipid content, as well as an increase in skeletal muscle Peroxisome proliferator-activated receptor-. coactivator (PGC)-1α. protein content.21 Although RSV is shown to affect ERK1/2 and RAR activities in different cancer cell lines,22,23 there are no studies of these end points in reference to embryonic development under diabetic conditions. We hypothesized that in diabetic pregnancy, there may be impairments in RAR and MAPK signaling and RSV may prevent these effects. In our prior studies, we found increased incidence of developmental delays in rat embryos from diabetic pregnancies, which included reduced mean embryo weight, reduced crown–rump length, and reduced somite number for gestational age.24 Resveratrol was able to prevent these delays in embryos from diabetic dams. In the prior studies, we also found that diabetes-induced oxidative stress and apoptosis might be the cause of developmental delays in the embryos.24 The current study was performed using embryos of diabetic dams exhibiting no visible physical defects other than reduced size. In the present study, RAR, RXR, and MAPK signaling were investigated and the protective roles of RSV on these signaling pathways were assessed.
Materials and Methods
Experimental Animal Model
All the experiments were performed using a protocol approved by the University of South Carolina Institutional Animal Care and Use committee (AUP #1601). Adult, age-matched (60-days old) female Sprague-Dawley (SD) rats (Charles River, Wilmington, Massachusetts), average weight 200 to 230 g, were placed with proven breeder male SD rats just before the end of a light cycle. The following morning, each female was examined for the presence of vaginal sperm. The day sperm was first observed was defined as day zero of gestation or embryonic day zero (E0).
Diabetes Induction
On day E1, a single dose of streptozotocin (STZ; Sigma, St Louis, Missouri; 55 mg/kg body weight dissolved in 100 mmol/L citrate buffer pH 4.5) was administered intraperitoneally to induce diabetes.24 Control rats received an equal volume of citrate buffer. After 48 hours (E3) of STZ administration, blood was drawn from the rats by tail snip, and glucose levels were measured using the TRUE2go glucose monitoring system (HOM Ediagnostics, Florida). Rats with blood glucose concentrations >200 mg/dL were considered diabetic.
Experimental Plan
Rats were randomly divided into 4 groups: (1) nondiabetic rats (C, control), (2) diabetic (D), (3) diabetic treated with RSV (DR), and (4) control treated with RSV (CR). Resveratrol (Sigma; 100 mg/kg body weight suspended in water) was administered by gavage feeding for 10 days (from day E3-E12). On gestation day 12, the pregnant rats were anesthetized using isoflurane for 2 minutes and then sacrificed, and finally embryos were isolated. Only embryos with no obvious physical defects (other than reduced size in the case of the diabetic group) were utilized. One third of the embryos were used for immunohistochemistry (IHC) and the remaining embryos for protein analysis by Western blot. For each parameter studied, we used 4 to 5 independent batches, each batch comprising 4 groups (C, D, DR, and CR) and each group having 2 dams. We obtained approximately 10 to 15 embryos per dam. In the diabetic group, only the embryos with reduced crown–rump length and body weight as noted previously were used for studies.24
Immunohistochemical Staining
For immunohistochemical staining, embryos were postfixed in 4% paraformaldehyde in phosphate-buffered saline ([PBS]; pH 7.4) overnight at 4°C with mild shaking. Embryos were embedded in paraffin and 5-µm thick sections of the hindbrain region of cranial neural tube were kept at 4°C until use. Sections were deparaffinized in xylene and rehydrated in a series of graded ethanol using Leica autostainer XL (Leica Microsystem Inc, Bannockburn, Illinois). Sections were boiled in microwave with 10 mmol/L citrate buffer (pH 6.0) for 20 minutes to expose the epitope of the antigen. After cooling for 20 minutes at room temperature and washing with distilled water, sections were permeablized in Tris-buffered saline (1× TBS, pH 7.5) with 0.025% Triton X-100 for 30 minutes. The embryo sections were blocked with 2% bovine serum albumin (BSA) in TBS for 3 hours at room temperature followed by incubation overnight at 4°C with primary antibodies diluted in 2% BSA-PBS. The primary antibodies used in this study were RARα, RARβ, RARγ, RXRα, RXRβ, and RXRγ from Santa Cruz Biotechnology (Santa Cruz, California), and antibodies to detect total and phosphorylated ERK, JNK, and p38 were from Cell Signaling (Danvers, Massachusetts). Embryo sections were washed 3 times (5 minutes each) with 1× TBS and incubated with fluorescein isothiocyanate or rhodamine-conjugated secondary antibodies (Santa Cruz Biotechnology) for 2 hours at room temperature. Nonspecific staining was detected using rabbit/mouse normal immunoglobulin ([IgG]; Santa Cruz Biotechnology) in place of primary antibody. Labeled sections were washed with 1× TBS, mounted in Vectashield mounting media (Vector Laboratories, Burlingame, California), and finally visualized with Nikon E-600 fluorescence microscope (Nikon Instruments Inc, Melville, New York). For each staining, images were captured using identical settings for all control and treatment groups.
Protein Analysis by Western Blot
Total embryonic tissue was homogenized in 1× RIPA (Radio-Immunoprecipitation Assay) buffer (Sigma, St Louis, Missouri) containing 1 mmol/L PMSF (phenylmethylsulfonyl fluoride). After incubation for 30 minutes, samples were centrifuged at 10 000g for 10 minutes and supernatant retained and used for BCA protein assay (Thermo Scientific, Waltham, Massachusetts). Equal amounts of extracted proteins were diluted with 5× Laemmli sample buffer and boiled for 5 minutes. These samples were subjected to electrophoresis, transferred to polyvinylidene fluoride membrane (Amersham Hybond-P) and immunoblotted with RARα, RARβ, RARγ, RXRα, RXRβ, and RXRγ antibodies to assess the expression levels of RARs and RXRs. The secondary antibodies used were goat anti-rabbit IgG-horseradish peroxidase (HRP) and goa anti-mouse IgG-HRP from Santa Cruz Biotechnology. The blots were stripped and reprobed with β-actin to check for loading differences. To study neural marker protein expression, Western blot was performed with GAP-43 (Santa Cruz Biotechnology), total Tau (Upstate-Millipore, Massachusetts), and neurofilament B (Neomarkers-Thermo Scientific) antibodies. For MAPKs, Western blot was carried out with antibodies recognizing the phosphorylated forms of ERK1/2, JNK1/2, and p38. Furthermore, membranes were stripped and reprobed with antibody against total cellular ERK, JNK, and p38 (Santa Cruz Biotechnology) for proper protein loading.
