Abstract
Modification of poly(A) tail length constitutes the main posttranscriptional mechanism by which gene expression is regulated during spermatogenesis. Embryonic poly(A)-binding protein (EPAB) and somatic cytoplasmic poly(A)-binding protein (PABPC1) are the 2 key proteins implicated in this pathway. In this study we characterized the temporal and spatial expression of Epab and Pabpc1 in immature (D6-D32) and mature (D88) mouse testis and in isolated spermatogenic cells. Both Epab and Pabpc1 expression increased during early postnatal life and reached their peak at D32 testis. This was due to an increase in both spermatogonia (SG) and spermatocytes. In the mature testis, the highest levels of Epab were detected in SG, followed by round spermatids (RSs), while the most prominent Pabpc1 expression was detected in spermatocytes and RSs. Our findings suggest that PABPC1 may play a role in translational regulation of gene expression by cytoplasmic polyadenylation, which occurs in spermatocytes, while both EPAB and PABPC1 may help stabilize stored polyadenylated messenger RNAs in RSs.
Keywords: EPAB, PABPC1, spermatogenesis, testis, polyadenylation
Introduction
Spermatogenesis is a complex process of proliferation and differentiation transforming spermatogonia (SG) into mature spermatozoa.1–4 During this tightly regulated process, male germ cells utilize unique mechanisms of transcription initiation/repression including dedicated transcription factors, tissue-specific promoters, and somatic gene silencing.5 Transcription factors unique to spermatogenic cells have been identified and include SPRM1, TAK-1, OCT-2, CREB, and CREM.6 Transcription ceases at mid-spermiogenesis (step 10 spermatids in mouse), as the remodeled condensed chromatin structure of the late-stage spermatids is incompatible with transcription.7–9 Thereafter, gene expression is regulated posttranscriptionally and is dependent upon dormant paternally stored messenger RNAs (mRNAs) that are polyadenylated within the nucleus and exported to the cytoplasm, where they await alteration of their poly(A) tails for subsequent activation.10,11
Interestingly, unlike in the oocyte where the extension of the poly(A) tail results in translational activation, shortening of poly(A) tails in spermatids seems to trigger translation.12 Indeed, stored mRNAs in spermatids usually have poly(A) tails longer than 150 nucleotides, but these polyadenylated mRNAs are not associated with ribosomes. They appear to undergo deadenylation until approximately 30 nucleotides of poly(A) remain; this is coincident with their association with ribosomes and an increase in translation.12,13
In addition, translational activation by cytoplasmic elongation of poly(A) tail, similar to that seen in oocytes, also occurs during spermatogenesis. Targeted disruption of cytoplasmic polyadenylation element-binding protein (CPEB)14 and testis-specific cytoplasmic poly(A) polymerase10 result in the arrest of spermatogenesis at the pachytene stage of the first meiotic division and during spermiogenesis (step 7 round spermatids [RSs]), respectively.
Overall, while posttranscriptional regulation of gene expression by polyadenylation or deadenylation seems to play a key role during spermatogenesis, molecular mediators of these processes in male germ cells remain to be elucidated. It is likely that poly(A)-binding proteins (PABPs)15–26 that bind and stabilize poly(A) tail in somatic and germ cells play a role in this process.
Embryonic poly(A)-binding protein (EPAB) has initially been identified in Xenopus as the predominant cytoplasmic PABP in oocytes and early embryos until zygotic genome activation (ZGA), when it is replaced by the somatic cytoplasmic poly(A)-binding protein, PABPC1.16 Orthologues of Xenopus EPAB have been identified and characterized in mouse and human.17,27 In the mouse, Epab mRNA is exclusively expressed in the gonads, oocytes, and 1- and 2-cell embryos but becomes undetectable in 4-cell, 8-cell, and blastocyst stage mouse embryos.17 By contrast, Pabpc1 mRNA is expressed at low levels in oocytes and in early preimplantation embryos, however, its expression is significantly increased at 8-cell and blastocyst stages.17 A similar expression pattern for Epab and Pabpc1 has been reported during human oocyte and early embryo development.27 As ZGA occurs at the 2-cell stage in mouse28 and 4- to 8-cell stage in human embryos,29 EPAB seems to be the predominant PABP during mammalian oocyte and early embryo development until ZGA, when it is replaced by PABPC1. This tightly controlled expression suggests that EPAB may play a key role in mediating translational regulation of gene expression during early development. Indeed, in Xenopus, EPAB is associated with protein complexes that stabilize maternally stored mRNAs and regulate their translational activation.16,30–32
Although Epab expression during mouse oogenesis and early embryo development has been characterized, its expression in the postnatal mouse testis and in specific spermatogenetic cell types has not yet been studied. In this study, we aimed to characterize Epab and Pabpc1 expression during male germ cell development and maturation by assessing immature and mature postnatal testis (D6-D88) and isolated spermatogenic cells.
Methods
Animals and Collection of Testes
The C57BL/6 mice were bred and maintained according to US Government Principles for the Utilization and Care of Vertebrate Animals Used in Testing, Research and Training, and all protocols were approved by Yale University’s Institutional Animal Care and Use Committee (IACUC #2008-11207). Mice were housed under a 12-hour light cycle with free access to food and water. Testes from immature (days of 6, 8, 16, 20, 29, and 32) and mature mice (day 88) were collected under sterile conditions following euthanasia. Absence of sperm cells in cauda epididymis of 32-day-old immature mice was confirmed by evaluation under inverted microscope, following mincing and incubation in Dulbecco Modified Eagle Medium (DMEM): F12 media (Gibco, Carlsbad, California) at 37°C for 30 minutes.
Determination of Relative Abundance of Germinal Epithelial Cell Types in Postnatal Male Mouse Testis
To determine the relative abundance of each germinal epithelial cell type in testis from immature and mature male mice, hematoxylin and eosin (HE) staining was performed. For this purpose, whole testes obtained from 6-, 8-, 16-, 29-, 32-, and 88-day-old mice were fixed in Bouin fixative for 12 hours at +4°C and dehydrated in increasing concentrations of ethanol. Then, they were cleared in xylene and embedded in paraffin. Paraffin blocks were cut serially at 5 μm thickness using the rotary microtome (Leica, Nussloch, Germany). For HE staining, slides were incubated at 60°C for 1 hour, treated twice with fresh xylene, and then rehydrated in decreasing ethanol series followed by wash in tap water. Slides were then stained with hematoxylin followed by eosin. After additional washes in distilled water, slides were dehydrated in increasing concentrations of ethanol and cleared in fresh xylene.
The HE-stained male germinal epithelial cells demonstrate well-defined morphological appearances. As previously described, SG were identified by stippled and deeply stained nucleus surrounded by a thin rim of cytoplasm; spermatocytes were identified by strongly stained woolly masses of chromatin around the nucleus and abundant cytoplasm; RSs were identified by a small round nucleus with a heavily stained irregular nucleolus in the center and abundant cytoplasm; elongated spermatids (ESs) were identified by hook-shaped nucleus surrounded by a small amount of cytoplasm at the surface and by the presence of a flagella.33–35 For each developmental stage studied (D6, D8, D16, D29, D32, and D88), randomly selected 10 seminiferous tubules from 4 testis sections were assessed by 2 independent investigators under bright field light microscope (Optiphot 300, Nikon, Japan). The percentage of each spermatogenic cell was then calculated.
