Abstract
Recent advances in telemedicine and robotically assisted telesurgery may offer advanced surgical care for the geographically remote patient. Similar advances in tele-anesthesia will be necessary to optimize perioperative care for these patients. Although many preliminary investigations into tele-anesthesia are underway, none involves remote performance of anesthesia-related procedures. Here we describe simulated robotically assisted fiberoptic intubations using an airway simulation mannequin. Both oral and nasal approaches to fiberoptic intubation were successful, but presented unique opportunities and challenges inherent to the robot’s design. Robotically assisted airway management is feasible using multipurpose surgical robotic systems.
Telemedicine has improved access to consultant-level medical care by minimizing geographic obstacles. Similar advances in telesurgery could offer expert surgical care for the geographically remote patient. Such surgical advances have been historically preceded by similar advances in anesthesia. True to this paradigm, early investigations into tele-anesthesia are already underway.1 However, none of these efforts has tackled one of the central tenets of anesthesiology: airway management.
In this report we present what we believe to be the first description of a simulated robotically assisted fiberoptic intubation. Instead of a procedure-specific device, we used the multipurpose DaVinci Surgical System type S (DVS) (Intuitive Surgical, Sunnyvale, California). This system incorporates 4 separate robotic arms, 1 of which is mated to a high-definition stereoscopic camera. The workstation allows the person performing the procedure to view the robot’s camera output, control the limbs, and receive simultaneous video input from third-party sources.2,3 The DVS is already in widespread clinical use for a variety of urologic, gynecologic, and cardiothoracic surgical procedures.4 In this study involving an airway mannequin, we successfully used the DVS for both oral and nasal fiberoptic intubation.
METHODS
An adult airway mannequin was placed at the head of a standard operating room bed. A stereoscopic video camera (DaVinci Surgical System, Intuitive Surgical) was mated to the first robotic arm. This arm was situated above the mannequin in a sagittal plane, angled to view the mannequin from a caudal to rostral fashion. The second and third arms were equipped with large and small graspers (Figs. 1 and 2). The fourth arm was equipped with a standard fiberoptic bronchoscope (Karl Storz Endoscopy, El Segundo, California). The trocars used for mounting standard robotic instruments were removed from this arm, allowing the bronchoscope handle to slide into the mounting bracket. The bronchoscope handle was oriented to keep the tip actuator lever facing away from the arm, with the suction port positioned to the side (Fig. 3). An external brace was required to keep the bronchoscope firmly seated within the arm during manipulation of the actuator.
Figure 1.
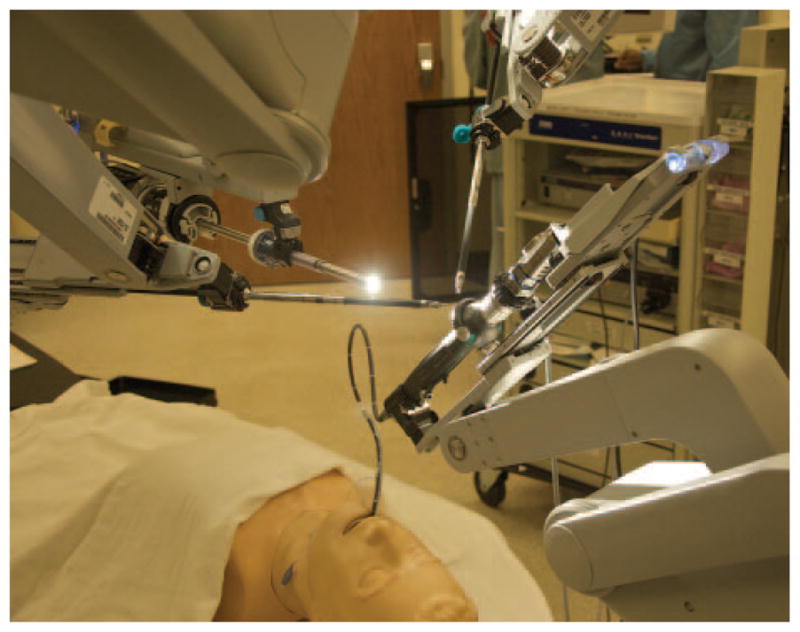
Schematic depicting the arrangement of the DaVinci Surgical System type S (DVS), bronchoscope, and airway mannequin.
Figure 2.
