Abstract
Background
A high frequency of hypogonadism has been reported in male patients with advanced cancer.
Objectives
To evaluate the association among low testosterone, symptom burden and survival in cancer patients.
Methods
119/131 (91%) consecutive male cancer patients had an endocrine evaluation of total/free/bioavailable testosterone (TT, FT, BT, respectively), high-sensitivity C-reactive protein (CRP), vitamin B12, thyroid stimulating hormone, 25-hydroxy vitamin D and cortisol levels when presenting with symptoms of fatigue and/or anorexia-cachexia. Symptoms were evaluated by the Edmonton Symptom Assessment Scale. We examined the correlation with Spearman test and survival with log rank test and Cox-regression analysis.
Results
The median age was 64; majority were white 85 (71%). Median TT was 209ng/dL (normal ≥200 ng/dL), FT was 4.4 ng/dL (normal ≥9 ng/dL), and BT was 22.0 ng/dL (normal ≥61ng/dL). Low TT, FT, and BT values were all associated with worse fatigue (p≤0.04), poor performance status (p≤0.05), weight loss (p≤0.01), and opioid use (p≤0.005). Low TT and FT were associated with increased anxiety (p≤0.04), decreased feeling of well-being (p≤0.04), and increased dyspnea (p≤0.05); while BT was only associated with anorexia (p=0.05). Decreased TT, FT, and BT values were all significantly associated with elevated CRP, low albumin and hemoglobin. In multivariate analysis, decreased survival was associated with low TT (HR 1.66; p=0.034), declining ECOG performance status (HR 1.55; p=0.004), high CRP (HR 3.28; p<0.001), and decreased albumin (HR 2.52; p<0.001).
Conclusion
In male cancer patients, low testosterone was associated with systemic inflammation, weight loss, increased symptom burden, and decreased survival.
Keywords: Hypogonadism, Palliative Care, Symptom Management, Advanced Cancer, Survival
Introduction
Low testosterone is reported in roughly two-thirds of male patients with advanced cancer. [1, 2] Even in recently diagnosed metastatic cancer patients, who have not received chemotherapy, low testosterone was noted in roughly half of the population tested. [3] The etiology of hypogonadism is most likely multifactorial. Hypogonadism may result from the dysfunction of the hypothalamic-pituitary-gonadal axis due to the underlying cancer, chronic inflammation, or treatment with chemotherapy, [4] corticosteroids, or appetite stimulants such as megestrol acetate. [5] In addition, opioid therapy, which is often required to treat pain in cancer patients, results in decreased testosterone levels. [6, 7]
A preliminary retrospective study conducted by our group found that hypogonadism was common in male cancer patients and was associated with fatigue, anorexia, depression, insomnia, and along with elevated high-sensitivity C-reactive protein (CRP), a poor prognosis for survival. [8] Limitations of the study included the absence of serum testosterone measurements in almost one-third of the patient population studied, the lack of testing for free or bio-available testosterone levels, and a small sample size. To address these limitations, the current study examined the association of testosterone - total, free, and bio-available testosterone (TT, FT, BT, respectively) - with symptoms including fatigue and weight loss, laboratory indicators of systemic inflammation, and survival in male patients with advanced cancer.
Materials and Methods
Approval by the Institutional Review Board at M D Anderson Cancer Center was obtained prior to collection of data with waiver of informed consent.
Male cancer patients who were evaluated and completed a laboratory workup for symptoms of cancer-related fatigue and/or cachexia in an outpatient supportive care clinic by a nurse and Palliative Medicine specialist at M D Anderson Cancer Center before October 31, 2012 were retrospectively identified. Male patients with prostate cancer, breast cancer, or who were being treated with testosterone supplementation, corticosteroids, or megestrol acetate were excluded. Demographic factors of the patient population including date of birth, age, sex, race, and the primary tumor diagnosis were recorded.
In addition, an electronic medical chart review recorded clinical data including, the symptom burden as measured by the Edmonton Symptom Assessment Scale (ESAS), laboratory tests, and history of weight loss over the past three months was obtained by documentation in the medical records or by patient history, if no records were available.
