Abstract
Our understanding of human papillomavirus (HPV) is still evolving. To further study the field, our laboratory has focused on determining the role of integrins in the initial steps of viral endocytosis into HaCaT cells. Our and others' previous findings have shown that α6 is necessary for infection. Here we show that α3 and β1 were dispensable, and we identified integrin α6β4 complex as necessary for infection in HaCaTs. β4 knock down resulted in a significant decrease in HPV16 PsV infection and perhaps most importantly resulted in defective post-translational α6 processing. We showed that the unprocessed α6 does not localize to the cell surface. We propose that the α6β4 complex is necessary for the formation of an endocytic complex that results in the signaling transduction events necessary for initial endocytosis.
Keywords: HPV, α6 Integrin, β4 Integrin, Virus entry, HaCaTs
Introduction
Papillomaviridae family consists of non-enveloped, 55 nm diameter, circular dsDNA viruses. To date, more than 150 human papillomavirus (HPV) types are identified by DNA sequencing and these types are commonly divided into five genera based on genotyping similarities (Doorbar, 2006; Doorbar et al., 2012). Infections occur in epithelial cells and can either cause benign warts on skin and mucosa (low-risk HPVs) or lead to cervical cancer (Scheffer et al., 2013). Out of the 15 identified oncogenic HPV types, HPV16 is the most common etiologic agent associated with cervical cancer (Laniosz et al., 2009; Schiffman, 2007). Although this disease-mediating agent has been under study for more than 40 years, the details of the infection process have not been clearly identified.
Research to identify the biological receptor(s) for HPVs has led to identification of some of the key players in HPV infection. The viral particle consists of the virally encoded L1 and L2 capsid protein. Many laboratories have shown data supporting that the initial HPV binding is L1 mediated, and occurs through heparan sulfate proteoglycans (HSPGs). Data have shown that removing the heparan sulfate glycosaminoglycans reduces infection; furthermore, addition of a soluble analog of heparan sulfate competes for HPV binding and thus blocks infection (Abban and Meneses, 2010; Giroglou et al., 2001; Johnson et al., 2009; Joyce et al., 1999). This initial binding has been suggested to occur in the extracellular matrix or at the cell surface. Upon binding of the virus to the HSPGs, a conformational change occurs. This change has been shown to be mediated by host cell protein cyclophilin B and results in the exposure of a hidden N-terminus of L2 (Bienkowska-Haba et al., 2009). This exposed region of L2 can be cleaved by members of the proprotein convertase family of peptidases, furin and/or PC 5/6 (Richards et al., 2006; Seidah and Prat, 2012). The cleavage of the L2 N-terminus is necessary for infection and is hypothesized to facilitate the transfer of the virus capsid to a secondary receptor on the keratinocyte plasma membrane (Day et al., 2008; Selinka et al., 2007). Search for this secondary receptor initially led to integrin α6 using HPV6b L1 viral like particles (VLP) (Evander et al., 1997). Using HPV16 pseudovirions, we confirmed the initial binding of viral particles to α6 and showed that siRNA-mediated silencing of integrin α6 decreases infection in HaCaTs (Abban and Meneses, 2010). In addition to the role of α6 integrin, the role for tetraspanins has been proposed by Dr. Florin's group; Dr. Ozbun's group has discussed the presence of an endocytic complex consisting of HPV16 PsVs, HSPGs and EGF; Annexin A2 and its S100A10 subunit have been identified as L2-specific receptor by Dr. Kast's group and they have further been characterized by Dr. Ozbun's group; and most recently Drs. DiMaio and Atwood's group have suggested the role of a retromer as necessary for infection and trafficking of incoming virus (Scheffer et al., 2013; Surviladze et al., 2012; Woodham et al., 2012; Dziduszko and Ozbun, 2013; Lipovsky et al., 2013).
In the aforementioned studies of Drs. Florin and Ozbun, the two common molecules seem to be the integrin α6 and tetraspanin 151 (Scheffer et al., 2013; Surviladze et al., 2012). Integrins are transmembrane receptors that have no intrinsic catalytic or enzymatic activity. To date, 18α and 8β integrin subunits have been identified–each of which is a single pass type I transmembrane protein–making up 24 identified integrin complexes. Upon ligand binding, integrins can mediate outside-in signaling with their ability to transduce signals through proteins that dock on their cytoplasmic tails, and also by regulating their ligand-binding affinity they can mediate inside-out signaling events (Takada et al., 2007). The proposed secondary HPV receptor integrin α6 can form complexes with either β1 or β4 integrins (Kligys et al., 2012). The possible roles of β subunits as a direct binding partner of HPV virions during infection were investigated, and these studies lead to the conclusion that neither β1 nor β4 showed any direct binding to viral particles (Evander et al., 1997; Huang and Lambert, 2012; Yoon et al., 2001).
In a previous study, we showed the importance of α6 in infection and we profiled the integrin subunits in human adult keratinocyte cell line (HaCaT). Our data showed that β1, α2, β2, α3, β4, β6 and α6 were present on HaCaT's cell surface (Abban and Meneses, 2010). Because integrins function as obligate heteromers, we addressed what complexes were formed in HaCaTs. We focused on four integrin subunits based on the profiling data: α3, α6, β1 and β4. These integrins have been implicated in wound healing (Goldfinger et al., 1999; Margadant et al., 2009). We identified several integrin complexes, and α6β4 was found to be involved in HaCaT infection by HPV. Our data showed that the level of β4 was directly correlated to the level of infection and for proper α6 integrin processing.
