Abstract
Objective
Determine transplacental transfer and metabolism of 17α-hydroxyprogesterone caproate (17-HPC) and its distribution between the tissue, maternal, and fetal circuits of the dually perfused placental lobule.
Study design
17-HPC (21ng/ml) and its dual labeled isotope, 17α-hydroxy-[3H]progesterone [14C] caproate were added to the maternal circuit. The concentrations of the drug and its metabolite in trophoblast tissue and both circuits were determined by High Performance Liquid Chromatography (HPLC) and liquid scintillation spectrometry.
Results
The data obtained revealed that 17-HPC is transferred from the maternal to fetal circuit. At the end of a 4 hour perfusion period, a metabolite of 17-HPC that retained both progesterone and caproate moiety was identified in the tissue, maternal and fetal circuits. Neither 17-HPC nor its metabolite, at the concentrations tested, had any adverse effect on the determined viability and functional parameters of placental tissue.
Conclusions
17α-hydroxyprogesterone caproate is metabolized by term placental lobule during its perfusion and both parent compound and its metabolite transferred to the fetal circuit.
Keywords: preterm delivery, 17α-hydroxyprogesterone caproate, dual perfusion, metabolism
Introduction
A recent clinical trail demonstrated that weekly administration of 17α-hydroxyprogesterone caproate (17HPC) significantly reduced the rate of recurrent preterm delivery in high-risk patients and reduced the occurrence of health complications in the newborns.1 These findings prompted investigations of the role of progesterone and its synthetic derivatives in prevention of preterm deliveries as well as the mechanism and site of action of 17-HPC.
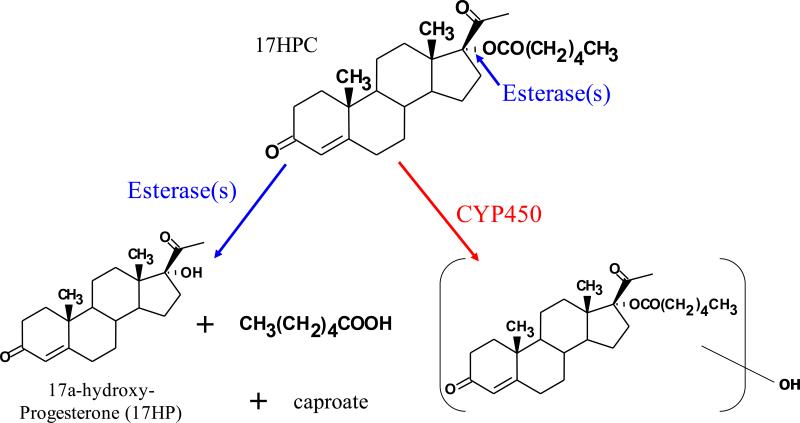
Recent investigation in the NICHD funded Obstetrics-Pharmacology Research Network (OPRU) focuses on the PK and PD of 17-HPC. In our laboratory, the focus is to determine whether 17HPC is a drug or prodrug. If a prodrug, then there are at least two possible pathways for its metabolism (Figure 1): First, hydrolysis of the ester bond in 17-HPC by plasma or tissue esterases and thus the formation of 17α-hydroxyprogesterone (17HP) and caproate; Second, the oxidation or conjugation of 17HPC by cytochrome P450 isozymes or other enzymes, respectively. Recent investigations in our laboratory revealed that 17HPC is not hydrolyzed in vitro by either human plasma or tissue-derived preparations obtained from the liver, term and preterm placenta2. However, the absence of 17HPC hydrolysis in vitro does not exclude that it could be hydrolyzed in vivo. Moreover, to the best of our knowledge, data on placental disposition of 17HPC which include its metabolism and transfer to the fetal circulation is scarce.
Figure 1.
Structure of 17HPC and its biotransformation pathways. First, hydrolysis of its ester bond by plasma or tissue esterases resulting in the formation of 17α- Hydroxyprogesterone (17HP) and caproate; Second, oxidation or conjugation of 17HPC by cytochrome P450 isozymes or other enzymes, respectively. Asterisk indicate the position of [3H] and [14C]- radiolabeles on progesterone and caproate moeties of parent compound.