The ESAS is a validated assessment tool which measures the response of cancer patients to 10 common symptoms over a 24-hour period including pain, fatigue, nausea, depression, anxiety, drowsiness, shortness of breath, appetite, sleep, and feeling of well-being). [9] Each of the 10 symptoms is rated by the patient on a scale of 0 (best) to 10 (worst).
Serum laboratory data values were recorded which included the following: TT, BT, and FT, CRP, thyroid stimulating hormone, 25-hydroxy vitamin D, and albumin. All patients were instructed to obtain laboratory testing in the morning hours or if they had altered sleep patterns, within 2 hours upon awakening.
TT is measured by liquid chromatography - tandem mass spectrometer (LC-MS/MS). [10] Secondary to abnormalities of sex-hormone-binding globulin (SHBG) noted in cancer patients, FT and BT were also ordered. FT is measured by equilibrium dialysis; FT results are expressed as a percentage of total testosterone which is multiplied by the value of TT to obtain an absolute free testosterone value. [11, 12] BT values were obtained by differential precipitation of SHBG by ammonium sulfate. [13] TT, FT, and BT were measured by Mayo Medical Laboratories, Rochester, Minnesota. CRP was measured by immunoturbidimetry, also at the Mayo Medical Laboratories.
We used descriptive statistics to summarize data, including medians, means, standard deviations, ranges, and frequencies, together with a 95% confidence interval. Spearman’s correlation test was used to determine the associations between lab abnormalities and symptom burden. In order to determine if there were significant differences between groups (normal vs. low testosterone), we used a two-sample t test when the data was approximately normally distributed, the Wilcoxon two-sample test if the data was skewed, and chi-square tests for categorical variables. The Kaplan-Meier method was used to analyze survival with comparisons of curves by the log rank test. Overall survival was calculated from the time of laboratory evaluation to death from any cause or date which patient was last known alive.
For exploratory purposes, we divided TT, FT, and BT into low, middle, and high tertiles and examined the association with symptom burden and survival using the Kruskal Wallis test. In addition, we evaluated the patient’s age and race, TT, FT, and BT levels, ECOG performance status, CRP, WBC count, hemoglobin, albumin, and corrected serum calcium levels in a multivariate survival analysis as potential indicators of a poor prognosis using the Cox regression model with backward selection. The Statistical Package for the Social Sciences (SPSS version 21.0, IBM Corporation, Armonk, New York) software was used for statistical analysis. A two-sided P value of less than 0.05 was considered statistically significant.
Results
Baseline Characteristics
A total of 119/ 131 consecutive male cancer patients (91%) were evaluable. Table 1 highlights the baseline characteristics of patients being treated for symptoms of cancer-related fatigue and/or cachexia. The symptom burden of the patient population included pain, anorexia, fatigue, and poor overall well-being. The mean age for these male cancer patients was 64 (range 31–91 years). The majority of patients who participated were white 85 (71%) and were diagnosed with a gastrointestinal (40%) or lung (25%) cancer. Seventy-two (62%) patients had a history of weight loss >5% in the past 6 months with a median of 7% (Q1-Q3:3.2–12.9%) loss of body weight.
Table 1.