Results
Assessment of possible integrin complexes on HaCaTs
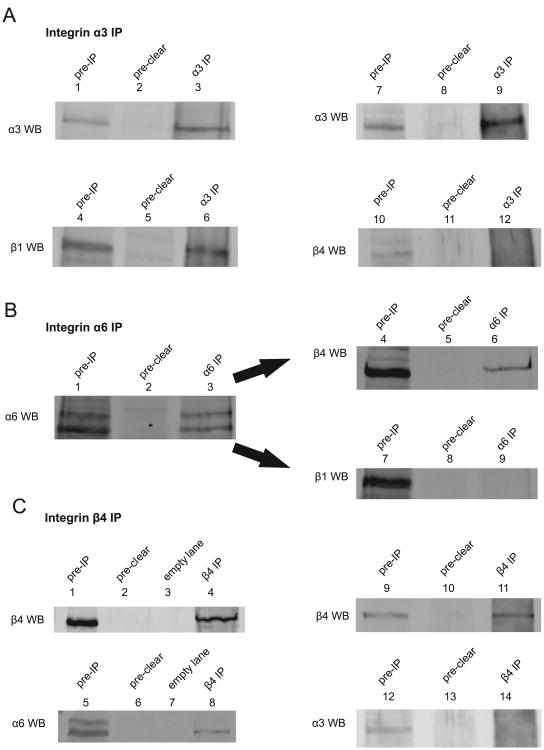
Integrin function requires a pairing of an α and a β subunit into a heterodimer. Based on our previous integrin profiling we addressed what heterodimers are found on HaCaTs. Using standard immunoprecipitation experiments with the α subunits as bait we showed that α3 was able to pull down β1 but not β4 (Fig. 1A, lanes 6 and 12) while α6 was able to pull down β4 but not β1 (Fig. 1B, lanes 6 and 9). We confirmed the presence of α6β4 and the lack of α3β4 complex by the reverse pulldown (Fig. 1C, lanes 8 and 14) These pulldowns showed that integrin complexes α3β1 and α6β4 were formed in HaCaTs.
Fig. 1. Integrin complexes in HaCaTs.
(A) HaCaT cell lysate was subjected to immunoprecipitation with integrin α3 antibody. Experiment in lanes 1–6 was analyzed for α3 and β1, experiment in lanes 7–12 was analyzed for α3 and β4. Lane 6 shows coimmunoprecipitation of β1 and α3 integrins, lane 12 shows lack of coimmunoprecipitation of α3 and β4. (B) HaCaT cell lysate was subjected to immunoprecipitation with integrin α6 antibody (sc-6596). Lanes 1–3 were blotted for α6, lanes 4–6 for β4, and 7–9 for β1 (same membrane was used). Lane 6 shows coimmunoprecipitation of β4 and α6 integrins, whereas lane 9 shows lack of α6β1 complex in HaCaT cells. (C) β4 integrin antibody was used to perform immunoprecipitation using HaCaT cell lysate. Experiment in lanes 1–8 was analyzed for β4 and α6, experiment in lanes 9–14 was analyzed for β4 and α3. Lane 8 shows the presence of α6β4 complex in HaCaT cells Lane 14 shows the lack of α3β4 complex in HaCaT cells.
Integrin β4, but not α3 or β1 are required for HPV16 pseudovirion infection
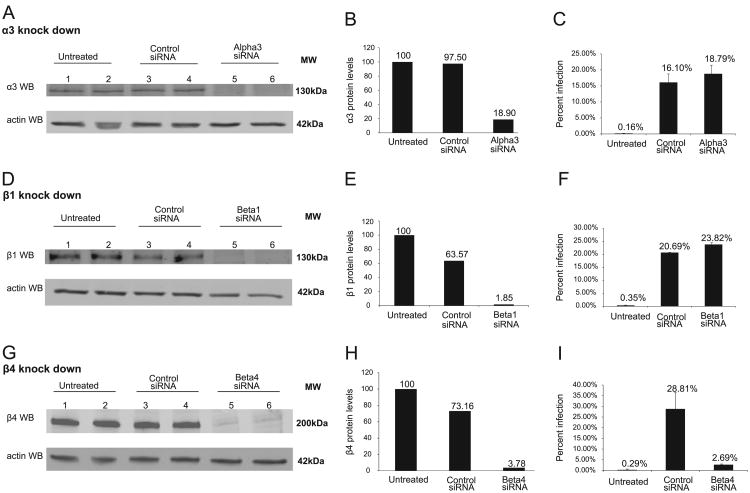
Having identified two heterodimeric integrin complexes, we tested the biological significance of the various integrins. HaCaT cells were treated with integrin specific siRNAs, subsequently incubated with HPV16 pseudovirion, and infection levels assessed by flow cytometry (GFP pseudogenome 8fwb plasmid was used in viral particles). The protein level of α3, β1, and β4 were reduced by 81%, 99%, and 96% compared to untreated HaCaTs (Fig. 2 western blots A, D, G and the measurements B, E, H). The necessity of α6 integrin data have been previously published by our group (Abban and Meneses, 2010). We did not observe any changes in infection in either α3 or β1 knockdown cells, but saw a 90% decrease in infection in β4 knockdown cells. Taken together with our previous α6 work, these data suggested that the necessary integrin complex for HPV16 infection in HaCaTs is α6β4.
Fig. 2. siRNA-mediated integrin β4 knock down, but not β1 or α3 knock down decreases HPV16 infection.