Separation of the Spermatogenic Cells From Immature and Mature Mouse Testes
Spermatogenic cell isolation was performed using previously described protocols33,36,37 with modifications. Testes from 2 mice for each of the postnatal ages of 6, 8, 16, 29, 32, and 88 days were analyzed. Briefly, testes were decapsulated and digested in DMEM:F12 medium containing 0.5 mg/mL of collagenase (Roche, Indianapolis, Indiana) for 20 minutes at 34°C. Following collagenase treatment, seminiferous tubules were washed twice with fresh media and were gently pipetted with glass Pasteur pipette for 20 minutes at 34°C in medium containing 0.25 mg/mL of trypsin to obtain mixed spermatogenic cells. Following centrifugation at 1600 rpm for 6 minutes, the pellet containing mixed spermatogenic cells was suspended in fresh DMEM:F12 medium containing 0.5% bovine serum albumin ([BSA] American Bioanalytical Inc, Natick, Massachusetts) and 1 μg/mL DNase (Roche). Following a second centrifugation at 1600 rpm for 6 minutes, the pellet was resuspended in media including 0.5% BSA and then filtered through 70 and 40 μm cell strainers (BD biosciences, California).
Spermatogenic cell types were fractionated using a discontinuous 2% to 4% BSA gradient prepared in a 15 mL tube (Falcon, BD biosciences) by first adding 1 mL of 10% BSA and then 1 mL of 4.0%, 3.8%, 3.6%, 3.4%, 3.2%, 3.0%, 2.8%, 2.6%, 2.4%, 2.2%, and 2.0% BSA. Filtered spermatogenic cells were counted using a Makler chamber (Sefi-Medical, Haifa, Israel), and 1 mL of mixed spermatognic cells (3.5 × 105 cells/mL) was placed onto the gradient. Finally, 1 mL of 0.2% BSA was added. The tube was then incubated at + 4°C for 4 hours with gentle rocking at the rate of 15 tilts/min to enhance gravity sedimentation. Following incubation, 1 mL fractions were collected and centrifuged at 1600 rpm for 6 minutes. Cells were then suspended and assessed for their morphology under inverted microscope (Diaphot 300, Nikon). In addition, cells from each fraction were stained with HE and their morphology was assessed as previously described using established criteria.33–35 Spermatocytes were found at fractions 3 to 6, RSs from spermiogenesis steps 1 to 13 at fractions 7 to 8, ESs from spermiogenesis steps 14 to 16 at fractions 12 to 14. Fractions with ≥77% purity for each specific spermatogenic cell type were pooled together and used for RNA isolation. In addition to morphologic parameters, mRNA expression for specific markers such as protamine 2 (Prm2) and synaptonemal complex 3 (Scp3) were assessed to confirm effective cell separation.
Mature spermatozoa were isolated from cauda epididymis of mature male mice of day 88 (D88). Dissected cauda epididymides were minced in 2.5 mL fresh DMEM:F12 medium and incubated at 37°C for 30 minutes to allow the release of spermatozoa into the medium. Following centrifugation at 1600 rpm for 6 minutes, the pellet was used for the total RNA isolation.38
RNA Isolation, Polymerase Chain Reaction, and Quantitative Real-Time PCR
Total RNA was isolated from immature (D6, D8, D16, D20, D29, and D32) and mature mice testes (D88) using Trizol (Invitrogen, Carlsbad, California) according to the manufacturer’s instructions and kept at −80°C until use. RNAqueous-Micro Kit (Ambion, Austin, Texas) was used for total RNA isolation from the isolated spermatogenic cells. RNA was treated for genomic DNA contamination using DNase I (Ambion). The quality and concentration of the isolated total RNA were determined at the absorbance 260 and 280 nm. Reverse transcription reactions were performed using the Omniscript kit (Qiagen, Valencia, CA, USA), according to the manufacturer’s instructions. Equal amounts of total RNA (2 µg) were reverse transcribed to complementary DNA (cDNA) using random decamer priming at 37°C for 1 hour.
Polymerase chain reactions (PCRs) were performed on cDNAs using Taq DNA polymerase (Qiagen). Amplifications were carried out with 27 to 35 cycles of PCR (32 cycles for Epab and Pabpc1, 27 for Prm2, 35 for Scp3, and 30 for β-actin [Actb]), in which the initial 5 minutes denaturation at 95°C was followed by a “touch-down” program for 10 cycles of 92°C for 30 seconds, 65°C for 30 seconds (−1°C per cycle), and 72°C for 30 seconds; and then 17 to 25 cycles of 92°C for 30 seconds, 55°C for 30 seconds, and 72°C for 30 seconds, and final extension at 72°C for 10 minutes, in a volume of 25 μL of reaction mixture containing 10 × PCR buffer (Qiagen), 0.125 mmol/L of each deoxyribonucleotide triphosphate (Roche), 0.5 μmol/L of each primer (Keck Facility, Connecticut), and 2 units of SuperTaq polymerase (Qiagen). All PCR products were separated on 1.5% agarose/Tris–acetate–EDTA gels and visualized by ethidium bromide staining.
Quantitative real-time PCRs (qRT-PCRs) were conducted on an iCycler (Bio-Rad, Hercules, California) and assayed in triplicate. Each 25 μL reaction mixture contained 12.5 μL of 2 × SybrGreen supermix (Biorad, Hercules, CA, USA), 0.4 μmol/L of each primer (Keck Facility, Connecticut) and 1 μL of cDNA template. Unique PCR products were confirmed by melting curve analysis. Relative amounts of the Epab, Pabpc1, and Actb genes were determined based on the standard curve generated using serially diluted mouse cDNA. The PCR efficiency was >92% for all qRT-PCR reactions. Relative fold change for the Epab and Pabpc1 genes were determined after normalization to Actb levels.
The primers used for PCR and RT-PCR reactions were Epab: forward: 5′-ATG AAC GGG CGC ATC GTG GGC-3′, reverse: 5′-ATG TGG CTG GGC TGT CCA CCT-3′; Pabpc1: forward: 5′-CCC AGC GCC CCC AGC TAC-3′, reverse: 5′-CAC GTT CCG CGT CC GCC-3′; Actb: forward: 5′-TGC GTG ACA TCA AAG AGA AG-3′, reverse: 5′-CGG ATG TCA ACG TCA CAC TT-3′, Prm2: forward: 5′-CGC TAC CGA ATG AGG AGC CCC AGT G-3′, reverse: 5′-TTA GTG ATG GTG CCT CCT ACA TTT CC-3′; Scp3: forward: 5′-TCA AAG CCA GTA ACC AGA-3′, reverse: 5′-TCG AAC ATT TGC CAT CTC-3′
RNA In Situ Hybridization
Testes obtained from immature and mature male mice were fixed in Bouin fixative for 4 hours at room temperature and dehydrated in increasing concentrations of ethanol. They were then cleared in xylene and embedded in paraffin. Paraffin blocks were stored at 4°C. Testis sections (5 μm) were cut using the rotary microtome (Leica).