The physicochemical properties of 17HPC (molecular weight of 428.6, lipophilicity, planar structure) suggest that it should be readily transferred across biological membranes, including that of the syncytiotrophoblast and endothelial cells of the fetal capillaries. Therefore, intrauterine exposure of the fetus to 17HPC should not be ruled out. A therapeutic agent administered during pregnancy could affect normal fetal growth and development either directly, as a result of its concentration in the fetal circulation, or indirectly by affecting the physiological functions of the placenta. Therefore, it is imperative to determine the extent of transplacental transfer of the drug, its retention by the tissue and effects on placental functions. These parameters are best determined in vitro by utilizing the technique of dual perfusion of placental lobule: a powerful tool in investigating transfer and metabolism of drugs under experimental conditions that most closely simulate in vivo conditions.
Therefore, the aims of this investigation are to determine the transfer of 17HPC across term human placenta, its metabolism by the tissue and its effect on trophoblast viability and functional parameters
Materials and Methods
Placentas from uncomplicated term pregnancies (n = 10) were obtained immediately after vaginal or abdominal deliveries from the Labor and Delivery ward of the University of Texas Medical Branch, Galveston, Texas, according to a protocol approved by the university's Institutional Review Board.
The technique of dual perfusion of placental lobule (DPPL) was used as described earlier by our laboratory 3 and according to the method of Miller et al.4 The concentration of 17HPC in the maternal reservoir (21ng/mL) was equal to the mean plasma concentration of the drug in patients who received a 1000 mg injection5. The dual-labeled radioactive isotope 17 α-hydroxyprogesterone[3H]-caproate[14C], (Specific activity: 105mCi/mmol and 37.3 mCi/mmol, respectively, custom synthesis by RTI International, Research Triangle Park, Durham, NC) was added to enhance the detection limit of the drug and account for any hydrolysis of the ester bond i.e. formation of 17HP[3H] and caproate[14C] or their derivatives. Human serum albumin (HSA) was added to both circuits at its approximate in vivo concentration of 30mg/mL.6 The perfusion system was used in its closed-closed configuration (recirculation of the medium). The amounts of radioactivity (using both the 3H and 14C- channels simultaniously) in placental tissue, maternal, and fetal perfusates were determined by liquid scintillation spectrometery (1900TR; Packard Instruments, Inc, Shelton, Ct). The concentration of 17HPC in all samples was calculated after correcting for the specific activity as previously reported3.
The effects of 17HPC on placental tissue was evaluated by determining its functional (human chorionic gonadotropin (hCG) release), and viability parameters (oxygen transfer and consumption)3.
Metabolism of 17HPC
Aliquots from the maternal (2 ml) and fetal (4 ml) perfusates were extracted twice with methylene chloride. The organic phase for each was combined, evaporated to dryness and reconstituted in acetonitrile. Recovery of total radioactivity was >95%. Standards of 17HPC (3H, 14C), 17HP (3H), and caproate (14C) were used to determine their retention times on a C18 HPLC column. The HPLC system (Waters, Milford, Ma) used was made of a multisolvent delivery system, a U.V. detector, an auto-sampler and an on line β-RAM flow-through detector (IN/US Systems, Tampa, Fl). Samples were eluted on using a mobile phase gradient starting with 100% (0.25% acetic acid/acetonitrile, 70/30, A) for 5 min followed by a linear increase to 100% of B (H2O/acetonitrile, 10/90) over a period of 30 min then maintained for 10 min at a flow rate of 1mL/min.
Data Analysis
The difference between compared values was determined by a two-tailed Student's t test and considered significant when P < .05.