(A) Integrin α3 antibody incubation shows the efficient integrin α3 knockdown in lanes 5 and 6. Actin was used as the loading control. (B) Measured integrin α3 band intensity of each lane was normalized to corresponding actin measurements. Integrin α3 protein levels decreased by ∼80% in α3 siRNA treated cells. (C) HPV16 PsV infection did not decrease in integrin α3 knockdown HaCaTs. (D) Integrin β1 antibody incubation shows the efficient integrin β1 knockdown in lanes 5 and 6. Actin was used as the loading control. (E) Measured integrin β1 band intensity of each lane was normalized to corresponding actin measurements. Integrin β1 protein levels decreased by %98 in β1 siRNA treated cells. (F) HPV16 PsV infection did not decrease in integrin β1 knockdown HaCaTs. (G) Integrin β4 antibody incubation shows the efficient integrin β4 knockdown in lanes 5 and 6. Actin was used as the loading control. (H) Measured integrin β4 band intensity of each lane was normalized to corresponding actin measurements. Integrin β4 protein levels decreased by %96 in β4 siRNA treated cells. (I) HPV16 PsV infection significantly decreased in integrin β4 knockdown HaCaTs. (B), (E), (H): Each bar shows the mean and normalized protein levels of two samples under each treatment (duplicate experiments). Integrin α3/β1/β4 expression in untreated HaCaT cells was considered as 100%. (C), (F) and (I): Percentage of infection was determined by flow cytometry. Error bars show the standard deviation of three experiments in which 10,000 cells were analyzed for GFP expression to obtain the percent of infected cells.
β4 Integrin is necessary for α6 integrin detection at the cell surface
We used confocal microscopy and flow cytometery to test the status of α6 and β4 integrins in β4 knockdown cells. As shown in Fig. 3A, control siRNA treated HaCaT cells have β4 and α6 integrin staining (Fig. 3A red and green, respectively; Fig. 3C lanes 1–2). By contrast, the level of α6 is minimal in β4 knock down cells (Fig. 3B red for β4, green for α6; Fig. 3C lanes 3–4). Flow cytometry analysis showed that the cell surface levels of β4 and of α6 are greatly decreased. Fig. 3D and E shows the shift to the left of both β4 and α6 staining indicative of a decrease in the surface expression of both integrins (Fig. 3D and E, compare the black trace-untreated vs. gray-β4 siRNA treated HaCaTs). Data are presented in graphical form in 3F (black-control cells, gray-β4siRNA treated cells, isotypic IgG controls were used in these experiments). These experiments showed that the detectable levels of both β4 and α6 within cells and at the cell surface is greatly reduced in β4 knockdown cells.
Fig. 3. siRNA-knockdown of integrin β4 causes loss of integrin α6 surface expression in HaCaT cells.
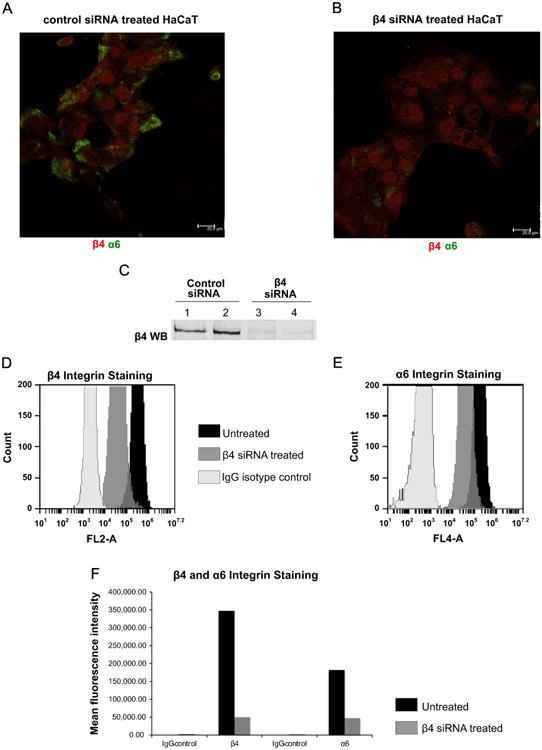
(A, B) Staining for integrin α6 with sc-6596 (green) and integrin β4 with ab-1922 (red) are shown in control siRNA treated and β4 siRNA treated cells. In β4 knock down cells α6 integrin staining was virtually lost. (C) β4 knock down was confirmed via western blot using the lysates of same control and β4 siRNA treated cells in Fig. 3A and B. (D) Surface expression levels of β4 integrin in untreated cells (black graph) and β4 siRNA treated cells (gray); IgG isotype staining of β4 siRNA treated HaCaT cells are shown in white. (E) Surface expression levels of α6 integrin in untreated cells (black graph), β4 siRNA treated cells (gray), and IgG isotype staining of β4 siRNA treated HaCaT cells (white) detected by flow cytometer. (F) Bar graph depiction of the mean fluorescence intensities of β4 and α6 integrins in untreated and β4 knock down cells. IgG isotype stainings of β4 and α6 integrins in β4 knock down cells are shown as well.