Epab and Pabpc1 cDNA probes for in situ hybridization (ISH) were produced by PCR, using cDNA from mouse ovary and testis. Amplifications were carried out in 35 cycles of PCR as above, with the exception of 1 minute extension steps in a volume of 50 μL reaction mixture as above including 0.02 mmol/L Dig-UTP (Roche). Both Epab and Pabpc1 primers were located on exons 1 and 2 of respective genes. The PCR products were fractionated on a 1.5% agarose gel and purified using QIAEX II gel extraction kit (Qiagen). The efficiency of labeling was confirmed by comparison with a labeled control DNA in a dot blot according to the manufacturer’s instructions (Roche). The labeled probe was stored at −20°C until use.
The ISH method was modified from Seli et al.17 Briefly, paraffin sections (5 μm) from mature male mice testis were incubated at 60°C for 1 hour prior to ISH. Then, sections were treated twice with fresh xylene and rehydrated in decreasing concentrations of ethanol followed by rinsing in water treated with 0.1% diethylpyrocarbonate (Sigma-Aldrich, St. Louis, MO, USA). Permeabilization with 5 μg/mL proteinase K (Roche) in 1 × phosphate-buffered saline (PBS) with 0.1% Tween-20 (PBST) at 37°C for 30 minutes was followed by postfixation in 4% paraformaldehyde in PBS for 5 minutes at room temperature. After washing with PBST, sections were incubated in prehybridization buffer (50% formamide, 5 mmol/L EDTA, 4 × SSC, 0.1% Tween-20, and 1% blocking reagent (Roche), 100 μg/mL of sonicated salmon sperm DNA (Stratagene, Jolla, CA, USA), 0.5% CHAPS (Sigma-Aldrich)] at 37°C for 2 hours. Hybridization with 300 ng/mL of digoxigenin-labeled Epab or Pabpc1 probes was performed at 42°C overnight. After hybridization, sections were washed twice in 2 × SSC, once in 0.1 × SSC for 20 minutes at 65°C, and twice in MABT (0.1 mol/L maleic acid [Sigma-Aldrich], 0.15 mol/L NaCl, pH 7.5, and 0.1% Tween-20) for 10 minutes at room temperature. Blocking was performed using 1% (wt/vol) blocking reagent (Roche) in MAB (0.1 mol/L maleic acid [Sigma-Aldrich]/0.15 mol/L NaCl) for 30 minutes at room temperature. Sections were then incubated in antidigoxigenin antibody conjugated to alkaline phosphatase (1:800, Roche) for 1 hour at room temperature. After six 10-minute washes with MABT, sections were equilibrated in NaCl-Tris-MgCl2-Tween-buffer (NTMT) buffer (0.1 mol/L NaCl, 0.1 mol/L Tris-HCl, 50 mmol/L MgCl2 [Sigma-Aldrich], 0.1% Tween-20) and incubated with 5-bromo-4-chloro-3-indolyl phosphate/nitroblue tetrazolium in NTMT (Roche) for ~16 hours. Color development was stopped with several washes in PBST, and slides were mounted with Immu-Mount (Thermo Shandon, Pittsburgh). Sections were analyzed under bright-field microscope (Carl Zeiss Inc, Thornwood, New York).
Statistical Analysis
Because the data were normally distributed (as determined by the Kolmogorov-Smirnov test), comparisons of samples were analyzed with one-way analysis of variance followed by a post hoc Student-Newman-Keuls test where appropriate. Statistical calculations were performed using SigmaStat for Windows, version 3.0 (Jandel Scientific Corp, San Rafael, California). Statistical significance was defined as P < .05.
Results
Expression Analysis of Epab and Pabpc1 in Postnatal Mouse Testis
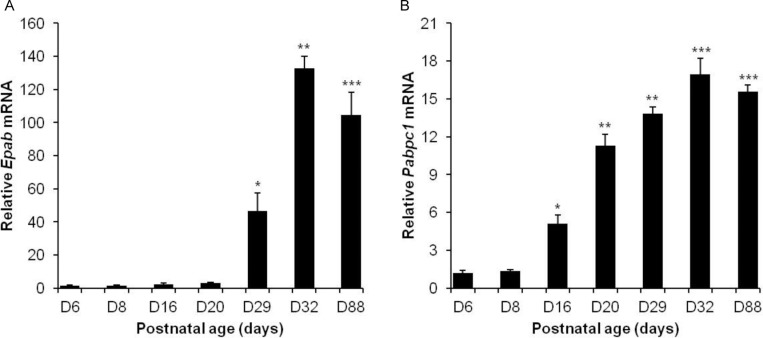
During postnatal development, testis undergoes maturation and the relative abundance of spermatogenic cells changes in a predictable manner. We first assessed Epab and Pabpc1 mRNA expression in postnatal mouse testis from D6, D8, D16, D20, D29, D32, and D88. Quantitative RT-PCR showed that the level of Epab mRNA was very low on D6, D8, D16, and D20, but increased on D29. A further 3-fold increase was detected on D32 (Figure 1A). The amount of Pabpc1 mRNA on D6 and D8 were low and increased significantly on D16. Further significant increases in Pabpc1 mRNA were observed on D20, D29, and D32 (Figure 1B). Both Epab and Pabpc1 mRNA expression reached their highest level at D32 and showed a slight decrease (statistically significant only for Epab [Epab written in a italic form]) on D88 (Figure 1).
Figure 1.
Expression analysis of Epab and Pabpc1 in postnatal mouse testis. Quantification of Epab or Pabpc1 mRNA expression in D6 to D88 postnatal mouse testes using real-time polymerase chain reaction (PCR). Epab or Pabpc1 expression was normalized to β-actin expression. A, Epab messenger RNA (mRNA) level was significantly increased at D29 and reached its highest level at D32. *P < .05 for D29 versus D20, D16, D8, and D6; **P < .05 for D32 versus D29; ***P < .05 for D88 versus D32. B, Pabpc1 mRNA level was significantly increased at D16 and reached its highest level at D32. *P < .05 for D16 versus D8, and D6; **P < .05 for D20 and D29 versus D16; ***P < .05 for D32 and D88 versus D29 and D20.