Electrophoretic Mobility Shift Assay
To determine the level of DNA-protein interaction of RAR and RXR with consensus RAREs, gel shift assays were performed. Ten micrograms of total embryonic protein extract was incubated with 2 µg of poly(dI-dC) and radiolabeled probes (~10 000 cpm) in 20 µL of 10 mmol/L HEPES (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid) (pH 7.9), 50 mmol/L NaCl, 1 mmol/L EDTA, and 10% glycerol for 30 minutes at 25°C. Previously published RAR and RXR probes were selected and synthesized from integrated DNA technologies.25 The probe sequences were as follows: RAR, 5′-AGGGTAGGGTTCACCGAAAGTTCACTC-3′ and RXR, 5′-AGCTTCAGGTCAGAGGTCAGAGAGCT-3′. The oligonucleotides were labeled with [γ-32P]ATP (PerkinElmer, Waltham, Massachusetts) using T4 polynucleotide kinase. One negative control (without protein) was included with each gel shift assay to evaluate the background. For competition experiments, excess unlabeled oligos were preincubated with the nuclear extract 15 minutes before the addition of labeled probe. Binding reactions were resolved on 4.5% native polyacrylamide gels containing 0.5× TBE (Tris/Borate/EDTA) buffer (45 mmol/L Tris base, 45 mmol/L boric acid, 1 mmol/L EDTA, pH 8.0) at 4°C for 2 hours at 150 V. The gels were then dried using gel drying film (Promega, Madison, Wisconsin) soaked in 40% methanol, 10% glycerol, and 7.5% acetic acid for 3 to 5 minutes. After overnight drying, the gels were placed in direct contact with x-ray film for autoradiography.
Data Analysis
All the Western blot data and electrophoretic mobility shift assay (EMSA) data were semiquantitatively analyzed by Adobe Photoshop 7.0. The scanned Western blot band intensity was measured by multiplying pixels with mean. The resultant absolute intensity was normalized against β-actin and relative intensity was plotted with GraphPad Prism 5 (GraphPad Software, California). For MAPKs, the resultant absolute intensity of phospho-ERK1/2, phospho-JNK1/2, and phospho-p38 were normalized against their total cellular level. The embryo data collected were from 5 different experiments, each representing data of control (C), diabetic (D), diabetic plus RSV (DR), and control plus RSV (CR). Mean data of at least 3 different groups were represented with ±2 standard errors for each estimated mean. The results were quantitatively analyzed in reference to the embryo data of diabetic versus diabetic treated with RSV using 1-way analysis of variance followed by Tukey multiple comparison test.
Results
Resveratrol Protects From Diabetes-Induced Suppression of Levels of RAR
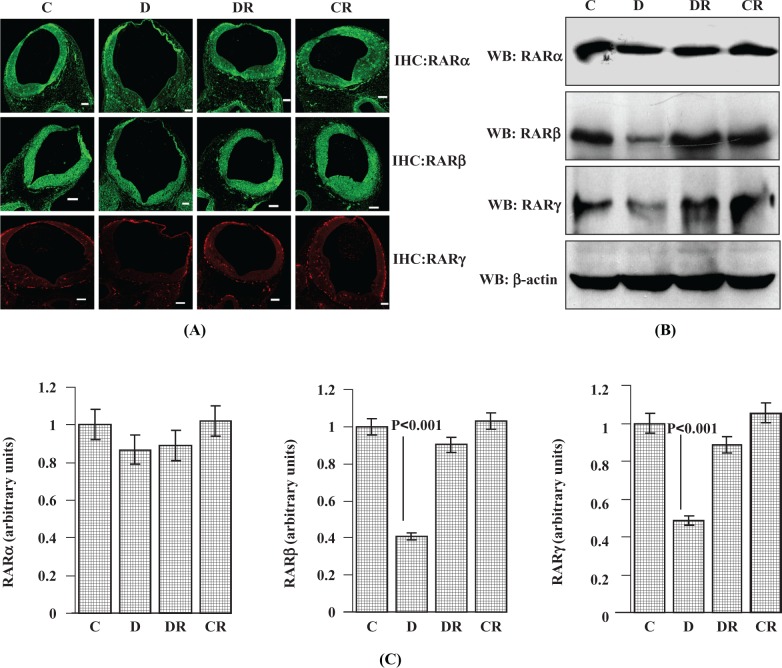
Diabetes impairs RA signaling.19 The RA plays major role during embryonic development in the control of patterning, neuronal differentiation, spinal cord anatomy, and its subsequent proliferative expansion.14 The effects of RA are mediated by both RARs and RXRs.26 The effect of diabetes on RARs during embryonic development was studied by analyzing the expression of different isoforms of RARs in E12 embryonic cranial neural tube regions (by IHC, Figure 1A) as well as in whole embryo lysates (by Western blot, Figure 1B and C). Twelve-day-old embryos were selected, as this is the age where rat organogenesis is near completion and similar diabetic embryos at this age have been characterized in our previous work.24 Immunoreactive RARα signal intensity was slightly decreased in cranial neural tube region (as evident from IHC data) in embryos of diabetic dams, although its level remains unchanged in whole embryonic lysates (as evident from Western blot data). The RARα expression in the RSV-treated diabetic and RSV control groups was similar to the vehicle control group. A significant decrease (P < .001) in RARβ expression in the whole embryos was measured by immunoblot and decreased signal intensity for RARβ was observed in the cranial region of the neural tube of diabetic dams; control levels were restored after RSV treatment. Whole embryo protein data clearly showed diabetes-induced suppression in RARγ (P < .001). Resveratrol treatments significantly restored the RARγ level in embryos from diabetic dams. The expression levels of RARα, RARβ, and RARγ in the control group treated with RSV were similar to the vehicle control group.
Figure 1.
Resveratrol protects against diabetes-induced suppression of RARs in E12 embryos of diabetic dams. Immunohistochemistry was performed with embryo sections to evaluate RARα, RARβ, and RARγ protein expression (A). Immunofluorescent photomicrographs (FITC-labeled RARα and RARβ and Rhodamine-labeled RARγ) were taken from the hindbrain region of cranial neural tube with a 10× objective. Scale bar = 100 µm. Controls without primary antibody did not show any changes (data not shown). Western blot analysis was performed with whole embryos to assess the RARα, RARβ, and RARγ expression status (B). Total embryonic protein (50 µg) was separated by SDS-PAGE and blotted with antibodies for different RAR isoforms (RARα, RARβ, and RARγ). Membranes were reprobed with β-actin antibody for protein loading normalization. Bar graph shows Western blot densitometry analysis for RARα, RARβ, and RARγ receptor expression (C). Error bars represent ±2 standard errors for each estimated mean. FITC indicates fluorescein isothiocyanate; SDS-PAGE, sodium dodecyl sulfate–polyacrlyamide gel electrophoresis; RAR, retinoic acid receptor.