RESULTS
Metabolism of 17HPC
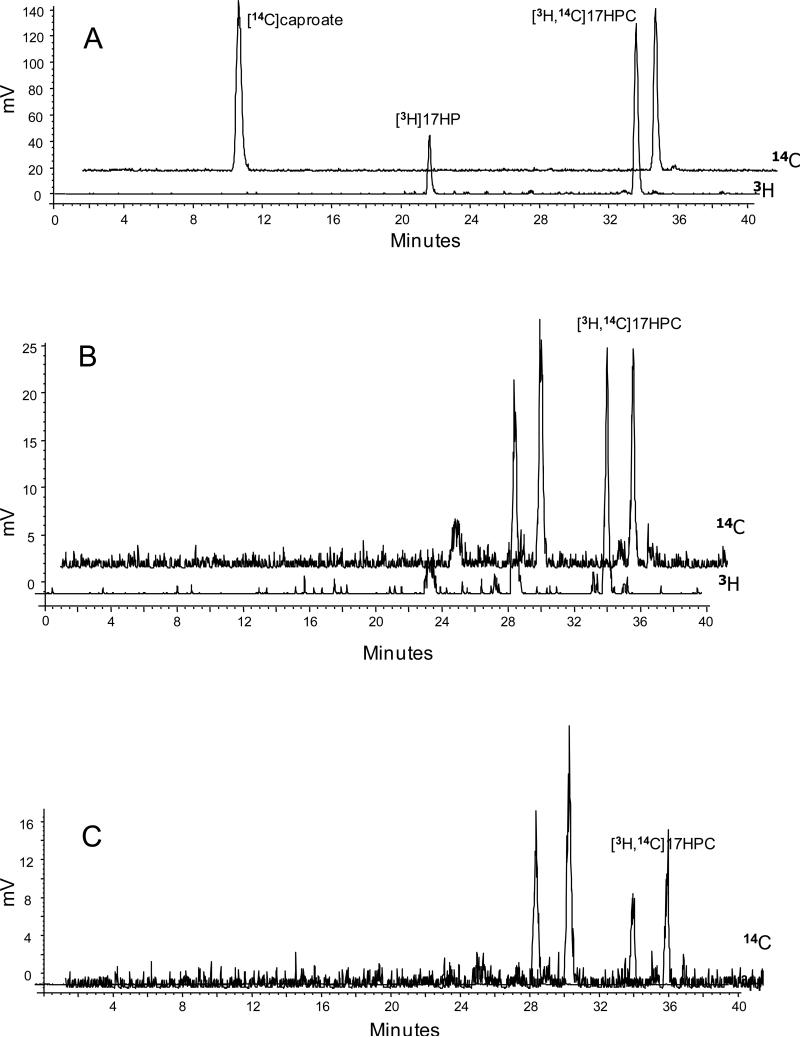
The retention times for the radiolabeled standards under our experimental conditions were as follows; 17HPC [14C and 3H], 33min; 17HP [3H], 21min.; caproate [14C], 11min (Figure 2A). The elution profile (chromatograms) of aliquots from the maternal and fetal perfusates revealed the following (Figures 2B&C): Two major peaks at 27min and 33min, each retaining both of the two radioactive ([3H] and [14C]) isotopes. One of these two peaks had a retention time of 33min which corresponds to that of the dual labeled standard of 17HPC. The second peak, retaining both radioisotopes, had a retention time of 27min and represents a more polar derivative/metabolite of 17HPC. Moreover, neither [3H]-17HP nor [14C]-caproate (retention times, 21 and 10min, respectively) were detected in the maternal nor fetal perfusates i.e. 17HPC was not hydrolyzed. .
Figure 2.
The radiochromatogram of the [14C]caproate, [3H]17HP and [3H,14C]17HPC. (A) The radiochromatogram of the retention times of standard compounds of the [14C]caproate (11 min) and [3H,14C]17HPC (33 min) detected on the [14C channel] and [3H]17HP (21 min) and [3H,14C]17HPC (33 min) detected on [3H] channele. (B-C) Radiochromatograms of aliquots from maternal and fetal circuits obtained at the end of the experiment, respectively. An additional peak appeared with an elution time of 27 min containing both radioactive [3H] and [14C].