Isolation of Spermatogenic Cells by Fractionation of Mixed Germ Cells From D6 to D88 Mouse Testis
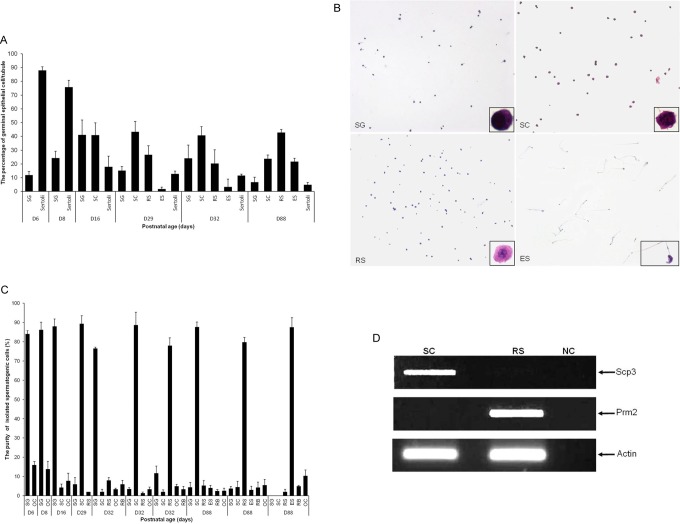
First, we determined the relative abundance of each germinal epithelial cell type in testis from immature and mature male mice. The HE staining was performed, assessing 10 tubules from 4 testis sections for each developmental stage (D6, D8, D16, D29, D32, and D88). We found that the relative abundance of male germinal epithelial cells changes during testicular maturation, with decreasing abundance of SG and sertoli cells and increasing abundance of RS and ES as previously described (Figure 2A).36
Figure 2.
A, Percentage of spermatogenic cell types in testis from D6 to D88 mouse testis. The age of the mice and counted spermatogenic cell types are specified for each fraction. B, Isolation of spermatogenic cells by fractionation of mixed germ cells from D6 to D88 mouse testis in a 2% to 4% bovine serum albumin (BSA) gradient. Photomicrographs of air-dried and hematoxylin–eosin-stained smears of spermatogenic cells isolated from immature or mature testis. The images were taken at an original magnification of ×100; inserts were at ×1000 magnification. C, Percentage of spermatogenic cell types in fractions obtained from D6 to D88 mouse testis by separation of mixed germ cells using a 2% to 4% BSA gradient. One hundred hematoxylin and eosin (HE)-stained cells were assessed for each fraction. The age of the mice and isolated spermatogenic cell types are specified for each fraction. D, Expression of Scp3 (synaptonemal complex 3) and Prm2 (protamine 2) in isolated SC and RS fractions. Scp3, a gene expressed specifically in spermatocytes, was detected in the SC fraction, but not in the RS fraction. Prm2, a gene expressed specifically in round spermatids, was detected in the RS fraction, but not in the SC fraction, suggesting efficient isolation. β-Actin was used as an endogenous control. SG indicates spermatogonia; SC, spermatocytes; RS, round spermatids; ES, elongated spermatids; RB, residual bodies; OC, other cells.
In order to study cell type-specific expression of Epab and Pabpc1, spermatogenic cells were isolated from immature (D6-D32) and mature mouse testes (D88) by fractionation using 2% to 4% BSA gradient. Isolated spermatogenic cells in fractions enriched for SG, spermatocytes, RSs, or ESs were stained with HE (Figure 2B), and percentage of spermatogenic cell types were determined using previously described morphologic characteristics (Figure 2C).
Enrichment of more than 77% was achieved for each spermatogenic cell type (Figure 2B), sufficient to assess cell type-specific Epab and Pabpc1 expression. In addition to morphologic characteristics, expression of cell-specific genes was tested by RT-PCR to confirm efficient cell isolation. Scp3, a marker of spermatocytes was expressed in the spermatocyte fraction and not in the RS fraction. Similarly, Prm2 expression was detected in the RS fraction and not in the spermatocyte fraction (Figure 2D). Morphologic assessment of HE stained slides and expression of the 2 cell-specific markers suggested efficient fractionation and isolation of spermatogenic cell types.
Quantification of Epab and Pabpc1 mRNA Expression in Isolated Spermatogenic Cells From Immature and Mature Mouse Testes
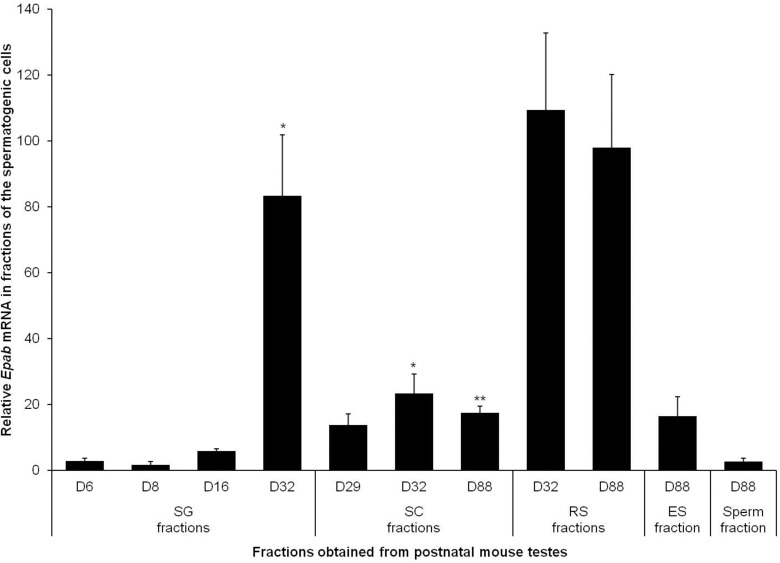
Epab and Pabpc1 expression in isolated spermatogenic cells obtained from immature and mature mouse testes was determined using qRT-PCR. Epab levels were low in SG isolated from D6, D8, and D16 testes but significantly increased in D32 (Figure 3). Similar to SG, Epab expression in the spermatocytes from D32 testis was remarkably higher than spermatocytes from D29. We found that Epab expression in spermatocytes decreased by D88 (Figure 3). Epab expression in RSs was high but did not differ significantly between D32 and D88 (Figure 3).
Figure 3.
Quantification of Epab messenger RNA (mRNA) expression in isolated spermatogenic cells from immature and mature mouse testes. Qualitative real-time polymerase chain reaction (qRT-PCR) in all samples was normalized to β-actin expression used as an internal control. In spermatogonia, Epab mRNA expression was low on D6, D8, and D16 (immature mice), and reached the highest value at D32. In spermatocytes Epab expression reached its highest level at D32. Epab mRNA expression in round spermatids isolated from 32- (immature) or 88-day-old (mature) mice was high, but there was no remarkable change between the 2 time points. SG indicates spermatogonia; SC, spermatocyte; RS, round spermatid; ES, elongated spermatid. SG: *P < .05 for D32 versus D16, D8, or D6; SC: *P < .05 for D32 versus D29; **P < .05 D88 and D32.