Resveratrol Normalizes Diabetes-Induced Suppression of RXR Levels
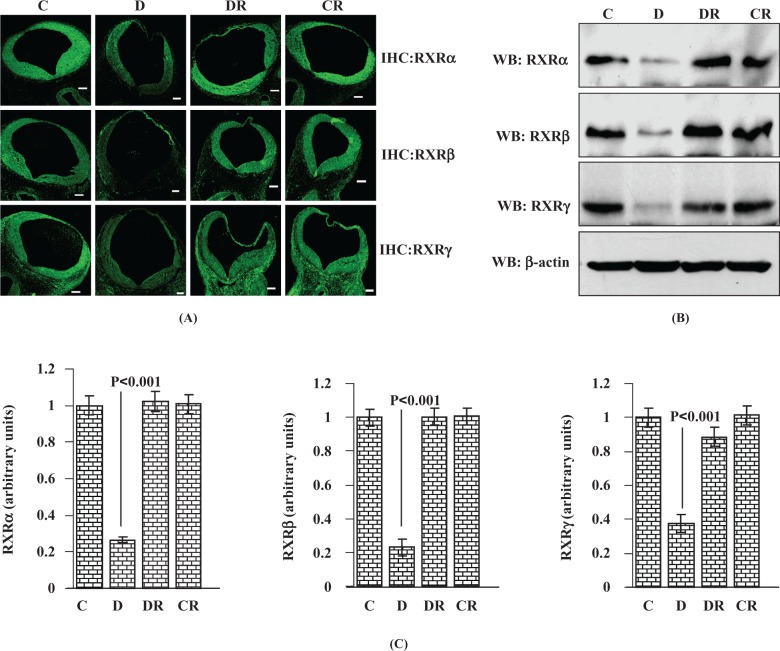
Like RARs, RXRs also play a major role during embryonic development.13 The RXR heterodimerizes with RAR and promotes RA signaling. The RXR also heterodimerizes with 7 other members of subfamily 1 nuclear receptors during embryonic and postnatal development.27 Immunoreactivity of all 3 isoforms of RXR (RXRα, RXRβ, and RXRγ) was downregulated in the cranial neural tube region as evidenced by IHC (Figure 2A) and significantly less (P < .001) in whole embryos of diabetic dams as evidenced by immunoblot (Figure 2B and C). Other than the neural tube region, RXRγ expression was also widely found in areas near the neural tube as well as in neural crest cells (as evident from IHC data for RXRγ). Whole embryos of RSV-treated diabetic dams exhibited almost normalized expression levels of RXRα, RXRβ, and RXRγ receptors. For each RXR receptor, the expression level of the control treated with RSV group remained unchanged in comparison with the vehicle-treated control group.
Figure 2.
Resveratrol normalizes diabetes-induced suppression of RXR in E12 embryos of diabetic dams. The RXRα, RXRβ, and RXRγ protein expression analysis was performed by immunohistochemistry and immunoblot. The FITC-labeled immunofluorescent photomicrographs (10× objective) were taken from embryonic cranial neural tube region (A). Scale bar = 100 µm. Controls without primary antibody did not show any changes (data not shown). Western blot analysis was carried out with 50 µg total embryonic protein and immunoblotted with RXRα, RXRβ, and RXRγ antibodies (B). Immunoblot for β-actin demonstrates similar protein loading between treatments. Bar graph shows Western blot densitometry analysis for RXRα, RXRβ, and RXRγ receptor expression (C). Error bars represent ±2 standard errors for each estimated mean. RXR indicates retinoid X receptor; FITC, fluorescein isothiocyanate.
Resveratrol Normalizes DNA-Protein Interactions for RAR and RXR in Embryos of Diabetic Dams
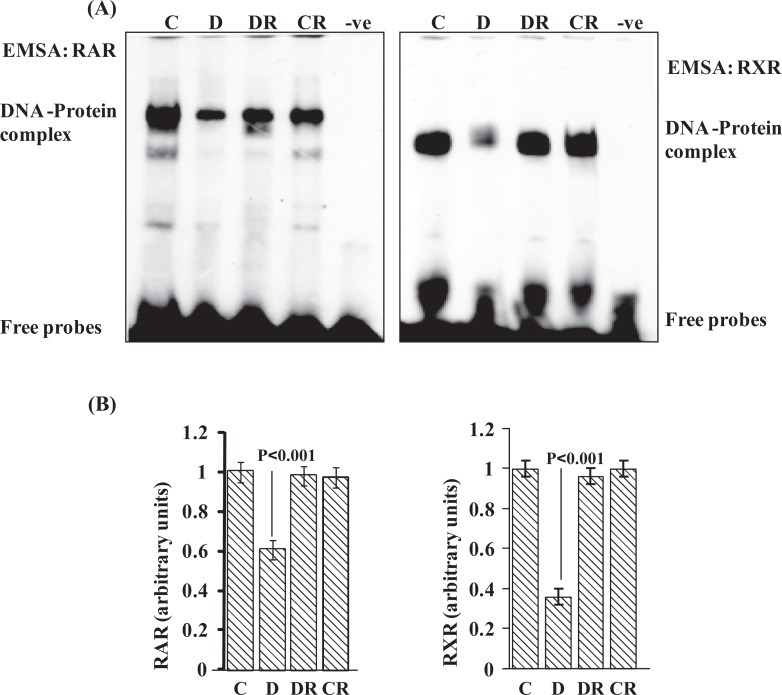
Nuclear receptors have the ability to directly bind to DNA and regulate the expression of adjacent genes. Both RAR and RXR isoforms are expressed in distinct patterns throughout development, and both RARs and RXRs form heterodimers that bind to RA-response elements to transduce RA signal during embryonic development.13 To check the status of DNA-protein interactions for RAR and RXR, EMSAs were performed. In embryos of diabetic dams DNA-protein complex intensity for both RAR and RXR was reduced (P < .001; Figure 3A and B). A significant improvement was found in RAR and RXR DNA-binding activity in embryos from diabetic dams treated with RSV. Controls treated with RSV exhibited similar DNA-binding activity as control–vehicle embryos. No shifted bands were observed in the absence of protein. In addition, competition assays were performed with excess unlabeled oligos which abolished DNA-protein complexes (data not shown).
Figure 3.
Resveratrol normalizes DNA-binding activity for RAR and RXR in embryos of diabetic dam. Electrophoretic mobility shift assay was performed to analyze the DNA-protein interaction for RAR and RXR family receptors (A). Ten micrograms of total embryonic protein extract was incubated with consensus oligonucleotides for retinoic acid response elements. Bar graph shows scanned densitometry analysis for RAR and RXR DNA-protein complex intensity (B). Error bars represent ±2 standard errors for each estimated mean. RAR indicates retinoic acid receptor; RXR indicates retinoid X receptor.