In β4 knock down HaCaTs, α6 integrin does not form a complex with β1
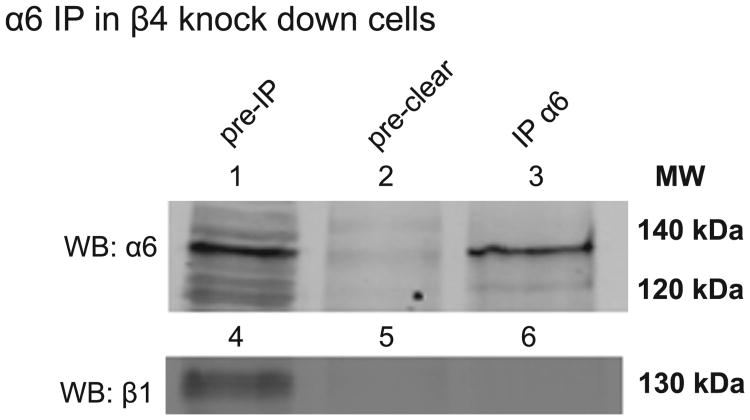
The observed decrease in levels of β4 by microscopy and flow cytometry were consistent with our western blot analysis of β4 knockdown cells. It was surprising to see a comparable loss of α6 expression. Based on the literature, one would theorize that in the absence of β4, α6 would pair with β1. Unexpectedly, our results showed that no such compensation occurs. The western blot of β4 knockdown cells clearly showed α6 expression but an immunoprecipitation with α6 in these samples did not immunoprecipitate β1 (Fig. 4). Thus, α6 does not complex with β1 in the absence of β4 in these experiments.
Fig. 4. β1 does not compensate as α6 partner in β4 knockdown cells.
β4 knock down HaCaT cell lysate (knock down shown in Fig. 1A) was subjected to immunoprecipitation with integrin α6 antibody (sc-6596). Lanes 1–3 show α6 integrin antibody incubation and lanes 4–6 show integrin β1 antibody incubation of the same membrane. Lane 6 indicates the lack of α6-β1 complex in β4 knock down HaCaT cells.
α6 processing is affected in the absence of β4 but not in the absence of β1 or α3 protein
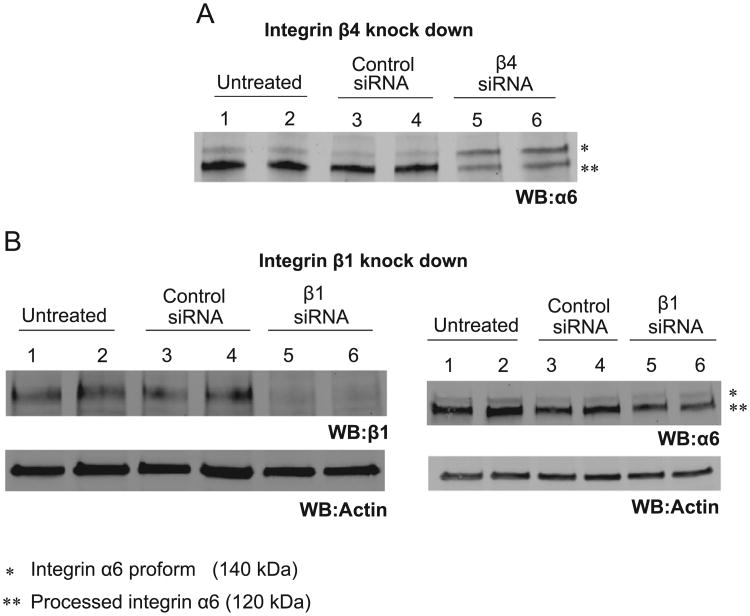
Analysis of α6 on the western blot of the β4 knockdown HaCaTs showed an α6 band at 140 kDa. This size was inconsistent with a processed α6 protein. A comparison of lanes 5, 6 to lanes 1–4 in Fig. 5A (control cells vs. β4 knockdown cells) shows that, in β4 knockdown cells the predominate α6 band detected is 140 kDa as compared to the 120 kDa band detected in control cells. The data lead us to conclude that lack of β4 protein results in the loss of α6 processing which results in its inability to be properly express at the cell surface and thus contributes to the loss in infection. The level of α6 processing was not disrupted in β1 or α3 knockdown cells, which did not have a loss of infection (Fig. 5 B and Fig. S4). α3 siRNA knockdown for experiment in Fig. S4 is shown in Fig. 2A. These final data are supportive although correlative with the importance of α6 processing.
Fig. 5. Atypical processing of α6 integrin in β4 knockdown cells.
(A) Untreated, control siRNA and integrin β4 targeted siRNA treated HaCaT cells lysates were subjected to western blotting using α6 antibody (β4 and actin incubation results of the same membrane were shown in Fig. 2G). In β4 siRNA treated cells, the enrichment of integrin α6 proform (140 kDa) over processed α6 (120 kDa) occurred. (B) Integrin β1 antibody incubation shows the efficient integrin β1 knockdown in lanes 5 and 6 (WB: β1). The same set of cell lysates were used for α6 antibody incubation. Actin was used as the loading control for both western blots. In α6 antibody incubation, there was no enrichment of integrin α6 proform (140 kDa) over processed α6 (120 kDa) in β1 knock down cells.
Discussion
The many improvements in the production of papillomavirus particles have allowed for a rapid development of data regarding the process of HPV virus binding, entry, trafficking and thus, onset of infection. In our laboratory we have taken advantage of the pseudovirion production technique that results in a viral particle containing an easily detectable reporter gene/protein, GFP. We used these pseudovirions to transduce the target cell model HaCaTs. In a previous work, we sought to determine if the finding that HPV16 VLPs bound to α6 integrin meant that α6 integrin was indeed a biological receptor for HPV16, i.e., absence of α6 integrin would result in a loss of infection (Evander et al., 1997; Yoon et al., 2001). We showed that α6 integrin was indeed crucial for HPV16 PsV infection in HaCaT cells (Abban and Meneses, 2010). We also characterized the integrin subunits that were expressed in HaCaTs with the thought that perhaps other integrin subunits played a role in HPV16 infection. We showed that the integrin subunits β1, α2, β2, α3, β4, β6 and α6 were expressed in HaCaT cells (Abban and Meneses, 2010). These data suggested that various integrin complexes (integrins are obligate heterodimers) could be formed in HaCaTs using the aforementioned integrins in the format of αXβY, were X and Y refer to the various subunit numbers (Takada et al., 2007).