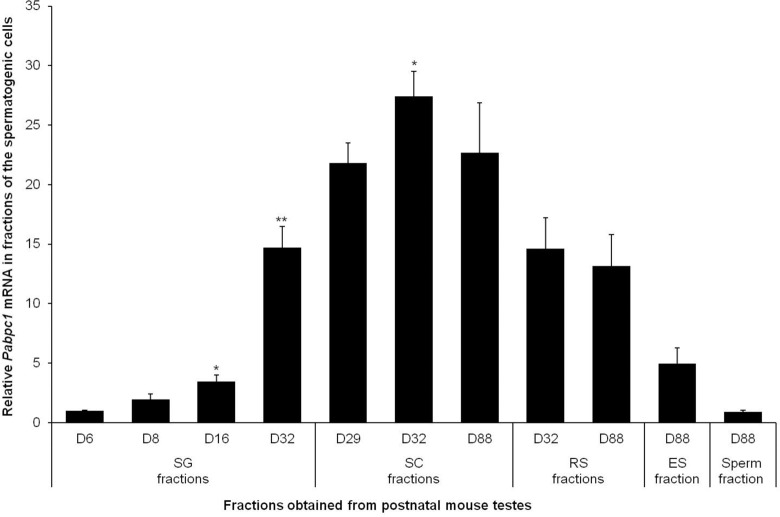
Pabpc1 mRNA expression in SG was low on D6, D8, and D16 testes and significantly increased by D32 (Figure 4). Similar to Epab, Pabpc1 expression in spermatocytes was found to be increased on D32 compared to D29, and decreased by D88 (Figure 4), and there was no significant difference in Pabpc1 expression of RSs between D32 and D88 (Figure 4).
Figure 4.
Quantification of Pabpc1 messenger RNA (mRNA) expression in isolated spermatogenic cells from immature and mature mouse testes. Qualitative real-time polymerase chain reaction (qRT-PCR) was normalized to β-actin expression used as an internal control. Pabpc1 expression began to rise at D8 and reached the highest value at D32. Pabpc1 mRNA expression reached its highest level at D32. In round spermatid fractions isolated from 32- (immature) and 88-day-old (mature) mice there was no remarkable change in Pabpc1 expression. SG indicates spermatogonia; SC, spermatocyte; RS, round spermatid; ES, elongated spermatid. SG: *P < .05 for D16 versus D8; **P < .05 for D32 versus D16; SC: *P < .05 for D32 versus D88 and D29.
Localization of Epab and Pabpc1 mRNA in Mature Male Mouse Testis
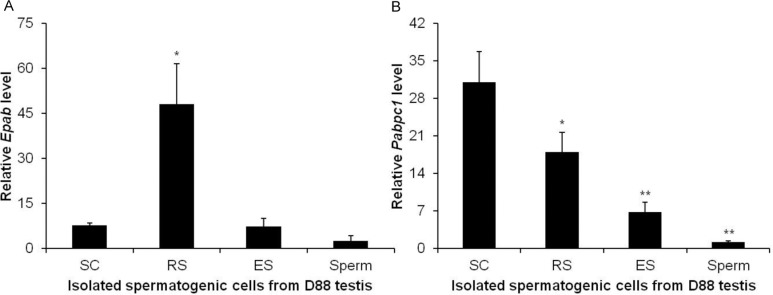
To assess cell-specific expression of Epab and Pabpc1 in mature male mice, we applied qRT-PCR and ISH. We first carried out qRT-PCR using spermatocytes, RSs, ESs isolated from testis, and spermatozoa isolated from cauda epididymis of mature male mice. We found that RSs have significantly higher Epab expression compared with spermatocytes, ESs, or spermatozoa (Figure 5A). Spermatogonia could not be isolated from adult mice because of very low abundance. Qualitative RT-PCR also revealed Pabpc1 expression in RSs and spermatocytes to be higher than ESs or spermatozoa (Figure 5B).
Figure 5.
Quantification of Epab and Pabpc1 messenger RNA (mRNA) expression in isolated spermatogenic cells from mature male mouse using Qualitative real-time polymerase chain reaction (qRT-PCR). Spermatocytes, round spermatids, and elongated spermatids were isolated from mature male testis, and spermatozoa were isolated from cauda epididymis. The qRT-PCRs for Epab and Pabpc1 were normalized to β-actin expression used as an internal control. A, Comparable with RNA in situ hybridization (ISH), round spermatids showed significantly higher Epab expression than spermatocytes, elongated spermatids, and spermatozoa. *P < .05 RS versus SC, ES, and spermatozoa. B, Comparable with RNA ISH, Pabpc1 expression in spermatocytes, and round spermatids was higher than elongated spermatids and spermatozoa. Moreover, qRT-PCR revealed that SC fractions had significantly more Pabpc1 mRNA than RS. SC indicates spermatocyte; RS, round spermatid; ES, elongated spermatid. *P < .05 RS versus SC; **P < .05 ES and spermatozoa versus RS.
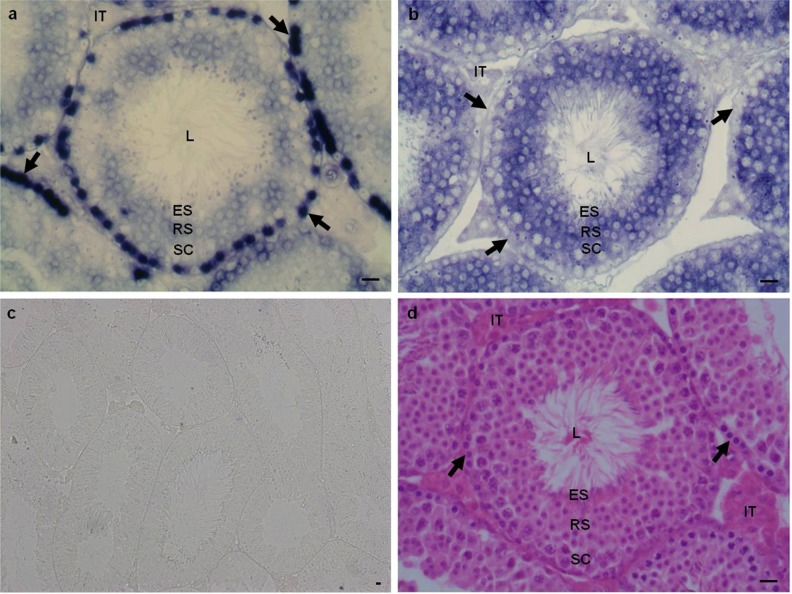
To confirm our findings and to assess expression of Epab and Pabpc1 in SG, we performed RNA ISH for Epab and Pabpc1 mRNAs in mature mouse testis. High expression of Epab mRNA was detected in SG settled at the basal layer of the seminiferous tubules. Epab was also expressed in spermatocytes, RSs, and ESs, at lower levels. It is noteworthy that, consistent with qRT-PCR findings, Epab mRNA expression seemed to be higher in RSs compared with spermatocytes or ESs (Figure 6A). In contrast to Epab expression, Pabpc1 was barely present in SG, but, consistent with qRT-PCR findings, its expression was remarkably increased in spermatocytes and RSs. Similar to Epab, Pabpc1 expression in ESs and spermatozoa was found to be very weak (Figure 5B). Therefore, using RNA ISH and cell separation combined with qRT-PCR, we established germ cell-specific expression of the 2 predominant poly(A)-binding proteins in mature mouse testis.
Figure 6.