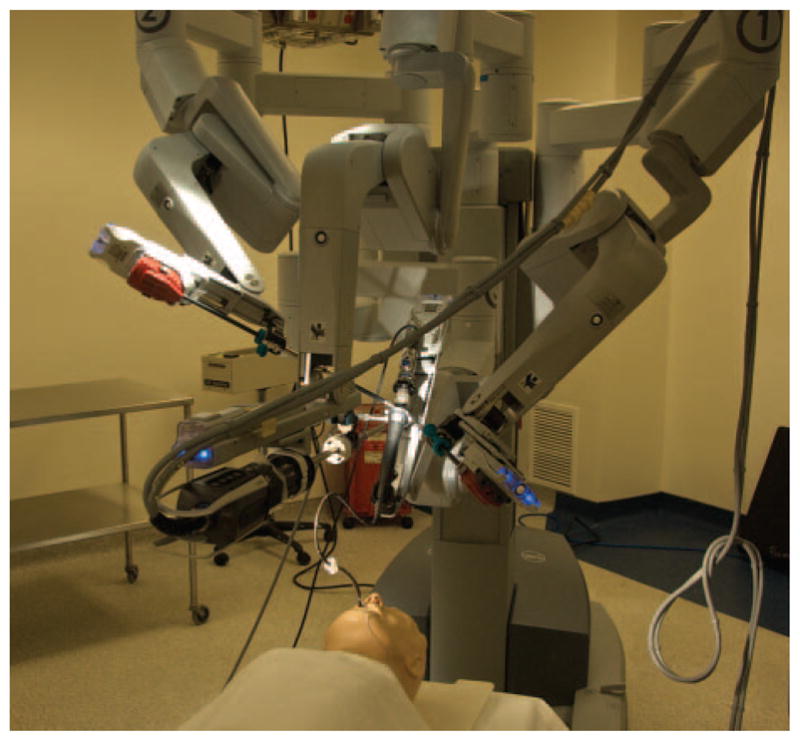
Schematic depicting the arrangement of the DaVinci Surgical System type S (DVS), bronchoscope, and airway mannequin.
Figure 3.
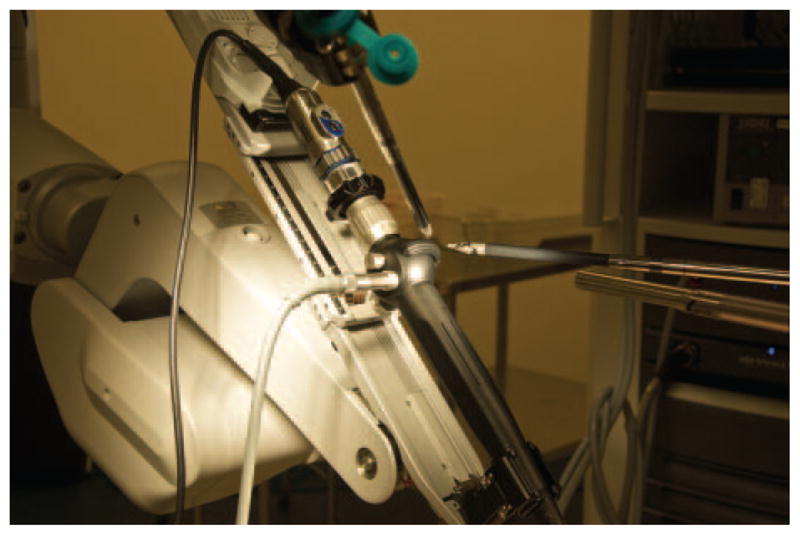
The handle of the bronchoscope was positioned into a robotic arm. Two other robotic arms were fitted with graspers and used to control the flexion and extension of the bronchoscope tip.
We manually loaded the endotracheal tube onto the bronchoscope. A camera was attached to the bronchoscope and connected to the DVS Tilepro multivideo input system (Intuitive Surgical, Sunnyvale, California), allowing simultaneous viewing of both the robot camera and bronchoscope camera in a single three-dimensional view (Fig. 4).
Figure 4.