Resveratrol Protects From Diabetes-Induced Reduction in Expression of Neuronal Marker Proteins
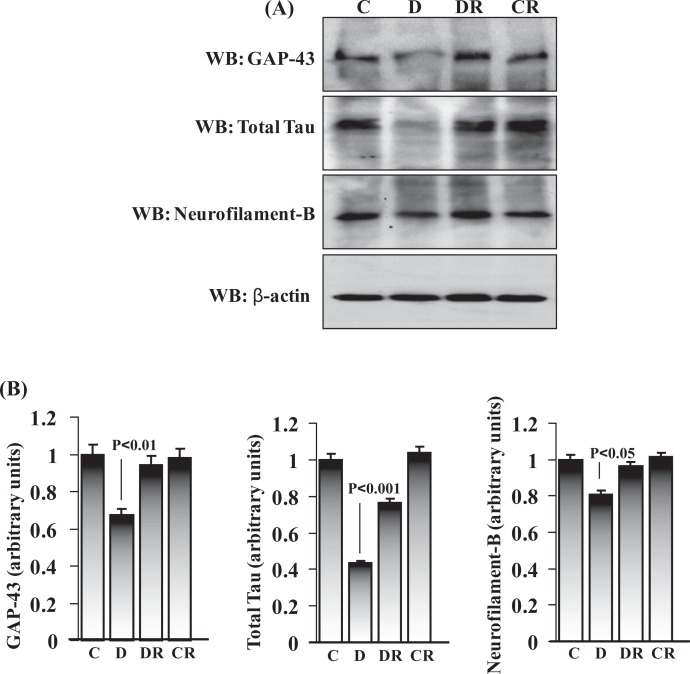
We also evaluated the expression patterns of neural marker proteins in E12 day rat embryos. GAP-43, total tau, and neurofilament B are neuronal marker proteins and normally produced by neurons during developmental growth and axonal regeneration and also in the adult nervous system. We evaluated the expression pattern of these 3 proteins by immunoblot using total embryonic protein and found reduced expression of all (comparatively less inhibition with neurofilament B) in the embryos of diabetic dams (Figure 4A and B). These data are consistent with a previous study where we found downregulation of neuronal marker expression in E16 embryonic cortex region of the brain.19 Embryos of RSV-treated diabetic dams significantly retained the expression level of GAP-43 (P < .01), total tau (P < .001), and neurofilament B (P < .05). No change in neuronal marker expression was observed in the RSV-treated control group.
Figure 4.
Resveratrol restored the expression of neuronal marker proteins in embryos of diabetic dams. Total embryonic protein (50 µg) was immunoblotted for neuronal markers (GAP-43, total tau, and neurofilament B) and β-actin (to evaluate protein loading) (A). The expression level of each neuronal marker was quantified by scanning densitometry and was expressed relative to actin in the corresponding embryonic protein (B). Error bars represent ±2 standard errors for each estimated mean.
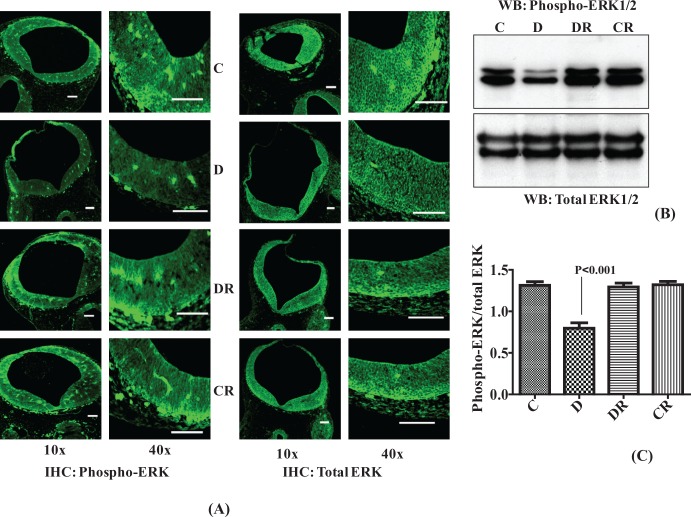
Resveratrol Improves Diabetes-Induced Reduction in Embryonic Extracellular Signal-Regulated Kinases
The ERK1/2 signaling pathway is preferentially activated in response to growth factors and regulates cell proliferation and cell differentiation.28 As embryos of diabetic dams were of relatively smaller size and lower weight, we hypothesized that there may be downregulation of ERK1/2 signaling resulting in less cell proliferation and differentiation. We analyzed the phosphorylation of ERK1/2 by IHC and Western blot and found less phospho-ERK1/2 in both cranial neural tube regions and whole embryo of diabetic dam. Resveratrol significantly improved the phosphorylation level of ERK1/2 in the embryos of diabetic dams (P < .001; Figure 5A, B, and C). The level of total ERK remained unchanged in all the groups. Resveratrol itself did not affect the phosphorylation of ERK1/2 as evident from the control group treated with RSV data.
Figure 5.
Resveratrol improves diabetes-induced reduction in embryonic extracellular signal-regulated kinases (ERK1/2). Immunohistochemistry was performed with embryo sections from the cranial neural tube region and fluorescein isothiocyanate-labeled immunofluorescence were photomicrographed using 10× and 40× objectives. Both total ERK and phosphorylated ERK are simultaneously evaluated in similar sections (A). Scale bar = 100 µm. Controls without primary antibody did not show any changes (data not shown). Western blot analysis was performed with total embryonic protein (40 µg) to check the level of ERK1/2 phosphorylation. Equal amounts of embryonic protein were immunoblotted with antibodies against phospho-ERK1/2. The same membranes were reprobed with an antibody against total ERK (B). Bar graph shows densitometry analysis and represents the ratio of phospho-ERK/total ERK (C). Error bars represent ±2 standard errors for each estimated mean.