Characteristics of Male Patients with Advanced Cancer
| Patient characteristics | N=119 (%)1 |
|---|---|
| Mean Age (years), range | 61 (31–91) |
| Male sex | 119 (100) |
| Race | |
| White | 85 (71) |
| Black | 13 (11) |
| Hispanic | 11 (9) |
| Asian | 10 (8) |
| Cancer Diagnosis | |
| Gastrointestinal | 48 (40) |
| Genitourinary | 10 (8) |
| Hematological | 3 (3) |
| Head & Neck | 18 (15) |
| Lung | 30 (25) |
| Other | 10 (8) |
| Median weight loss % in last 6 months, (Q1-Q3) | 7.0 (3.2–12.9) |
| Weight loss >5% in last 6 months | 72 (62) |
| Median 25OH Vitamin D level (ng/mL), (Q1-Q3) | 21 (13–31) |
| Median TSH (mcU/mL), (Q1-Q3) | 2 (1–3) |
| Median Total Testosterone (ng/dl), (Q1-Q3) | 209 (98–378) |
| Median Free Testosterone (ng/dl), 2 (Q1-Q3) | 4.4 (1.9–7.5) |
| Median Bio-available Testosterone (ng/dL), 3 (Q1-Q3) | 22.0 (8.1–41.5) |
| Median hs CRP (mg/L), (Q1-Q3) | 33.3 (9.5–91.0) |
| Median WBC (K/UL), (Q1-Q3) | 6.6 (4.6–10.0) |
| Median Hemoglobin (g/dL), median (Q1-Q3) | 11.3 (10.2–12.6) |
| Median Albumin (mg/dL), median (Q1-Q3) | 3.8 (3.3–4.1) |
| Median MEDD (mg/d), median (Q1-Q3) | 30 (0–100) |
| Median Edmonton Symptom Assessment Score, (Q1-Q3) | |
| Pain | 4 (2–6) |
| Fatigue | 5 (3–7) |
| Nausea | 1 (0–2) |
| Depression | 2 (0–3) |
| Anxiety | 2 (0–4) |
| Drowsiness | 3 (1–5) |
| Appetite | 5 (2–7) |
| Well Being | 5 (3–6) |
| Dyspnea | 2 (0–5) |
| Sleep | 4 (2–7) |
| Median Survival (days), 95% CI | 151 (116–186) |
unless otherwise specified;
N=116;
N=118; TSH – thyroid stimulating hormone; hs CRP – high-sensitivity C-reactive protein; WBC – white blood cell count; MEDD – morphine equivalent daily dosing.
Associations Among Testosterone, CRP level, Albumin, and Vitamin D
The median level of TT was 209 ng/dL (Q1-Q3:98–378 ng/dL), normal (≥ 200ng/dL). Table 1. The median value of FT was 4.4 ng/dL (Q1-Q3:1.9–7.5), normal range of ≥ 9ng/dL, and the median value of BT was 22.0 ng/dL (Q1-Q3:8.1–41.5 ng/dL), normal range of ≥61ng/dL, as specified by normal reference range for each appropriate assay conducted by the Mayo Clinic Laboratory. Fifty-two patients (44%) had TT levels below 200 ng/dL, 100 patients (86%) with FT below the cutoff value of 9ng/dL, and 104 patients (89%) with BT below the lowest limit of 61ng/dL.
The median CRP was 21mg/dL (Q1-Q3:9.5–91.0 mg/L), reference range <13.00 mg/L when used to assess for an inflammatory response as specified by the Cleveland Clinic laboratory. Table 2 summarizes the associations among testosterone and other laboratory values. We found a significant correlation between decreased levels of TT, FT, and BT and elevated levels of CRP ([ρ −0.40; P< 0.001], [ρ −0.37; P< 0.001], [ρ −0.27; P< 0.001], respectively). TT was significantly associated with an increased white blood cell count (ρ −0.20; P =0.03), which was not associated with either FT or BT values.
Table 2.
Association Among Testosterone and Other Laboratory Values in Male Cancer Patients
| Laboratory Value | Total Testosterone (ng/dl; n=119) rho, (p-value) |
Free Testosterone (ng/dl; n=116) rho, (p-value) |
Bio-available Testosterone (ng/dl; n=118) rho, (p-value) |
|---|---|---|---|
| CRP (MG/L) | −0.40, (<0.001) | −0.37, (<0.001) | −0.27, (<0.001) |
| WBC (K/UL) | −0.20, (0.027) | −0.16, (0.086) | −0.04, (0.668) |
| Hemoglobin (gm/dl) | 0.35, (<0.001) | 0.32, (<0.001) | 0.33, (0.003) |
| Albumin (gm/dl) | 0.28, (0.002) | 0.31, (0.001) | 0.32, (<0.001) |
| 25OH Vitamin D level (ng/ml) | 0.13, (0.165)* | 0.10, (0.302) | 0.12, (0.212)** |
n=117,
n=116; CRP – high-sensitivity C-reactive protein; WBC – white blood cell count
There was a direct correlation between levels of TT, FT, and BT with hemoglobin and albumin levels which were significant, summarized in Table 2. Fifty (43%) male cancer patients vitamin D deficiency, 25-hyrdroxy vitamin D level < 20 ng/dL. No significant associations were noted between testosterone values and vitamin D levels.