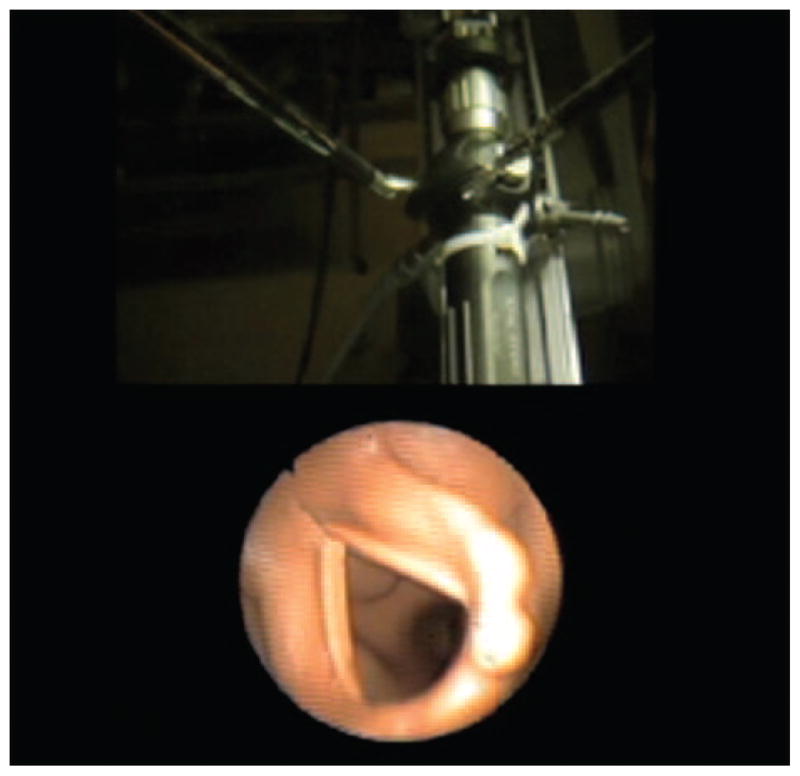
Image from the DaVinci workstation during the robotically assisted fiberoptic intubation. The robot operator can simultaneously visualize video output from both the bronchoscope and the robot’s main camera without turning attention away from the workstation.
Before attempting intubation, the urologist spent approximately 2 hours performing robotic dexterity exercises with the robotic surgical instruments and operator console. After training was complete, an anesthesiologist manually placed the bronchoscope tip within the oropharnyx, and a urologist (S. Parekattil) used robot arms 2 and 3 to adjust the bronchoscope actuator and steer the bronchoscope tip into the hypopharynx and through the vocal cords (Video 1; see Supplemental Digital Content 1, http://links.lww.com/AA/A171; see the Appendix for video legend). Next the bronchoscope tip was placed external to the nare, an anesthesiologist manually advanced and rotated the bronchoscope, and an urologist used the robot to steer the tip into the hypopharynx and through the vocal cords.
RESULTS
Two intubation attempts were completed for this demonstration. During oral intubation, it took 75 seconds to advance the bronchoscope tip from the oropharynx to carinal visualization. For nasal intubation, it took 67 seconds to advance the tip from nasal entry to carinal visualization.
DISCUSSION
Fiberoptic intubation is feasible with robotic equipment. We did not encounter significant differences between the nasal and oral intubation routes. Even if optimized for anesthetic practice, robotic-assisted anesthetic procedures are not likely to become a part of routine anesthetic practice. Their optimal role may ultimately be for environments hazardous to routine anesthetic practice, such as the battlefield or space-based environments.5
Our initial attempts at robotic-assisted intubation focused on direct laryngoscopy. However, we had considerable difficulty using the DVS robotic graspers to lift and manipulate both Macintosh and Miller laryngoscope light handles. Direct endotracheal tube manipulation with the DVS was quite challenging. On the other hand, the focus of the DVS on small-scale manipulation, coupled with its multiaxis flexibility and unifying video input, suggested that fiberoptic approaches to airway management would capitalize on the capabilities of the DVS.
The robot operator controls the DVS through a dedicated workstation in a corner of the operating room. The workstation could easily be placed in another room, building, or continent.6,7 Traditionally, such distances have been limited by latencies between user input, robot action, and robot-user feedback.8 Progress in adapting data packet transmission along existing telecommunication systems has minimized such limitations,9 allowing long-distance robotic telesurgery with acceptable latencies. 10
Ideally, this exercise would have required zero nonrobotic interventions. However, because of the cost of the bronchoscope, we elected to accept a loss in simulation fidelity to minimize potential damage to the costly equipment. Further work will be necessary to explore how well the DVS can rotate and advance a bronchoscope tip.
Our experience indicates that because of the support and preparation required for robotically assisted intubation, current systems are not ready for such deployment into complex operating environments. The bedside presence of the anesthesiologist was necessary even in this focused simulation to assist with important maneuvers necessary during robotically assisted fiberoptic intubation. Furthermore, this exercise did not include important factors such as patient preparation, positioning, topical and systemic medication administration, and patient monitoring.