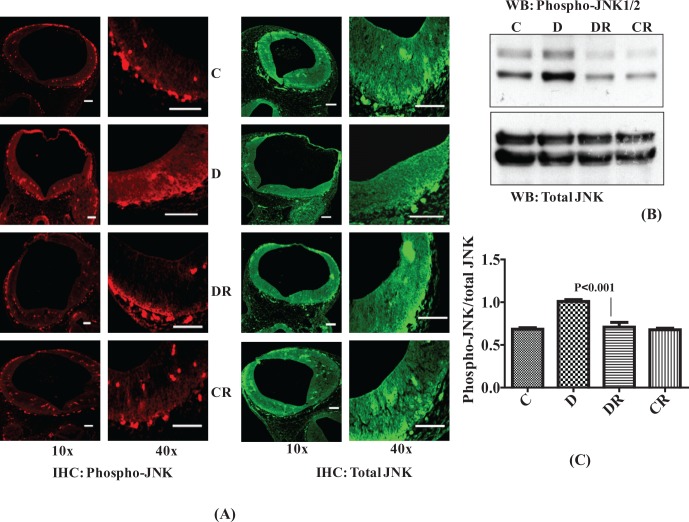
Resveratrol Prevents Diabetes-Induced Activation of JNK1/2
Activation of JNK1/2 has been shown to accompany embryonic dysmorphogenesis in the embryos of diabetic dams, and it has also been shown that embryos can be partially rescued by antioxidant supplementation.29,30 We analyzed JNK1/2 activation by measuring the phosphorylation level of JNK1/2. The IHC data showed greater intensity of phospho-JNK1/2 and Western blot data confirmed a significantly higher level of phospho-JNK1/2 in whole embryos of diabetic dams. Resveratrol treatment of diabetic dams restored the embryonic phospho-JNK1/2 level to the control levels (P < .001; Figure 6A, B, and C). The total cellular level of JNK remained the same in all the groups. Levels of phospho-JNK1/2 of the control group treated with RSV were equal to the control group treated with vehicle.
Figure 6.
Resveratrol prevents diabetes-induced activation of stress-activated protein kinases (JNK1/2). Immunohistochemistry was performed for phosphorylated JNK (Rhodamine labeled) and total JNK (FITC labeled) with embryonic sections and its expression was analyzed in cranial neural tube region using 10× and 40× objectives (A). Scale bar = 100 µm. Controls without primary antibody did not show any changes (data not shown). Western blot analysis was performed with embryonic protein (40 µg) to evaluate the level of JNK1/2 phosphorylation and reblotted with an antibody to total JNK for comparison (B). Bar graph shows scanned densitometry ratio analysis for phospho-JNK/total JNK (C). Error bars represent ± 2 standard errors for each estimated mean. JNK indicates c-Jun N-terminal kinase; FITC, fluorescein isothiocyanate.
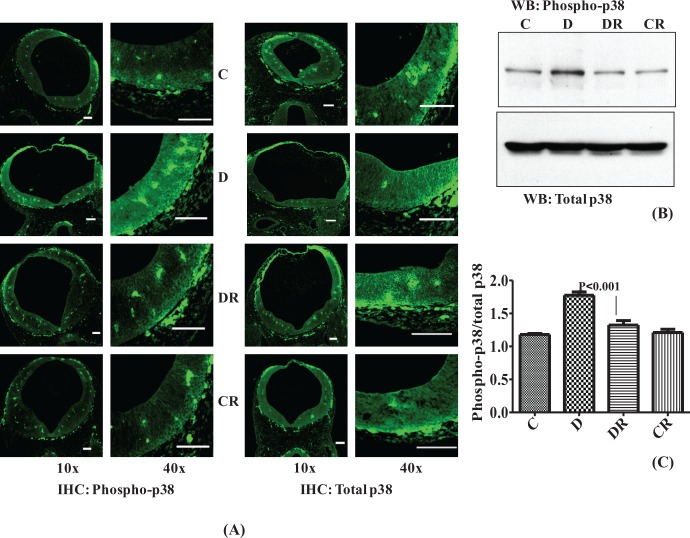
Resveratrol Decreases the Diabetes-Induced Activation of p38 Level
p38 signaling pathways are responsive to stress stimuli and its role reported in cell differentiation and apoptosis.17 Phosphorylated p38 (phospho-p38) was also found to be increased in neural tube sections of embryos of diabetic dams as evidenced by greater immunoreactive intensity with IHC and by Western blot (P < .001; Figure 7A, B, and C). No change in total p38 was observed. Resveratrol treatment normalized the level of phospho-p38 in the diabetic group treated with RSV. Phospho-p38 levels of the control treated group with RSV were the same as the vehicle control group.
Figure 7.
Resveratrol decreases the diabetes-induced activation of p38 MAPK. Immunohistochemistry was performed with embryo sections from cranial neural tube regions for phospho-p38 and total p38, and fluorescein isothiocyanate-labeled immunofluorescence were photomicrographed using 10× and 40× objectives (A). Scale bar = 100 µm. Controls without primary antibody did not show any changes (data not shown). Western blot was performed with 40 µg embryonic protein to check the level of p38 phosphorylation in comparison to total p38 (B). Bar graph represents scanned densitometry ratio analysis for phospho-p38/total p38 (C). Error bars represent ±2 standard errors for each estimated mean.
Discussion
The results presented here suggest that RSV could play a promising role in protecting embryos from the harmful effects of diabetes on the normal development. It impacts a large array of biological events and exerts a protective role for several diseases in animal models. In a recent study, it was shown to exhibit calorie restriction-like effects on energy metabolism and metabolic profile in obese humans.20,21,31–33 It is a safe and natural supplement.34 Resveratrol also protects against the teratogenic effects of dioxin in pregnancy.35,36 We found diabetes induced impairments in RAR, RXR, and MAPK signaling in the developing embryos of diabetic dams, which might contribute to a long-term developmental misprogramming.37 Gavage feeding of RSV (100 mg/kg body weight) to the diabetic dams corrected RAR, RXR, and MAPK signaling in the developing embryos.
In our study, there was a significant downregulation of RARs and RXRs in the embryos of diabetic dams. It is well accepted that the effects of RA in embryonic morphogenesis are modulated by heterodimers of RAR-RXR.13 It is possible that downregulation of these receptors would suppress the expression of many developmental genes. Indeed, a microarray study showed that expression of 128 NTDs-associated genes were decreased (>2-fold change) in the embryos of diabetic rats.38 The NTD and other embryonic malformations are extreme outcomes of diabetic pregnancy and are associated with great variability in the expression of dysregulated genes.38 In addition to these defects, embryos undergo developmental delays in diabetic pregnancies as measured by reduced embryo weight, crown–rump length, and somite number compared with nondiabetic pregnancies.24 Maternal diabetes can cause delays in development of the embryos.24,39–41 It has also been suggested that diabetes-induced developmental delays may impair postnatal development.39,41 However, the underlying mechanisms are not well understood for such impairments. Decreased expression of RAR-RXR in our study indicates impaired RA signaling. Suppression of RAR receptors is involved in embryonic skeleton hypoplasia in embryos of vitamin A-deficient rats.15 During embryonic development, RA is required for generating the correct anatomy of the spinal cord and for its subsequent proliferative expansion.14 The RA plays an important role in normal embryogenesis. However, changes in RA levels during embryonic development may also induce various malformations such as exencephaly and spina bifida. In a related study, maternal hyperglycemia was demonstrated to potentiate the caudal regression property of RA.42 Therefore, RA supplementation cannot be used to correct the RA signaling in embryos during diabetic pregnancy. In our studies, RSV prevented diabetes-induced changes in the expression of RARs and RXRs without any obvious adverse effects as RAR/RXR expression in the CR group was the same as that of vehicle controls.