Placental Transfer of 17 HPC
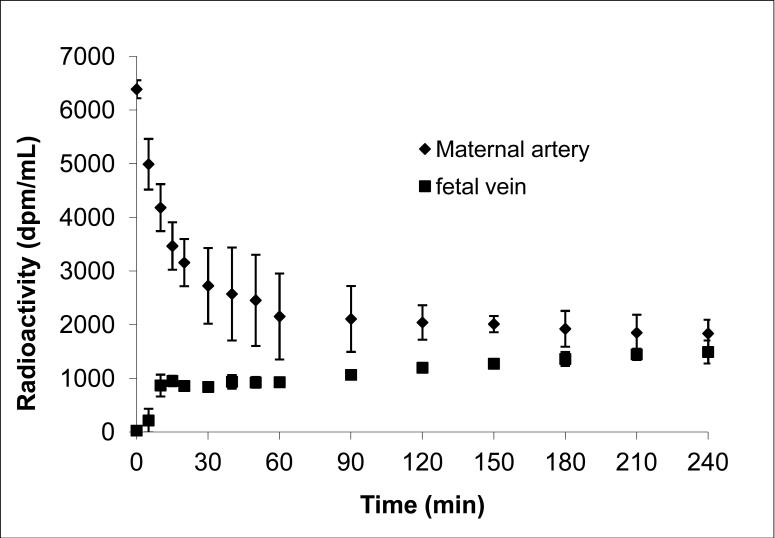
The amounts of radioactivity in the maternal circuit declined with time during the 4hour experimental period (Figure 3). The decline was biphasic, the first initial phase (15minutes) was rapid and accounted for approximately 50% of the amount of 17HPC added to the maternal reservoir. However, only 15% appeared in the fetal circuit during this initial period, indicating that approximately 2/3 of the amount of radioactivity was retained by the perfused tissue.
Figure 3.
The time concentration curve of radioactivity determined in the maternal and fetal circuits during 4 hours of perfusion. Each time point represents the sum of the radioactivity for 17HPC and its metabolite.
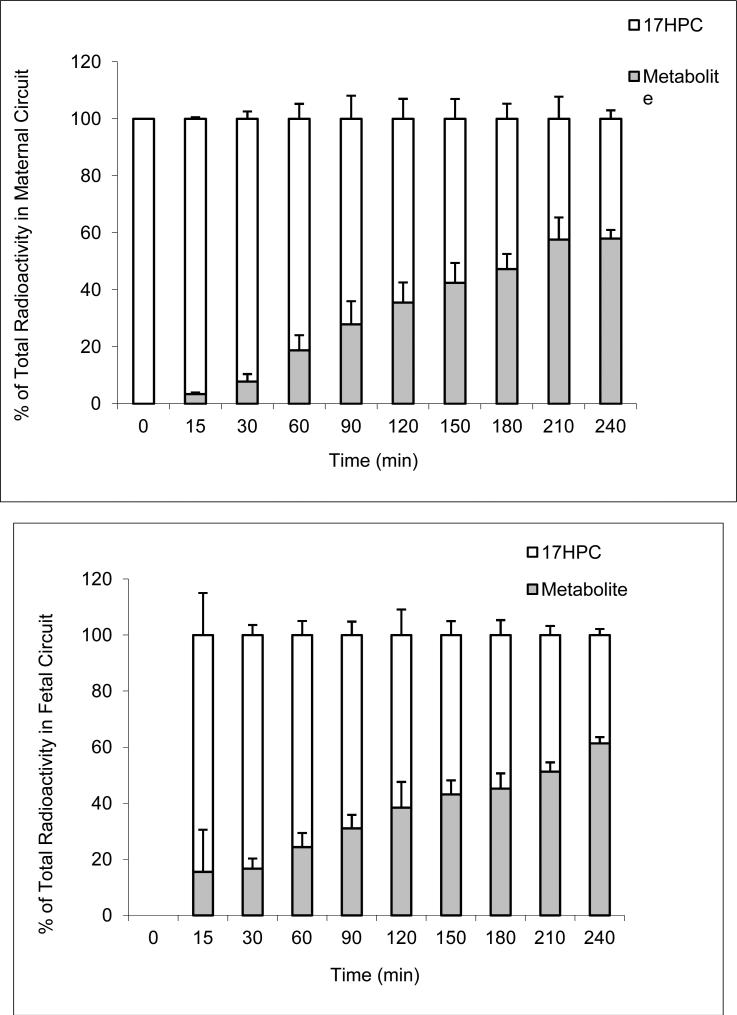
The second phase of the decline in maternal perfusate was slower and reached a plateau after 60 minutes. It should be emphasized here that the amounts of radioactivity determined in the tissue, maternal and fetal circuits represents the sum of 17HPC and its derivative/metabolite. It is apparent (Figure 4A) that the metabolite of 17HPC was detected in the maternal circuit after 15 minutes and steadily increased during the entire 4 hour period. At the end of the experimental period, the ratio of metabolite to 17HPC was 3:2.