In this manuscript, we addressed if HPV16 infection was dependent on other integrins and examined what beta partners were necessary for α6s role–as integrins are obligate heterodimers. Our data showed that in HaCaTs, α6 integrin forms complex with β4 integrin and α3 integrin forms complex with β1 integrin, but we saw no evidence of β1 interaction with α6 (Fig. 1). Using siRNA, we individually knocked down α3, β1,and β4 integrins. It is important to note that these integrins, along with α6, are the most prominently expressed during the process of wound healing (Goldfinger et al., 1999; Margadant et al., 2009). Data and biology of papillomavirus infection suggest that infection by HPV is most likely to occur during the process of wound healing (Roberts et al., 2007). Our experiments showed that a decrease of over 85% of either α3 or β1 protein did not negatively impact infection levels (Fig. 2C, F). In contrast, similar level of β4 integrin knock down showed a 90% decrease in HPV16 PsV infection in HaCaT cells (Fig. 2I).
In the process of performing our experiments, Dr. Florin's group showed that α3 knock down causes a decrease in HPV16 PsV infection in HeLa cells (Scheffer et al., 2013). Our results in HaCaT cells are different from their finding in HeLa cells; i.e., knock down of α3 does not decrease HPV16 PsV infection in HaCaT cells (Fig. 2C).
We previously published the importance of α6 during infection, and here we showed that it is the partner of β4 in HaCaT cells. Using an antibody to the surface exposed segment of α6, our flow cytometry data presented here showed that in addition to a loss of β4, β4 knock down cells have decreased cell surface α6 integrin expression (decrease of ∼75%) (Fig. 3E). β4 integrin depletion did not interfere with α3 integrin's surface localization (Fig. S1) and α6 integrin was able to localize to the cell surface in β1 integrin knock down HaCaTs (Fig. S2). The loss of α6 integrin in the β4 integrin knockdown cell was also observed by microscopy using an antibody targeting the intracellular portion of α6 (Fig. 3B). However, in western blots we were able to detect normal levels of α6 integrin protein in these cells (Fig. 5A). These data suggested that the epitopes detected by these two in-vivo antibodies under normal conditions are not properly formed. Data also suggest that in the absence of β4 there is no compensatory mechanism to properly process and express α6 at the cell surface. It has been suggested that in the absence of β4, β1 can serve as heterodimer partner for α6 (Kligys et al., 2012). Our data showed that β1 did not compensate for the absence of β4, i.e., no α6β1 complex was formed in the absence of β4 (Fig. 4A).
Further analysis of our data pointed out that in our immunoprecipitation western blots, there was an α6 doublet. Integrin α6 is known to be processed post-translationally, including cleavage, in order for proper signaling (Delwel et al., 1996). The cleavage of the 140 kDa proform of α6 results in the production of the heavy chain (∼120 kDa) and light chain (∼20 kDa) that are covalently associated. In our SDS-PAGE/western blot assays, we observed the unprocessed proform (∼140 kDa), and the processed heavy chain (∼120 kDa). The presence of these two bands is in agreement with other groups' studies (Lehmann et al., 1996; Lissitzky et al., 2000). The relative levels of these two forms differed between normal and β4 knockdown cells. The control cells had primarily the processed band (lower), in contrast to cells with β4 knockdown, the balance shifted to an accumulation of the proform. These data suggest that in the absence of β4, there is diminished or lack of α6 processing. In addition to α6, α3 integrin is also known to be post-translationally processed (Lehmann et al., 1996). Therefore we also analyzed the effect of β4 knock down on α3 processing. As opposed to its interference with α6 processing, β4 knock down did not have a similar effect on α3 processing (Fig. S3). We analyzed our other integrin knockdown experiments (i.e., α3, β1) for relative level of α6 processing, and we did not observe a change as compared to control cells (Fig. 5B and Fig. S4). Our results thus suggested the inability to have α6 properly processed results in HPV16 infection loss in HaCaT cells.
It has been previously shown that inhibiting the role of furin and PC 5/6 results in the loss of HPV16 infection. In HPV biology, the published data show that the N-terminus of HPV minor capsid protein L2 is the target for the aforementioned proteases (Richards et al., 2006). This L2 N-terminus cleavage facilitates transfer of the virus to a secondary receptor on the basal keratinocyte before its internalization into cells (Day et al., 2008). It is of interest to point out that furin and PC 5/6 are also involved in post-translational α integrin processing (Lissitzky et al., 2000). Studies with inhibitors of furin-like PCs resulted in a dramatic decrease in papillomavirus infection (Richards et al., 2006). The effects of those inhibitors on α6 integrin processing are being addressed in our laboratory in order to broaden our understanding of the roles of these PCs in HPV biology.