Localization of Epab and Pabpc1 messenger RNA (mRNA) by in situ hybridization in mature male mouse testis. A, Epab expression in mature male mouse testis. Epab expression was assessed by in situ hybridization using a Epab exon 2 probe. Epab expression was highest in spermatogonia (arrows) localized at the periphery of the tubules. In addition, RSs had higher expression compared with spermatocytes or elongated spermatids. B, Pabpc1 expression in mature male mouse testis. Pabpc1 expression was assessed by in situ hybridization using a Pabpc1 exon 1-2 probe. Pabpc1 expression was cytoplasmic and was localized to the seminiferous tubules as well as the intertubular areas. In seminiferous tubules, spermatocytes and RSs had higher Pabpc1 expression than spermatogonia or elongated spermatids. C, Negative control for in situ hybridization. D, Hematoxylin and eosin stained mature male mouse testis. L indicates lumen of the seminiferous tubule; ES, elongated spermatid; RS, round spermatid; SC, spermatocyte; IT, intertubular area.
Localization of Epab mRNA in Immature Male Mouse Testis
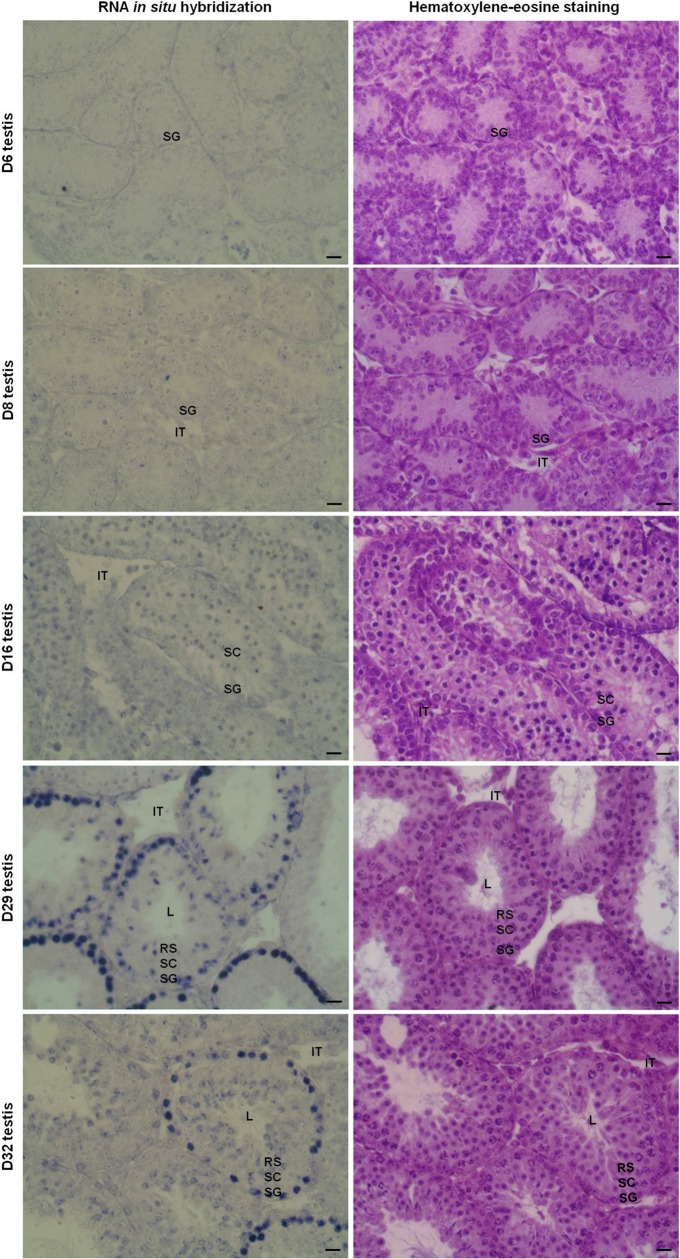
We detected a high expression of Epab in SG in mature male mouse testis using ISH. However, qRT-PCR revealed that Epab was present at very low levels in postnatal D6 or D8 (Figure 1A), when SG is the predominant germ cell in the seminiferous tubule (Figure 2A). To determine whether there is an increase in spermatogonial Epab expression in spermatogonial cells during postnatal development, as suggested by the qRT-PCR findings (Figure 1A), we performed ISH to assess Epab expression in testis from 6-, 8-, 16-, 29-, and 32-day-old mice (Figure 7). While Epab expression in SG was very weak on D6, D8, and D16, it increased significantly by D29 and D32. This finding was consistent with qRT-PCR findings presented in Figure 1.
Figure 7.
Localization of Epab messenger RNA (mRNA) by in situ hybridization in postnatal male mouse testis from D6 to D32. Hematoxylin–eosin staining was also done for each stage. Epab expression was assessed by in situ hybridization using a Epab exon 2 probe. While Epab expression in spermatogonia was very weak on D6, D8, and D16, it increased significantly by D29 and D32. Spermatocytes had slightly higher Epab expression in D16 than D29 or D32 testes. In RSs, Epab expression in D32 testis was found to be more than D29 testis. L indicates lumen of the seminiferous tubule; ES, elongated spermatid; RSs, round spermatid; SC, spermatocyte; IT, intertubular area.
Discussion
In this study, we characterized temporal and spatial expression of Epab in male germ cells and gonads in mouse and compared and contrasted Epab expression to that of Pabpc1. Immature testes from 6-, 8-, 16-, 29-, and 32-day-old mice were analyzed as these time points are characterized by a predictable spermatogenic cell type content of increasing maturity (Figure 2A).36 We found that, Epab mRNA expression was low at D6, D8, D16, and D20 testes, but increased significantly at D29, reaching the highest level at D32 and declining at D88 (Figure 1A). Pabpc1 mRNA levels were also low at D6 and D8 and began to increase at D16. Similar to Epab, Pabpc1 expression reached its highest level at D32 testis and decreased at D88 (Figure 1B). Our Pabpc1 findings are consistent with a study by Kimura et al, which examined Pabpc1 expression during mouse postnatal testicular development at 5 to 60 days using Northern blot and found Pabpc1 mRNA to be remarkably increased from D18 onward.39 The peak expression observed for both Epab and Pabpc1 at D32 strongly suggests that these PABPs may play important roles in the process of mature spermatozoa production that starts at around D35 postpartum.40
Pabpc1 and Epab are expressed in different types of cells and at different time points during development: Pabpc1 is present in all somatic cells and in the embryonic cells following ZGA, while Epab is expressed in gametes and early embryos.16,17 Therefore, we wanted to determine whether these 2 PABPs differ in the type of germ cells in which they are expressed. Expression analysis of Epab and Pabpc1 in isolated spermatogenic cells was performed between D6 and D88, on days selected based on the elegant study carried out by Bellve36 as well as our own findings (Figure 2A). We have isolated spermatogenic cell types at high purity from immature and mature testes at specific ages with the aim of obtaining adequate number of a specific cell type for analysis. For example, RSs first appear at D18 mouse testis, when all spermatogenic cell types become detectable.36 However, the numbers of RSs are very low at D18; therefore, RSs isolation was performed at D32. Similarly, as SG constitute the great majority of spermatogenic cells in early immature mouse testis with decreasing abundance at D32, isolation of SG was performed at D6, D8, D16, and D32. However, due to the low number of SG in mature testis, adequate purity for molecular analysis of SG could not be achieved at D88 testis samples. Other methods for spermatogonial cell isolation include culture on laminin matrix41, flow cytometry,42,43 or Magnetic-activated cell sorting (MACS) methods.44 In this study, we used RNA ISH to assess Epab and Pabpc1 expression in SG from mature testes and to compare with other germ cells.