Figure 4.
The ratio of metabolite to 17HPC in maternal (A) and fetal (B) circuits during perfusion period.
The parent compound (17HPC) appeared in the fetal circuit within 5 minutes of its perfusion (Figure 3). Similar to the maternal circuit, the metabolite was detected in the fetal circuit after 15 minutes (Figure 4B). The amount of metabolite in the fetal circuit increased steadily until the end of the 4 hour perfusion period at which time it accounted for approximately 60% of the total radioactivity (Figure 4B).
These data indicate that 17HPC is transferred across the human placenta to the fetal circuit, and that more than half of it is retained by the placental tissue. Moreover, the metabolite formed by the tissue is released into both the maternal and fetal circuits.
Placental Distribution of 17HPC and its derivative/metabolite
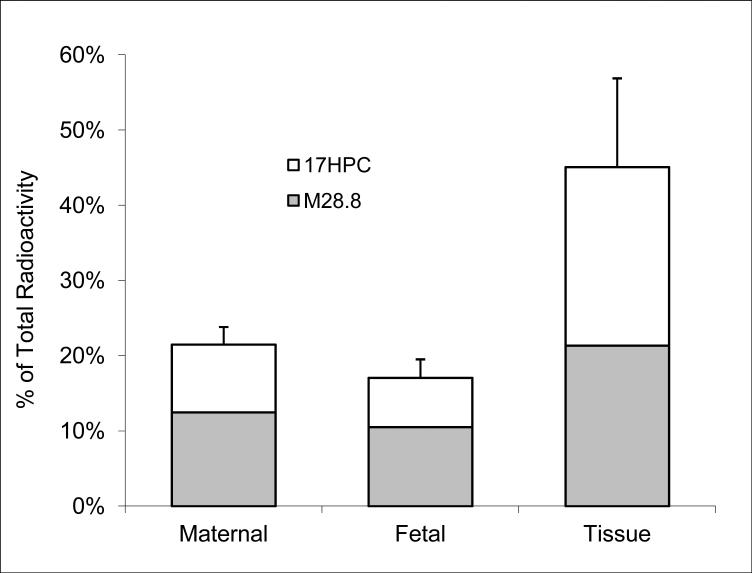
At the end of the experimental period (4 hours), the distribution of radioactivity between placental tissue, maternal, and fetal circuit was as follows: 46.3 ± 17.5% was retained by the tissue, 21.6 ± 2.3% remained in the maternal circuit and 17.2 ± 2.2% was transferred to the fetal circuit (Figure 5). Therefore, the majority of radioactivity was retained by the perfused tissue. Unlike the maternal and fetal circuits, the ratio of the metabolite to17HPC retained in the tissue was 1:1.
Figure 5.
The distribution of 17HPC and its metabolite between placental tissue, maternal and fetal circuits at the end of the perfusion period.