Three of the retinoid receptors (RARα, RXRα, and RXRβ) tend to be ubiquitously expressed, while the others (RARβ, RARγ, and RXRγ) show complex, tissue-specific expression.26 Previous studies observed that RARγ is expressed specifically in the open neural plate, while RARβ is expressed in the closed neural tube.43 In our study, whole embryos from diabetic dams had a significantly reduced level of RARγ. Level of RARβ was drastically decreased in embryos of diabetic dams. DNA-protein interactions for RAR were also found suppressed in embryos from diabetic pregnancies. Changes in the relative abundance of these receptors might lead to decreased cell proliferation and premature neuronal differentiation. Corrected RAR expression as well as RAR DNA-protein complex formation was found in embryos of diabetic dams treated with RSV. The relative amount of RXR protein downregulation as well as suppression of RXR DNA-protein interaction was greater than that of RARs. The RXRs also heterodimerize with other nuclear receptors including RAR, thyroid hormone receptor, vitamin D receptor-like, and liver X receptor-like factors among others.27 These receptors exert their action by binding as heterodimers to response elements in the promoters of target genes and regulating their transcription. Both RXRα and RXRβ show ubiquitous expression in the developing rodent brain, whereas RXRγ appears in specific regions of newborn brain.44,45 Downregulation of these receptors due to maternal hyperglycemia might lead to improper or misprogrammed embryonic development and RSV treatment may be correcting or blocking these events.
The RA signaling is linked with the expression of neuronal markers.18 Diabetes significantly suppressed the expression of neuronal markers GAP-43, total tau, and neurofilament B in the embryos of diabetic dams, which might be an indicator of morphological and/or functional alteration in the developing central nervous system (Figure 4). Previously, we have shown that diabetes inhibits neurite formation and decreases the expression of neuronal markers in E16 cortical neurons cultured in hyperglycemic conditions.19 Here, we demonstrated that RSV restored the neuronal marker expression levels of embryos of diabetic dams up to that of the vehicle control group. We further screened for changes in other possible RAR/RXR target proteins such as PI3 kinase (P85α, P110α, P110β, and P110γ), Rac1, and c-SRC.18 However, we did not find significant changes in any of these end points (data not shown). This indicated that protein levels of these signaling components may not mediate the effects. Their activation status might be impaired under diabetic conditions and these impairments may be prevented by RSV.
The MAPKs play crucial roles during normal embryonic development. In particular, ERK1/2 are preferentially activated by growth factors and mitogens and regulate cell proliferation and differentiation during development.28 Various stimuli such as growth factors, intracellular pH changes, ultraviolet irradiation promote the activation of the p38 and JNK MAPKs.46,47 MAPK signaling controls stress/defense responses that determine whether cells have to survive, differentiate, or undergo apoptosis. We found relatively lower levels of ERK1/2 phosphorylation in the embryos of diabetic dams in the cranial neural tube as well as in whole embryo, implying growth inhibition during embryonic development resulting in developmental delay. Activation of JNK1/2 has been shown as one of the mechanisms of hyperglycemia-induced diabetic embryopathy.30 However, JNK also plays an important role in neuronal development signaling as shown by the deletion of Jnk1/2 in mice, which is lethal at mid-gastrulation, with embryos showing evidence of NTDs.48 We found higher levels of JNK1/2 and p38 phosphorylation which might be contributing to neuronal apoptosis in the developing embryonic brain.
Many studies support that hyperglycemia-induced oxidative stress and apoptosis as the main causes of complications resulting from diabetes.49 Hyperglycemia increases reactive oxygen species (ROS), and it has been shown to modulate MAPK signaling.50 In the embryo, oxidative stress results in DNA, protein, and lipid modifications that disrupt normal development. These effects can be due to altered MAPK signaling and can result in the misregulated expression of genes necessary for embryonic development. Resveratrol can also selectively inhibit stress-activated MAPK signaling.51 We demonstrated increased phospho-ERK1/2 and decreased phospho-JNK1/2 and phospho-p38 in the embryos of RSV-treated diabetic dams compared with those not exposed to RSV.
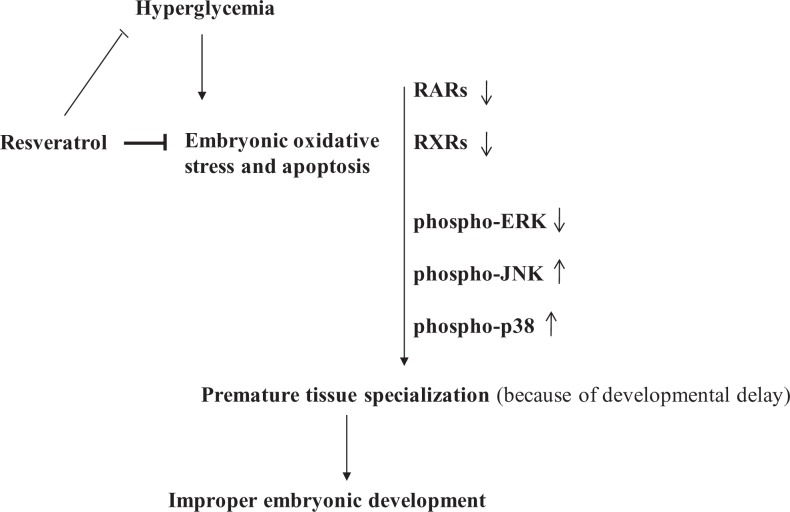
In a previous study, we have shown that RSV efficiently reduced embryonic oxidative stress and apoptosis associated with diabetic embryopathy possibly via maternal glucose homeostasis as well as by directly acting on embryos. We also reported that the developmental delays in the embryos of diabetic dams were prevented by RSV, however, the signaling mechanisms mediating RSV effects remain to be determined.24 Here, using the embryos with developmental delays in whole body growth, we studied the possible mechanisms underlying these effects of RSV. By preventing impairments in RAR, RXR, and MAPK signaling under diabetic conditions, RSV may prevent developmental misprogramming resulting in proper embryonic development (Figure 8). However, further studies are warranted to determine the mechanism and explore the potential of RSV supplementation to prevent other defects such as neural tube and heart defects under diabetic conditions.
Figure 8.