We found both Epab and Pabpc1 expression to increase in SG and spermatocytes during postnatal life reaching their peak at D32 (Figures 3 and 4). This suggests that rather than an increased expression in a specific spermatogenic cell type, an overall increase in PABP expression in spermatogenic cells together with an increase in the number of germ cells is responsible for the observed increase in Epab and Pabpc1 expression in maturing testis (Figure 1A and B), possibly in preparation for spermatogenesis. Interestingly, in testis, Epab and Pabpc1 expression showed a moderate but statistically significant decrease at D88 compared with D32.
In mature male mouse testis, the highest Epab expression was in SG (Figure 6A). Conversely, Pabpc1 mRNA levels in SG were low compared to other spermatogenic cells in mature testis (Figure 6B). This observation suggests that during the adult life, EPAB may play an important role in SG, possibly helping expression of genes responsible for initiating spermatogenesis. Additional studies are required to determine specific functions of EPAB and its interactions with other proteins and mRNAs in the SG. In addition, as Pabpc1 is also expressed in SG (especially in immature testes) and as SG have not been reported to require posttranscriptional regulation, it would be important to determine whether spermatogonial function is dependent on the presence of EPAB, or whether EPAB’s functions can be compensated by other RNA-binding proteins.
In addition, Epab mRNA levels were significantly higher in isolated RSs compared with spermatocytes, ESs, or sperm cells (Figures 5 and 6). As is known, a set of translationally repressed mRNAs that have long poly(A) tails are stored in the RSs.12 Therefore, increased Epab expression in the RSs could play a role in the maintenance of the poly(A) tail length of stored mRNAs. Transcription becomes suppressed in ESs, where gene expression is regulated primarily posttranscriptionally. In these cells, unlike that observed in oocytes or spermatocytes, translational activation of mRNAs requires shortening of poly(A) tail, coinciding with a decrease in Epab expression. It is noteworthy that, while at very low levels, spermatozoa contain Epab mRNA.
Unlike Epab, the highest Pabpc1 mRNA expression was detected in spermatocytes, followed by RSs; both cell types exhibited significantly higher Pabpc1 expression compared with SG, ESs, or spermatozoa. Our findings are consistent with a study by Kleene et al,20 which demonstrated that enriched pachytene spermatocyte and RS fractions have higher Pabpc1 expression than ESs in mouse, using Northern blot analysis. Expression of Pabpc1 in pachytene spermatocytes and RSs has also been demonstrated in human, where SG and ESs also express Pabpc1.19 Increased Pabpc1 expression in spermatocytes suggests that Pabpc1 may play an important role in regulating poly(A) tail length in spermatocytes, where translational activation is associated with cytoplasmic lengthening of poly(A) tail and depends on CPEB.14 In addition, similar to Epab, elevated levels of Pabpc1 in RSs may play a role in maintaining polyadenylated mRNAs stored in RSs for later activation, and the decrease in the ES stage may facilitate translational activation of paternally stored mRNAs by ploy(A) tail shortening.
Overall, our findings reveal that both Epab and Pabpc1 have a distinct temporal and cell-specific expression in mouse testes. The expression of these 2 key cytoplasmic PABPs seems to be tightly regulated during postnatal testicular development as well as mature spermatogenesis. As posttranscriptional mechanisms seem to play an important role in regulating gene expression at multiple stages of male germ cell development, further characterization of the role of these proteins, identification of the mRNAs that they stabilize and activate, and isolation of the proteins that they bind would be beneficial in understanding the intricate mechanisms governing male reproduction.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no conflicts of interest with respect to the authorship and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Awards K08 HD046581-01 and R01HD059909 to ES from the National Institute of Health (NIH), and by Akdeniz University Research Fund (Project no: 2010.03.0122.003) and the Scientific and Technological Research Council of Turkey (TUBITAK) Grant (109S280) to ND. SO is supported by the 2214-Abroad Research Fellowship for PhD students from TUBITAK. ML is partially supported by CTSA Grant Number UL1 RR024139 from the National Center for Research Resources (NCRR), a component of the NIH.
References
- 1. Nayernia K, Adham I, Kremling H, et al. Stage and developmental specific gene expression during mammalian spermatogenesis. Int J Dev Biol. 1996;40(1):379–383 [PubMed] [Google Scholar]
- 2. Holstein AF, Schulze W, Davidoff M. Understanding spermatogenesis is a prerequisite for treatment. Reprod Biol Endocrinol. 2003;1:107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Kerr JB, Loveland KL, O’Bryan MK, et al. Cytology of the testis and intrinsic control mechanisms. In: Neill JD, et al. eds. Knobil and Neill's Physiology of Reproduction. Vol. 1, 3rd ed New York, NY: Academic Press; 2006:827–947 [Google Scholar]
- 4. Yan W. Male infertility caused by spermiogenic defects: lessons from gene knockouts. Mol Cell Endocrinol. 2009;306(1-2):24–32 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. DeJong J. Basic mechanisms for the control of germ cell gene expression. Gene. 2006;366(1):39–50 [DOI] [PubMed] [Google Scholar]
- 6. Eddy EM. Regulation of gene expression during spermatogenesis. Semin Cell Dev Biol. 1998;9(4):451–457 [DOI] [PubMed] [Google Scholar]
- 7. Hecht NB. Molecular mechanisms of male germ cell differentiation. Bioessays. 1998;20(7):555–561 [DOI] [PubMed] [Google Scholar]
- 8. Steger K. Transcriptional and translational regulation of gene expression in haploid spermatids. Anat Embryol (Berl). 1999;199(6):471–487 [DOI] [PubMed] [Google Scholar]
- 9. Kierszenbaum AL, Tres LL. RNA transcription and chromatin structure during meiotic and postmeiotic stages of spermatogenesis. Fed Proc. 1978;37(11):2512–2516 [PubMed] [Google Scholar]
- 10. Kashiwabara S, Noguchi J, Zhuang T, et al. Regulation of spermatogenesis by testis-specific, cytoplasmic poly(A) polymerase TPAP. Science. 2002;298(5600):1999–2002 [DOI] [PubMed] [Google Scholar]
- 11. Elliott D. Pathways of post-transcriptional gene regulation in mammalian germ cell development. Cytogenet Genome Res. 2003;103(3-4):210–216 [DOI] [PubMed] [Google Scholar]
- 12. Kleene KC. Poly(A) shortening accompanies the activation of translation of five mRNAs during spermiogenesis in the mouse. Development. 1989;106(2):367–373 [DOI] [PubMed] [Google Scholar]