Effect of 17HPC and its Metabolite on Tissue Function/Viability
The effect of 17 HPC and its metabolite on placental tissue viability and functional parameters (oxygen transfer, oxygen consumption, and the release of human chorionic gonadotropin (hCG)) were determined. Oxygen transfer ranged between 0.35±0.02 to 0.62±0.12mL/min × kg, and was not affected by 17HPC. In addition, oxygen consumption was also not affected by the drug or its metabolite. There was no difference in hCG release from perfused tissue in presence or absence (medium only) of 17HPC. Therefore, neither 17HPC nor its metabolite had an effect on placental tissue viability and functional parameters determined.
Comment
The aim of this investigation was to determine placental transfer, metabolism and distribution of 17HPC between the tissue, maternal and fetal circuits of the dually perfused term placental lobule. This technique retains placental tissue anatomical and functional integrity and is the most reliable in vitro method for investigating the bidirectional transfer, distribution and metabolism of drugs. However, the lack of metabolism of a drug during its perfusion could be attributed to the limited area of the tissue in contact with it, which represents 5% of the total placenta, as previously discussed7. Accordingly, the metabolism of a drug should be further investigated utilizing subcellular fractions of trophoblast tissue before conclusions on its biotransformation by placental tissue can be made. On the other hand, the metabolism of a drug during its perfusion suggests high activity of the responsible major enzyme catalyzing the reaction. Moreover, information obtained on the transfer of a drug utilizing the technique of dual perfusion of placental lobule can be extrapolated to in vivo conditions for placentas of the same gestational age.
In this investigation, the dual labeled radioactive isotope of 17HPC, [3H]-progesterone and [14C]-caproate, was utilized for the following two reasons: First, to enhance the detection limits of 17HPC during its perfusion. Second, to determine either the hydrolysis of 17HPC—to progesterone and caproate—or its metabolism during its perfusion period of 4 hours. Therefore, the recirculating mode of the technique was utilized to allow accumulation of metabolite formed.
Data cited here on placental transfer and distribution of the lipophillic 17HPC (log P 5.7 and mol. wt. 428) is consistent with those compounds with similar physichochemical properties in being a two step/phase process. During the first phase (short duration), the drug is transferred from the maternal circuit to the tissue and is characterized by a steep decline in drug concentration in the maternal circuit with minimal transfer to the fetal circuit. During the second phase (longer duration), the drug is released from the tissue to the fetal circuit and is characterized by continuous but shallow decline in drug concentration in the maternal circuit with an increase in concentration in the fetal until steady state is reached (Figure 3). However, it should be noted that the data on the amounts of radioactivity in the maternal and fetal circuits during the 4 hour perfusion period of 17HPC (Figure 3) represent both the parent compound and its metabolite. Specifically, the metabolite of 17HPC appeared in the maternal and fetal circuits after 15 minutes (Figure 4 A&B). The metabolite was formed by the tissue during the entire perfusion period and was released, resulting in a steady increase in its concentration in both circuits (Fig 4 A&B). At the end of the experimental period, the amount of metabolite in both circuits was approximately 1.5 times that of 17HPC while in the tissue they were approximately equal to each other (Figure 5). In addition, the total amount of 17HPC plus its metabolite in the tissue were approximately equal to the sum of those in the maternal and fetal circuits. Taken together, it is apparent that tissue retention of the metabolite formed is similar to that of 17HPC. Moreover, data revealed that neither 17HPC nor its metabolite had any apparent adverse effects on the prefused tissue as determined by the viability (oxygen transfer and consumption) and functional (hCG release) parameters.
Data cited here indicate that 17HPC was not hydrolyzed to 17HP and caproate as revealed by the HPLC chromatograms (Fig 1 B&C). This data is in agreement with recent reports on the lack of 17HPC hydrolysis by S9 fractions obtained from term2,8 and preterm placentas2.
On the other hand, the same chromatograms revealed the formation of a metabolite/compound with a retention time less than that of 17HPC by 4 minutes. This compound/metabolite contained both the progesterone nucleus and its caproate moiety as revealed by the detection of both the 3H and 14C in one peak. Taken together, these data indicate that a polar group was introduced into the parent compound, most likely in the progesterone moiety (though the caproate can not be ruled out at this time). It should be noted here that during the last six years, our laboratory reported on placental transfer of methadone, LAAM, buprenorphine, glyburide, and metformin utilizing the same technique and none of these drugs were metabolized during their perfusion for the same experimental period of 4 hours. Therefore, as discussed above, the observed metabolism of 17HPC suggests that it is a substrate of an enzyme with high activity.