In summary our study is the first to show the roles of integrins α3, β1 and β4 in HPV16 PsV infection in HaCaT cells. Our results clearly indicated that, as opposed to its crucial role in HPV16 PsV infection in HeLa cells, α3 integrin is, in fact, dispensable for infection in HaCaT cells. β1, the beta partner of α3 is also not required for HPV16 PsV infection HaCaTs. Based on the data presented in this manuscript, we articulate that β4 knock down causes loss of functional integrin α6 from the cell surface; therefore, α6 can no longer function as an HPV receptor. Loss of functional α6 at the cell surface may prevent the formation of the proposed endocytic complex or may lead to a loss of proper signal transduction leading to viral entry. Further studies are ongoing to determine the events mediated by the α6β4 heterodimer and the processing of α6.
Experimental procedures
Cell culture and HPV16 PsV production
HaCaT cells (in vitro spontaneously transformed keratinocytes from histologically normal skin) were purchased from AddexBio (San Diego, CA). Cells were cultured in Dulbecco's Modified Eagle's media (DMEM) supplemented with 10% Fetal Bovine Serum (FBS, DMEM-10). DMEM was purchased from Thermo Scientific (Waltham, MA) and FBS was purchased from Gemini Bio-products (West Sacramento, CA).
HPV16 pseudovirion (PsV) production and purification were performed as previously described (Abban and Meneses, 2010). Briefly, 8fwb (GFP expressing plasmid) and p16sheLL (HPV16 L1 and L2 plasmid) were transfected into 293TT cells. Cells were harvested, and after high salt extraction, PsV were purified on an optiprep gradient (27–39%). In our experiments, infection is detected by the expression of GFP at a multiplicity of infection (MOI) of 0.15, i.e., 0.15 infectious units/cell using flow cytometer (BD Accuri C6 flow cytometer, Franklin Lakes, NJ). 293TT cells were a generous gift from Dr. Ozbun (The University of New Mexico School of Medicine, NM).
Antibodies
Rabbit ab1922 (anti-integrin β4), rabbit ab1952 (anti-integrin β1), mouse ab2057 (anti-integrin α3) and rabbit ab1920 (anti-integrin α3) antibodies were purchased from EMD Millipore (Billerica, MA). Goat sc-6596 (anti-integrin α6) was purchased from Santa Cruz Biotechnology (Dallas, TX). Integrin α6 rabbit recombinant oligoclonal antibody (Cat. no. 710209) was purchased from Novex-Life Technologies (Grand Island, NY).
siRNA-mediated knock down of integrins β4, β1 and α3
Accell SMARTpools (set of 4 siRNAs) were purchased from Dharmacon (Waltham, MA). Lipofectamine RNAiMax was purchased from Life Technologies (Norwalk, CT). Negative control siRNA was purchased from IDT (Coralville, IA) (sense: CGUUAA-UCGCGUAUAAUACGCGUAT, antisense: AUACGCGUAUUAUACGC-GAUUAACGAC). HaCaT cells were plated on 12-well plates, at 60% confluency; cells were transfected with 2 μl lipofectamine and 40 nM of Accell SMARTpool for integrin β4, 2 μl lipofectamine and 20 nM of Accell SMARTpool for integrin β1, and 2 μl lipofectamine and 20 nM of Accell SMARTpool for integrin α3. Cells were harvested at 24 h post-transfection with α3 siRNA pool and at 48 h for integrin β1 and β4 siRNA pools with the following lysis buffer: Nonidet P-40 (United States Biological, Salem, MA) and 50 × protease inhibitor cocktail (BD Biosciences, San Jose, CA). The cell lysates were then subjected to western blot analysis. After western blot transfer, the nitrocellulose membrane (Thermo Scientific) was blocked for 30 min with 5% milk and incubated overnight with corresponding primary antibodies. Odyssey Imaging System (Li-Cor, Lincoln, NE) was used to scan the membrane and the band intensities were analyzed using the built-in software.
HPV16 PsV infection of HaCaT cells and flow cytometry analysis
After siRNA-mediated knock down of aforementioned integrin subunits was achieved, the cells were incubated on ice for 30 min in fresh DMEM-10. The cells were then infected with HPV16 PsV at an MOI of 0.15 and incubated on ice for 2 h. After 2 h, media was removed and the cells were washed three times with 1 × PBS. Infected cells were incubated for 48 h at 37°C. The cells were then collected via trypsinization and they were washed three times with 1 × PBS (MP biomedicals, Solon, OH) by spinning at 3000 × g for 5 min. Percentage of infected HaCaT cells was calculated based on GFP positive cells that were measured by flow cytometer.
Co-immunoprecipitation experiments
HaCaT cells were plated on 100 mm tissue culture dishes and at 80% confluency, the cell lysates were harvested in NP40 and 50 × protease inhibitor mix. α6, β4, β1 and α3 integrins were immunoprecipitated with protein A and protein G magnetic beads (EMD Millipore) and α6, β4 and α3 antibodies according to the manufacturer's instructions. HaCaT cell lysate that was not subjected to IP is referred as pre-IP; cell lysate that was incubated with magnetic beads for 1 h in the absence of corresponding antibodies, (before antibody was added) is referred as pre-clear.