This study demonstrated that a multipurpose surgical robot could be adapted for use in airway management. Although limited in its approach to direct laryngoscopy, the DVS was able to assist with both oral and nasal fiberoptic intubation. Future studies will be necessary to optimize robotic interfaces with other airway management techniques.
Supplementary Material
Acknowledgments
Funding was provided by the University of Florida College of Medicine, Department of Anesthesiology, as well as by National Institutes of Health grant UL1 RR029890 Clinical and Translational Science Award, NIH (NCRR).
APPENDIX: VIDEO CAPTIONS
Video 1. This video demonstrates how the robotic manipulation arms adjusted the flexion and extension of the bronchoscope tip, and how the bronchoscope itself was secured to a third arm. Both oral and nasal intubations are demonstrated. The bronchoscope advancement was performed manually to avoid damage to the bronchoscope and required very minimal human input. Advancement was performed at the direction of the robot operator.
Footnotes
Supplemental digital content is available for this article. Direct URL citations appear in the printed text and are provided in the HTML and PDF versions of this article on the journal’s Web site (www.anesthesia-analgesia.org).
References
- 1.Hemmerling TM. Automated anesthesia. Curr Opinion Anaesthesiol. 2009;103:811–6. doi: 10.1097/ACO.0b013e328332c9b4. [DOI] [PubMed] [Google Scholar]
- 2.Bhayani S, Snow D. Novel dynamic information integration during da Vinci robotic partial nephrectomy and radical nephrectomy. J Robotic Surg. 2008;2:67–9. doi: 10.1007/s11701-008-0083-9. [DOI] [PubMed] [Google Scholar]
- 3.Rogers CG, Laungani R, Bhandari A, Krane LS, Eun D, Patel MN, Boris R, Shrivastava A, Menon M. Maximizing console surgeon independence during robot-assisted renal surgery by using the fourth arm and TilePro. J Endourol. 2009;23:115–22. doi: 10.1089/end.2008.0416. [DOI] [PubMed] [Google Scholar]
- 4.Palep JH. Robotic assisted minimally invasive surgery. J Minim Access Surg. 2009;5:1–7. doi: 10.4103/0972-9941.51313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Harnett BM, Doarn CR, Rosen J, Hannaford B, Broderick TJ. Evaluation of unmanned airborne vehicles and mobile robotic telesurgery in an extreme environment. Telemed e-Health. 2008;14:539–44. doi: 10.1089/tmj.2007.0087. [DOI] [PubMed] [Google Scholar]
- 6.Sterbis JR, Hanly EJ, Herman BC, Marohn MR, Broderick TJ, Shih SP, Harnett B, Doarn C, Schenkman NS. Transcontinental telesurgical nephrectomy using the da Vinci robot in a porcine model. Urology. 2008;71:971–3. doi: 10.1016/j.urology.2007.11.027. [DOI] [PubMed] [Google Scholar]
- 7.Marescaux J, Leroy J, Gagner M, Rubino F, Mutter D, Vix M, Butner SE, Smith MK. Transatlantic robot-assisted telesurgery. Nature. 2001;413:379–80. doi: 10.1038/35096636. [DOI] [PubMed] [Google Scholar]
- 8.Anvari M, Broderick T, Stein H, Chapman T, Ghodoussi M, Birch DW, Mckinley C, Trudeau P, Dutta S, Goldsmith CH. The impact of latency on surgical precision and task completion during robotic-assisted remote telepresence surgery. Comp Aided Surg. 2005;10:93–9. doi: 10.3109/10929080500228654. [DOI] [PubMed] [Google Scholar]
- 9.Rayman R, Primak S, Patel R, Moallem M, Morady R, Tavakoli M, Subotic V, Galbraith N, van Wynsberghe A, Croome K. Effects of latency on telesurgery: an experimental study. Medical image computing and computer-assisted intervention MICCAI. 2005;8:57–64. doi: 10.1007/11566489_8. [DOI] [PubMed] [Google Scholar]
- 10.Rayman R, Croome K, Galbraith N, McClure R, Morady R, Peterson S, Smith S, Subotic V, Wynsberghe AV, Primak S. Long-distance robotic telesurgery: a feasibility study for care in remote environments. Intl Med Robotics Comp Assist Surg. 2006;2:216–24. doi: 10.1002/rcs.99. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