- 13. Kleene KC, Distel RJ, Hecht NB. Translational regulation and deadenylation of a protamine mRNA during spermiogenesis in the mouse. Dev Biol. 1984;105(1):71–79 [DOI] [PubMed] [Google Scholar]
- 14. Tay J, Richter JD. Germ cell differentiation and synaptonemal complex formation are disrupted in CPEB knockout mice. Dev Cell. 2001;1(2):201–213 [DOI] [PubMed] [Google Scholar]
- 15. Stambuk RA, Moon RT. Purification and characterization of recombinant Xenopus poly(A)(+)-binding protein expressed in a baculovirus system. Biochem J. 1992;287(Pt 3):761–766 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Voeltz GK, Ongkasuwan J, Standart N, et al. A novel embryonic poly(A) binding protein, ePAB, regulates mRNA deadenylation in Xenopus egg extracts. Genes Dev. 2001;15(6):774–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Seli E, Lalioti MD, Flaherty SM, et al. An embryonic poly(A)-binding protein (ePAB) is expressed in mouse oocytes and early preimplantation embryos. Proc Natl Acad Sci U S A. 2005;102(2):367–372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Yang H, Duckett CS, Lindsten T. iPABP, an inducible poly(A)-binding protein detected in activated human T cells. Mol Cell Biol. 1995;15(12):6770–6776 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Feral C, Guellaen G, Pawlak A. Human testis expresses a specific poly(A)-binding protein. Nucleic Acids Res. 2001;29(9):1872–1883 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Kleene KC, Wang MY, Cutler M, et al. Developmental expression of poly(A) binding protein mRNAs during spermatogenesis in the mouse. Mol Reprod Dev. 1994;39(4):355–364 [DOI] [PubMed] [Google Scholar]
- 21. Wahle E. A novel poly(A)-binding protein acts as a specificity factor in the second phase of messenger RNA polyadenylation. Cell. 1991;66(4):759–768 [DOI] [PubMed] [Google Scholar]
- 22. Good PJ, Abler L, Herring D, Sheets MD. Xenopus embryonic poly(A) binding protein 2 (ePABP2) defines a new family of cytoplasmic poly(A) binding proteins expressed during the early stages of vertebrate development. Genesis. 2004;38(4):166–175 [DOI] [PubMed] [Google Scholar]
- 23. Wahle E, Lustig A, Jeno P, Maurer P. Mammalian poly(A)-binding protein II. Physical properties and binding to polynucleotides. J Biol Chem. 1993;268(4):2937–2945 [PubMed] [Google Scholar]
- 24. Bienroth S, Keller W, Wahle E. Assembly of a processive messenger RNA polyadenylation complex. EMBO J. 1993;12(2):585–594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Cosson B, Couturier A, Le Guellec R, et al. Characterization of the poly(A) binding proteins expressed during oogenesis and early development of Xenopus laevis. Biol Cell. 2002;94(4-5):217–231 [DOI] [PubMed] [Google Scholar]
- 26. Gray NK, Coller JM, Dickson KS, Wickens M. Multiple portions of poly(A)-binding protein stimulate translation in vivo. EMBO J. 2000;19(17):4723–4733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Guzeloglu-Kayisli O, Pauli S, Demir H, Lalioti MD, Sakkas D, Seli E. Identification and characterization of human embryonic poly(A) binding protein (EPAB). Mol Hum Reprod. 2008;14(10):581–588 [DOI] [PubMed] [Google Scholar]
- 28. Flach G, Johnson MH, Braude PR, Taylor RA, Bolton VN. The transition from maternal to embryonic control in the 2-cell mouse embryo. EMBO J. 1982;1(6):681–686 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Braude P, Bolton V, Moore S. Human gene expression first occurs between the four- and eight-cell stages of preimplantation development. Nature. 1988;332(6163):459–461 [DOI] [PubMed] [Google Scholar]
- 30. Kim JH, Richter JD. RINGO/cdk1 and CPEB mediate poly(A) tail stabilization and translational regulation by ePAB. Genes Dev. 2007;21(20):2571–2579 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Padmanabhan K, Richter JD. Regulated Pumilio-2 binding controls RINGO/Spy mRNA translation and CPEB activation. Genes Dev. 2006;20(2):199–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Cao Q, Richter JD. Dissolution of the maskin-eIF4E complex by cytoplasmic polyadenylation and poly(A)-binding protein controls cyclin B1 mRNA translation and oocyte maturation. EMBO J. 2002;21(14):3852–3862 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Meistrich ML, Bruce WR, Clermont Y. Cellular composition of fractions of mouse testis cells following velocity sedimentation separation. Exp Cell Res. 1973;79(1):213–227 [PubMed] [Google Scholar]
- 34. Vasco C, Zuccotti M, Redi CA, Garagna S. Identification, isolation, and RT-PCR analysis of single stage-specific spermatogenetic cells obtained from portions of seminiferous tubules classified by transillumination microscopy. Mol Reprod Dev. 2009;76(12):1173–1177 [DOI] [PubMed] [Google Scholar]
- 35. Kotaja N, Kimmins S, Brancorsini S, et al. Preparation, isolation and characterization of stage-specific spermatogenic cells for cellular and molecular analysis. Nat Methods. 2004;1(3):249–254 [DOI] [PubMed] [Google Scholar]
- 36. Bellve AR. Purification, culture, and fractionation of spermatogenic cells. Methods Enzymol. 1993;225:84–113 [DOI] [PubMed] [Google Scholar]
- 37. Romrell LJ, Bellve AR, Fawcett DW. Separation of mouse spermatogenic cells by sedimentation velocity. A morphological characterization. Dev Biol. 1976;49(1):119–131 [DOI] [PubMed] [Google Scholar]
- 38. Ganaiem M, AbuElhija M, Lunenfeld E, et al. Effect of interleukin-1 receptor antagonist gene deletion on male mouse fertility. Endocrinology. 2009;150(1):295–303 [DOI] [PubMed] [Google Scholar]
- 39. Kimura M, Ishida K, Kashiwabara S, Baba T. Characterization of two cytoplasmic poly(A)-binding proteins, PABPC1 and PABPC2, in mouse spermatogenic cells. Biol Reprod. 2009;80(3):545–554 [DOI] [PubMed] [Google Scholar]
- 40. Forand A, Messiaen S, Habert R, Bernardino-Sgherri J. Exposure of the mouse perinatal testis to radiation leads to hypospermia at sexual maturity. Reproduction. 2009;137(3):487–495 [DOI] [PubMed] [Google Scholar]
- 41. Hamra FK, Chapman KM, Wu Z, Garbers DL. Isolating highly pure rat spermatogonial stem cells in culture. Methods Mol Biol. 2008;450:163–179 [DOI] [PubMed] [Google Scholar]
- 42. Izadyar F, Wong J, Maki C, et al. Identification and characterization of repopulating spermatogonial stem cells from the adult human testis. Hum Reprod. 2011;26(6):1296–1306 [DOI] [PubMed] [Google Scholar]
- 43. Choi YH, Park CH, Kim W, et al. Vaccinia-related kinase 1 is required for the maintenance of undifferentiated spermatogonia in mouse male germ cells. PLoS One. 2010;5(12): e15254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Gassei K, Ehmcke J, Schlatt S. Efficient enrichment of undifferentiated GFR alpha 1 + spermatogonia from immature rat testis by magnetic activated cell sorting. Cell Tissue Res. 2009;337(1):177–183 [DOI] [PubMed] [Google Scholar]