Immunofluorescence experiments
To visualize integrin α6 and β4 subunits in β4 knock down and control siRNA treated HaCaT cells, the cells were plated on 12-well plates containing glass coverslips. Knock down of integrin β4 was performed with siRNA transfection and negative control siRNA was used for control siRNA treated cells (as described above). At 48 h post-transfection, media was aspirated and cells were washed three times with 1 × PBS. The cells were then fixed in 4% paraformaldehyde for 10 min on ice and washed three times with 1 × PBS. Cells on coverslips were permeabilized using blocking buffer (0.2% fish skin gelatin (Sigma, St. Louis, MO), 0.2% Triton X-100 in PBS) for 15 min at room temperature. The coverslips were then incubated with corresponding primary antibodies (integrin α6:sc-6596, and integrin β4: ab-1922) at a dilution of 1:50 for 1 h. After primary antibody incubation, coverslips were washed three times with 1 × PBS and incubated with secondary antibodies (Alexa-Fluor donkey anti-rabbit 647 and donkey anti-goat 488) at 1:2000 dilutions for 30 min. Antibody dilutions were prepared in blocking buffer. After secondary antibody incubation, the coverslips were washed three times with 1 × PBS and mounted on slides using ProLong Gold Antifade Reagent.
Detection of integrin α6 and β4 surface level expressions with flow cytometer
HaCaT cells were seeded on two 12-well plates. siRNA-mediated knock down of integrin β4 was performed on one of the plates. Media was aspirated from the wells and the cells were washed three times with 1 × PBS. After that, the cells were harvested by trypsinization and they were washed three times with 1 × PBS by spinning at 3000 × g for 5 min. Following the washing steps, α6 integrin was stained with PE-Cy 5 Rat antihuman antibody CD49f (BD Biosciences) and β4 integrin was stained with PE Rat anti-human CD104 (BD Biosciences). IgG2a PE-Cy 5 Rat anti-human isotype and IgG2b PE Rat anti-human isotype antibodies were used as controls for non-specific fluorescence. The cells were incubated with corresponding antibodies for 30 min at 4°C. Then the cells were washed three times with 1 × PBS by spinning at 3000 × g for 5 min. Surface expression levels of α6 and β4 integrins were monitored via flow cytometry analysis (BD Accuri C6 flow cytometer).
Author summary
Human papillomavirus (HPV) is a major cancer causing agent worldwide. Infection by HPV can lead to cancers including cervical, anal, and oropharyngeal. Although much is known about the virus, there are still questions as to the process by which the virus gains entry into the target cells, i.e., how does the virus infection first begins. Our research laboratory is working at trying to identify how the virus first attaches to the target cells, and how this leads to the process of the virus entering the cell. Preventing viral entry would decrease the level of HPV mediated diseases including cancers. Here, using a skin cell line we defined cellular molecules that are involved in the initial viral entry and show that proper cellular processing of one of these is necessary. Our studies provide new avenues for viral interference, and show the importance of protein processing in the expression of viral binding partners.
Supplementary Material
Acknowledgments
The authors would like to thank Meneses laboratory members, Dr. Everly, and B. Arman Aksoy for their comments on the manuscript; and Drs. Ozbun, and Buck for reagents. Supported by RSG-12-021-01-MPC from the American Cancer Society. Supported by R21CA153096 from the National Institutes of Health/National Cancer Institute.
Footnotes
Appendix. Supporting information: Supplementary data associated with this article can be found in the online version at http://dx.doi.org/10.1016/j.virol.2013.10.034.
References
- Abban CY, Meneses PI. Usage of heparan sulfate, integrins, and FAK in HPV16 infection. Virology. 2010;403:1–16. doi: 10.1016/j.virol.2010.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bienkowska-Haba M, Patel HD, Sapp M. Target cell cyclophilins facilitate human papillomavirus type 16 infection. PLoS Pathogens. 2009;5:e1000524. doi: 10.1371/journal.ppat.1000524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day PM, Gambhira R, Roden RB, Lowy DR, Schiller JT. Mechanisms of human papillomavirus type 16 neutralization by 12 cross-neutralizing and l1 type-specific antibodies. J Virol. 2008;82:4638–4646. doi: 10.1128/JVI.00143-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwel GO, Hogervorst F, Sonnenberg A. Cleavage of the alpha6A subunit is essential for activation of the alpha6Abeta1 integrin by phorbol 12-myristate 13-acetate. J Biol Chem. 1996;271:7293–7296. doi: 10.1074/jbc.271.13.7293. [DOI] [PubMed] [Google Scholar]
- Doorbar J. Molecular biology of human papillomavirus infection and cervical cancer. Clin Sci (Lond) 2006;110:525–541. doi: 10.1042/CS20050369. [DOI] [PubMed] [Google Scholar]
- Doorbar J, Quint W, Banks L, Bravo IG, Stoler M, et al. The biology and life-cycle of human papillomaviruses. Vaccine. 2012;30(5):F55–F70. doi: 10.1016/j.vaccine.2012.06.083. [DOI] [PubMed] [Google Scholar]