Putative model explaining maternal diabetes-induced impairment in the signaling mechanisms affecting the embryonic development and how resveratrol may be preventing the effects.
Conclusions
Diabetes produces various types of defects in the developing embryos. Most commonly studied defects are NTDs. We have studied a condition where the development of the embryos is delayed (reduced crown–rump length and weight) under diabetic conditions. Our studies for the first time indicate that RSV is a potential natural product to minimize the effects of diabetes on embryonic developmental delays (present study and Singh et al24). The present study also demonstrates that RSV may be targeting RARs and MAPKs to prevent the effects. Whether effects of RSV are direct or indirect due to the sugar-lowering effects remain to be determined. Also, whether RSV may prevent other defects such as NTDs or heart defects and similar mechanisms are targeted needs further investigation.
Acknowledgments
We thank Tobias Handschin and Parth Patel for helpful discussion and technical support. We also thank members of the SOM instrumentation resource facility for their assistance in immunofluorescence microscopy.
Footnotes
Declaration of Conflicting Interests: The authors declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: supported in part by National Institutes of Health R21AA016121 (US) and Health Sciences Distinguished Professorship (DJD).
References
- 1. Zabihi S, Loeken MR. Understanding diabetic teratogenesis: where are we now and where are we going? Birth Defects Res A Clin Mol Teratol. 2010;88(10):779–790 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Oyama K, Sugimura Y, Murase T, et al. Folic acid prevents congenital malformations in the offspring of diabetic mice. Endocr J. 2009;56(1):29–37 [DOI] [PubMed] [Google Scholar]
- 3. Zhao Z, Reece EA. Experimental mechanisms of diabetic embryopathy and strategies for developing therapeutic interventions. J Soc Gynecol Investig. 2005;12(8):549–557 [DOI] [PubMed] [Google Scholar]
- 4. Cleves MA, Hobbs CA. Collaborative strategies for unraveling the complexity of birth defects. J Matern Fetal Neonatal Med. 2004;15(1):35–38 [DOI] [PubMed] [Google Scholar]
- 5. Wroblewska-Seniuk K, Wender-Ozegowska E, Szczapa J. Long-term effects of diabetes during pregnancy on the offspring. Pediatr Diabetes. 2009;10(7):432–440 [DOI] [PubMed] [Google Scholar]
- 6. Dabelea D. The predisposition to obesity and diabetes in offspring of diabetic mothers. Diabetes Care. 2007;30(suppl 2):S169–S174 [DOI] [PubMed] [Google Scholar]
- 7. Pavlinkova G, Salbaum JM, Kappen C. Maternal diabetes alters transcriptional programs in the developing embryo. BMC Genomics. 2009;10:274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Fu J, Tay SS, Ling EA, et al. High glucose alters the expression of genes involved in proliferation and cell-fate specification of embryonic neural stem cells. Diabetologia. 2006;49(5):1027–1038 [DOI] [PubMed] [Google Scholar]
- 9. Dheen ST, Tay SS, Boran J, et al. Recent studies on neural tube defects in embryos of diabetic pregnancy: an overview. Curr Med Chem. 2009;16(18):2345–2354 [DOI] [PubMed] [Google Scholar]
- 10. Simeoni U, Barker DJ. Offspring of diabetic pregnancy: long-term outcomes. Semin Fetal Neonatal Med. 2009;14(2):119–124 [DOI] [PubMed] [Google Scholar]
- 11. Van Lieshout RJ, Voruganti LP. Diabetes mellitus during pregnancy and increased risk of schizophrenia in offspring: a review of the evidence and putative mechanisms. J Psychiatry Neurosci. 2008;33(5):395–404 [PMC free article] [PubMed] [Google Scholar]
- 12. Palha JA, Goodman AB. Thyroid hormones and retinoids: a possible link between genes and environment in schizophrenia. Brain Res Rev. 2006;51(1):61–71 [DOI] [PubMed] [Google Scholar]
- 13. Mark M, Ghyselinck NB, Chambon P. Function of retinoic acid receptors during embryonic development. Nucl Recept Signal. 2009;7:e002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Wilson L, Gale E, Maden M. The role of retinoic acid in the morphogenesis of the neural tube. J Anat. 2003;203(4):357–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Li N, Sun S, Wang D, et al. Suppression of retinoic acid receptors may contribute to embryonic skeleton hypoplasia in maternal rats with chronic vitamin A deficiency. J Nutr Biochem. 2010;21(8):710–716 [DOI] [PubMed] [Google Scholar]
- 16. Lenhard JM. PPAR gamma/RXR as a molecular target for diabetes. Receptors Channels. 2001;7(4):249–258 [PubMed] [Google Scholar]
- 17. Yan J, Hales BF. p38 and c-Jun N-terminal kinase mitogen-activated protein kinase signaling pathways play distinct roles in the response of organogenesis-stage embryos to a teratogen. J Pharmacol Exp Ther. 2008;326(3):764–772 [DOI] [PubMed] [Google Scholar]
- 18. Pan J, Kao YL, Joshi S, et al. Activation of Rac1 by phosphatidylinositol 3-kinase in vivo: role in activation of mitogen-activated protein kinase (MAPK) pathways and retinoic acid-induced neuronal differentiation of SH-SY5Y cells. J Neurochem. 2005;93(3):571–583 [DOI] [PubMed] [Google Scholar]
- 19. Guleria RS, Pan J, Dipette D, et al. Hyperglycemia inhibits retinoic acid-induced activation of Rac1, prevents differentiation of cortical neurons, and causes oxidative stress in a rat model of diabetic pregnancy. Diabetes. 2006;55(12):3326–3334 [DOI] [PubMed] [Google Scholar]
- 20. Pervaiz S, Holme AL. Resveratrol: its biologic targets and functional activity. Antioxid Redox Signal. 2009;11(11):2851–2897 [DOI] [PubMed] [Google Scholar]