In summary, data obtained in this investigation demonstrated, for the first time, that 17HPC is biotransformed by placental tissue to a metabolite that is lipophilic but more polar than the parent compound. In absence of information on the identity, rate of formation, and pharmacological activity of the metabolite, it is difficult to predict whether it contributes to the reported effects of 17HPC on prevention of recurrent preterm deliveries in a specific patient population. Alternatively, if the metabolite formed is pharmacologically inactive and if its rate of formation- which would be the rate of 17HPC inactivation- varies between individuals, then these patients can be described as poor metabolizers of 17HPC. Therefore, treatment of preterm deliveries in the latter patient population with 17HPC will be successful. Both issues are currently under investigation in our laboratory as well as by other sites of OPRU network.
Condensation.
17α-Hydroxyprogesterone caproate is metabolized by perfused placental lobule and both parent compound and its metabolite transferred to the fetal circuit.
Acknowledgements
The authors would like to thank the Perinatal Research Division for their assistance and as well as the assistance of the Publication, Grant, & Media Support of the Department of Obstetrics and Gynecology.
This work was supported by the NIH Obstetrics-Fetal Pharmacology research Units network (U10-HD047891; G.D.V. Hankins, P.I.).
Footnotes
Source of Support:
Presentation information: Presented at the 27th Annual Meeting of the Society of Maternal Fetal Medicine, San Francisco, California, February 5-10th, 2007.
REFERENCES
- 1.Meis P, Klebanoff M, Thom E, et al. Prevention of recurrent preterm delivery by 17 Alpha-Hydroxyprogesterone Caproate. N Engl J Med. 2003;348:2379–2385. doi: 10.1056/NEJMoa035140. [DOI] [PubMed] [Google Scholar]
- 2.Yan Ry, Fokina V, Hankins GDV, Ahmed MS, Nanovskaya TN. The effect of esterases on 17α-hydroxyprogesterone caproate. Am J Obstet & Gynecol. doi: 10.1016/j.ajog.2007.07.038. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Nanovskaya T, Deshmukh S, Brooks M, Ahmed M. Transplacental transfer and metabolism of buprenorphine. J Pharmacol Exp Ther. 2002;300:26–33. doi: 10.1124/jpet.300.1.26. [DOI] [PubMed] [Google Scholar]
- 4.Miller R, Wier P, Maulik D, Di Sant'Agnese P. Human placenta in vitro: characterization during 12 hours of dual perfusion. Contrib Gynecol Obstet. 1985;13:77–84. [PubMed] [Google Scholar]
- 5.Onsrud M, Paus E, Haug E, Kjorstad K. Intramuscular administration of hydroxyprogesterone caproate in patients with endometrial carcinoma. Pharmacokinetics and effects on adrenal function. Acta Obstet Gynecol Scand. 1985:519–523. doi: 10.3109/00016348509156732. [DOI] [PubMed] [Google Scholar]
- 6.Pritchard JA, Macdonald PC. Williams obstetrics. 15th ed. Appleton-Century-Crofts; New York: 1976. [Google Scholar]
- 7.Nekhayeva IA, Nanovskaya TN, Deshmukh SV, Zharikova OL, Hankins GDV, Ahmed MS. Bidirectional transfer of methadone across human placenta. Biochem Pharmacol. 2005;69:187–197. doi: 10.1016/j.bcp.2004.09.008. [DOI] [PubMed] [Google Scholar]
- 8.Wiener M, Lupu CI, Plotz EJ. Metabolism of 17α-hydroxyprogesterone-4-14C-17α-caproate by homogenates of rat liver and human placenta. Acta Endocrinologica. 1961;36:511–526. [PubMed] [Google Scholar]