- Dziduszko A, Ozbun MA. Annexin A2 and S100A10 regulate human papillomavirus type 16 entry and intracellular trafficking in human keratinocytes. J Virol. 2013;87:7502–7515. doi: 10.1128/JVI.00519-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evander M, Frazer IH, Payne E, Qi YM, Hengst K, et al. Identification of the alpha6 integrin as a candidate receptor for papillomaviruses. J Virol. 1997;71:2449–2456. doi: 10.1128/jvi.71.3.2449-2456.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giroglou T, Florin L, Schafer F, Streeck RE, Sapp M. Human papillomavirus infection requires cell surface heparan sulfate. J Virol. 2001;75:1565–1570. doi: 10.1128/JVI.75.3.1565-1570.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldfinger LE, Hopkinson SB, deHart GW, Collawn S, Couchman JR, et al. The alpha3 laminin subunit, alpha6beta4 and alpha3beta1 integrin coordinately regulate wound healing in cultured epithelial cells and in the skin. J Cell Sci. 1999;112(Pt 16):2615–2629. doi: 10.1242/jcs.112.16.2615. [DOI] [PubMed] [Google Scholar]
- Huang HS, Lambert PF. Use of an in vivo animal model for assessing the role of integrin alpha(6)beta(4) and syndecan-1 in early steps in papillomavirus infection. Virology. 2012;433:395–400. doi: 10.1016/j.virol.2012.08.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson KM, Kines RC, Roberts JN, Lowy DR, Schiller JT, et al. Role of heparan sulfate in attachment to and infection of the murine female genital tract by human papillomavirus. J Virol. 2009;83:2067–2074. doi: 10.1128/JVI.02190-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joyce JG, Tung JS, Przysiecki CT, Cook JC, Lehman ED, et al. The L1 major capsid protein of human papillomavirus type 11 recombinant virus-like particles interacts with heparin and cell-surface glycosaminoglycans on human keratinocytes. J Biol Chem. 1999;274:5810–5822. doi: 10.1074/jbc.274.9.5810. [DOI] [PubMed] [Google Scholar]
- Kligys KR, Wu Y, Hopkinson SB, Kaur S, Platanias LC, et al. alpha6beta4 integrin, a master regulator of expression of integrins in human keratinocytes. J Biol Chem. 2012;287:17975–17984. doi: 10.1074/jbc.M111.310458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laniosz V, Dabydeen SA, Havens MA, Meneses PI. Human papillomavirus type 16 infection of human keratinocytes requires clathrin and caveolin-1 and is brefeldin a sensitive. J Virol. 2009;83:82218232. doi: 10.1128/JVI.00576-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehmann M, Rigot V, Seidah NG, Marvaldi J, Lissitzky JC. Lack of integrin alpha-chain endoproteolytic cleavage in furin-deficient human colon adenocarcinoma cells LoVo. Biochem J. 1996;317(Pt 3):803–809. doi: 10.1042/bj3170803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipovsky A, Popa A, Pimienta G, Wyler M, Bhan A, et al. Genome-wide siRNA screen identifies the retromer as a cellular entry factor for human papillomavirus. Proc Natl Acad Sci USA. 2013;110:7452–7457. doi: 10.1073/pnas.1302164110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lissitzky JC, Luis J, Munzer JS, Benjannet S, Parat F, et al. Endoproteolytic processing of integrin pro-alpha subunits involves the redundant function of furin and proprotein convertase (PC) 5A, but not paired basic amino acid converting enzyme (PACE) 4, PC5B or PC7. Biochem J. 2000;346(Pt 1):133–138. [PMC free article] [PubMed] [Google Scholar]
- Margadant C, Raymond K, Kreft M, Sachs N, Janssen H, et al. Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J Cell Sci. 2009;122:278–288. doi: 10.1242/jcs.029108. [DOI] [PubMed] [Google Scholar]
- Richards RM, Lowy DR, Schiller JT, Day PM. Cleavage of the papillomavirus minor capsid protein, L2, at a furin consensus site is necessary for infection. Proc Natl Acad Sci USA. 2006;103:1522–1527. doi: 10.1073/pnas.0508815103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts JN, Buck CB, Thompson CD, Kines R, Bernardo M, et al. Genital transmission of HPV in a mouse model is potentiated by nonoxynol-9 and inhibited by carrageenan. Nat Med. 2007;13:857–861. doi: 10.1038/nm1598. [DOI] [PubMed] [Google Scholar]
- Scheffer KD, Gawlitza A, Spoden GA, Zhang XA, Lambert C, et al. Tetraspanin CD151 mediates papillomavirus type 16 endocytosis. J Virol. 2013;87:3435–3446. doi: 10.1128/JVI.02906-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffman M. Integration of human papillomavirus vaccination, cytology, and human papillomavirus testing. Cancer. 2007;111:145–153. doi: 10.1002/cncr.22751. [DOI] [PubMed] [Google Scholar]
- Seidah NG, Prat A. The biology and therapeutic targeting of the proprotein convertases. Nat Rev Drug Discov. 2012;11:367–383. doi: 10.1038/nrd3699. [DOI] [PubMed] [Google Scholar]
- Selinka HC, Florin L, Patel HD, Freitag K, Schmidtke M, et al. Inhibition of transfer to secondary receptors by heparan sulfate-binding drug or antibody induces noninfectious uptake of human papillomavirus. J Virol. 2007;81:10970–10980. doi: 10.1128/JVI.00998-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Surviladze Z, Dziduszko A, Ozbun MA. Essential roles for soluble virion-associated heparan sulfonated proteoglycans and growth factors in human papillomavirus infections. PLoS Pathogens. 2012;8:e1002519. doi: 10.1371/journal.ppat.1002519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takada Y, Ye X, Simon S. The integrins. Genome Biol. 2007;8:215. doi: 10.1186/gb-2007-8-5-215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodham AW, Da Silva DM, Skeate JG, Raff AB, Ambroso MR, et al. The S100A10 subunit of the annexin A2 heterotetramer facilitates L2-mediated human papillomavirus infection. PLoS One. 2012;7:e43519. doi: 10.1371/journal.pone.0043519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoon CS, Kim KD, Park SN, Cheong SW. alpha(6) Integrin is the main receptor of human papillomavirus type 16 VLP. Biochem Biophys Res Commun. 2001;283:668–673. doi: 10.1006/bbrc.2001.4838. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.