- 21. Timmers S, Konings E, Bilet L, et al. Calorie restriction-like effects of 30 days of resveratrol supplementation on energy metabolism and metabolic profile in obese humans. Cell Metab. 2011;14(5):612–622 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Banerjee Mustafi S, Chakraborty PK, Raha S. Modulation of Akt and ERK1/2 pathways by resveratrol in chronic myelogenous leukemia (CML) cells results in the downregulation of Hsp70. PLoS One.5(1):e8719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Kang MR, Lee SW, Um E, et al. Reciprocal roles of SIRT1 and SKIP in the regulation of RAR activity: implication in the retinoic acid-induced neuronal differentiation of P19 cells. Nucleic Acids Res. 2010;38(3):822–831 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Singh CK, Kumar A, Hitchcock DB, et al. Resveratrol prevents embryonic oxidative stress and apoptosis associated with diabetic embryopathy and improves glucose and lipid profile of diabetic dam. Mol Nutr Food Res. 2011;55(8):1186–1196 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Kumar A, Singh CK, DiPette DD, et al. Ethanol impairs activation of retinoic acid receptors in cerebellar granule cells in a rodent model of fetal alcohol spectrum disorders. Alcohol Clin Exp Res. 2010;34(5):928–937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Dolle P. Developmental expression of retinoic acid receptors (RARs). Nucl Recept Signal. 2009;7:e006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Germain P, Chambon P, Eichele G, et al. International Union of Pharmacology. LXIII. Retinoid X receptors. Pharmacol Rev. 2006;58(4):760–772 [DOI] [PubMed] [Google Scholar]
- 28. Corson LB, Yamanaka Y, Lai KM, et al. Spatial and temporal patterns of ERK signaling during mouse embryogenesis. Development. 2003;130(19):4527–4537 [DOI] [PubMed] [Google Scholar]
- 29. Reece EA, Wu YK, Zhao Z, et al. Dietary vitamin and lipid therapy rescues aberrant signaling and apoptosis and prevents hyperglycemia-induced diabetic embryopathy in rats. Am J Obstet Gynecol. 2006;194(2):580–585 [DOI] [PubMed] [Google Scholar]
- 30. Yang P, Zhao Z, Reece EA. Involvement of c-Jun N-terminal kinases activation in diabetic embryopathy. Biochem Biophys Res Commun. 2007;357(3):749–754 [DOI] [PubMed] [Google Scholar]
- 31. Ates O, Cayli SR, Yucel N, et al. Central nervous system protection by resveratrol in streptozotocin-induced diabetic rats. J Clin Neurosci. 2007;14(3):256–260 [DOI] [PubMed] [Google Scholar]
- 32. Palsamy P, Subramanian S. Resveratrol, a natural phytoalexin, normalizes hyperglycemia in streptozotocin-nicotinamide induced experimental diabetic rats. Biomed Pharmacother. 2008;62(9):598–605 [DOI] [PubMed] [Google Scholar]
- 33. Kutuk O, Poli G, Basaga H. Resveratrol protects against 4-hydroxynonenal-induced apoptosis by blocking JNK and c-JUN/AP-1 signaling. Toxicol Sci. 2006;90(1):120–132 [DOI] [PubMed] [Google Scholar]
- 34. Williams LD, Burdock GA, Edwards JA, et al. Safety studies conducted on high-purity trans-resveratrol in experimental animals. Food Chem Toxicol. 2009;47(9):2170–2182 [DOI] [PubMed] [Google Scholar]
- 35. Jang JY, Park D, Shin S, et al. Antiteratogenic effect of resveratrol in mice exposed in utero to 2,3,7,8-tetrachlorodibenzo-p-dioxin. Eur J Pharmacol. 2008;591(1-3):280–283 [DOI] [PubMed] [Google Scholar]
- 36. Singh NP, Singh US, Nagarkatti M, et al. Resveratrol (3,5,4′-trihydroxystilbene) protects pregnant mother and fetus from the immunotoxic effects of 2,3,7,8-tetrachlorodibenzo-p-dioxin. Mol Nutr Food Res. 2011;55(2):209–219 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. El-Osta A, Brasacchio D, Yao D, et al. Transient high glucose causes persistent epigenetic changes and altered gene expression during subsequent normoglycemia. J Exp Med. 2008;205(10):2409–2417 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Salbaum JM, Kappen C. Neural tube defect genes and maternal diabetes during pregnancy. Birth Defects Res A Clin Mol Teratol. 2010;88(8):601–611 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Petersen MB, Pedersen SA, Greisen G, et al. Early growth delay in diabetic pregnancy: relation to psychomotor development at age 4. Br Med J (Clin Res Ed). 1988;296(6622):598–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wilson GN, Howe M, Stover JM. Delayed developmental sequences in rodent diabetic embryopathy. Pediatr Res. 1985;19(12):1337–1340 [DOI] [PubMed] [Google Scholar]
- 41. Van Assche FA, Holemans K, Aerts L. Long-term consequences for offspring of diabetes during pregnancy. Br Med Bull. 2001;60:173–182 [DOI] [PubMed] [Google Scholar]
- 42. Chan BW, Chan KS, Koide T, et al. Maternal diabetes increases the risk of caudal regression caused by retinoic acid. Diabetes. 2002;51(9):2811–2816 [DOI] [PubMed] [Google Scholar]
- 43. Chen WH, Morriss-Kay GM, Copp AJ. Genesis and prevention of spinal neural tube defects in the curly tail mutant mouse: involvement of retinoic acid and its nuclear receptors RAR-beta and RAR-gamma. Development. 1995;121(3):681–691 [DOI] [PubMed] [Google Scholar]
- 44. Dolle P, Fraulob V, Kastner P, et al. Developmental expression of murine retinoid X receptor (RXR) genes. Mech Dev. 1994;45(2):91–104 [DOI] [PubMed] [Google Scholar]
- 45. Zetterstrom RH, Lindqvist E, Mata de Urquiza A, et al. Role of retinoids in the CNS: differential expression of retinoid binding proteins and receptors and evidence for presence of retinoic acid. Eur J Neurosci. 1999;11(2):407–416 [DOI] [PubMed] [Google Scholar]
- 46. Kyriakis JM, Avruch J. Sounding the alarm: protein kinase cascades activated by stress and inflammation. J Biol Chem. 1996;271(40):24313–24316 [DOI] [PubMed] [Google Scholar]
- 47. Rana A, Gallo K, Godowski P, et al. The mixed lineage kinase SPRK phosphorylates and activates the stress-activated protein kinase activator, SEK-1. J Biol Chem. 1996;271(32):19025–19028 [DOI] [PubMed] [Google Scholar]
- 48. Sabapathy K, Jochum W, Hochedlinger K, et al. Defective neural tube morphogenesis and altered apoptosis in the absence of both JNK1 and JNK2. Mech Dev. 1999;89(1-2):115–124 [DOI] [PubMed] [Google Scholar]
- 49. Ornoy A. Embryonic oxidative stress as a mechanism of teratogenesis with special emphasis on diabetic embryopathy. Reprod Toxicol. 2007;24(1):31–41 [DOI] [PubMed] [Google Scholar]
- 50. Torres M, Forman HJ. Redox signaling and the MAP kinase pathways. Biofactors. 2003;17(1-4):287–296 [DOI] [PubMed] [Google Scholar]
- 51. Malemud CJ. Inhibitors of stress-activated protein/mitogen-activated protein kinase pathways. Curr Opin Pharmacol. 2007;7(3):339–343 [DOI] [PubMed] [Google Scholar]