Association among Testosterone, Symptom Burden, Weight Loss, and Opioid Use
Table 3 summarizes the association among TT, FT, and BT levels, symptom burden as measured by the ESAS, ECOG performance status, history of >5% weight loss, and opioid use in male patients with advanced cancer.
Table 3.
Association Among Testosterone, Symptom Burden, ECOG performance status, Weight Loss, and Opioid Use in Male Cancer Patients
| Laboratory Value | Total Testosterone (ng/dl; n=119) rho, (p-value) |
Free Testosterone (ng/dl; n=116) rho, (p-value) |
Bio-available Testosterone (ng/dl; n=118) rho, (p-value) |
|---|---|---|---|
| Pain | −0.15, (0.09) | −0.14, (0.14) | −0.07, (0.47) |
| Fatigue | −0.21, (0.02) | −0.24, (0.01) | −0.19, (0.04) |
| Nausea | −0.07, (0.46) | −0.13, (0.17) | −0.14, (0.14) |
| Depression | −0.07, (0.46) | −0.08, (0.42) | −0.06, (0.52) |
| Anxiety | −0.21, (0.02) | −0.19, (0.04) | −0.11, (0.26) |
| Drowsiness | −0.06, (0.55) | −0.13, (0.17) | −0.08, (0.36) |
| Appetite | −0.17, (0.07) | −0.13, (0.17) | −0.19, (0.05) |
| Well Being | −0.24, (0.01) | −0.20, (0.04) | −0.13, (0.17) |
| Dyspnea | −0.18, (0.05) | −0.21, (0.02) | −0.11, (0.25) |
| Sleep | 0.04, (0.64) | 0.09, (0.32) | 0.10, (0.26) |
| >5% weight loss/6 months | −0.27, (<0.001)* | −0.24, (<0.001)** | −0.23, (0.01)* |
| ECOG performance status | −0.20, (0.03)* | −0.25, (<0.001)** | −0.19, (0.05)* |
| Morphine Equivalent Daily Dosing (MEDD) | −0.36, (0.004) | −0.33, (0.005) | −0.27, (0.002) |
n=117;
n=115; ECOG – Eastern Cooperative Oncology Group
Both low TT and FT correlated with worsening fatigue ([ρ = −0.21;P ≤ 0.02], [ρ = −0.24;P=0.01]), respectively), increased anxiety ([ρ = −0.21;P ≤ 0.02], [ρ = −0.19;P=0.04]), decreased feeling of well-being ([ρ = −0.24;P ≤ 0.01], [ρ = −0.20;P=0.04]), and increased dyspnea ([ρ = −0.18;P=0.05], [ρ = −0.21;P=0.02]). BT had significant correlation with fatigue (ρ = −0.19;P=0.04), appetite (ρ = −0.19;P=0.045), weight loss history (ρ = −0.23;P=0.01), and ECOG performance status (ρ = −0.19;P=0.045) but was not significantly associated with symptoms of anxiety, feeling of well-being, or dyspnea, unlike TT and FT values.
Patients were divided into tertiles based on the following TT values (ng/dL): low ≤ 127.2,127.2< middle ≤ 331.8, and high >331.8; FT values (ng/dL): low ≤ 2.6, 2.6 < middle ≤ 6.38, and high > 6.38; and BT values (ng.dL): low ≤ 12, 12 < middle ≤ 33, and high >33. Male cancer patients with the lowest tertile for TT, FT, and BT values were all significantly associated with weight loss >5% over the past 6 months (p≤0.03). The lowest tertile of TT was significantly associated with increased anxiety (p=0.047) and poor sense of well-being (p=0.045). Male cancer patients with the lowest tertile of FT revealed a trend for worse fatigue (p=0.07). For patients with the lowest tertile of BT, a trend for worse fatigue (p=0.09) and poor appetite (p=0.08) was also noted.
TT, FT, and BT levels were all inversely associated with opioid use (morphine equivalent daily dosing (MEDD)) ([ρ = −0.36; P=0.004], [ρ = −0.33; P=0.005], [ρ = −0.27; P=0.002], respectively).
Multivariate Survival Analysis
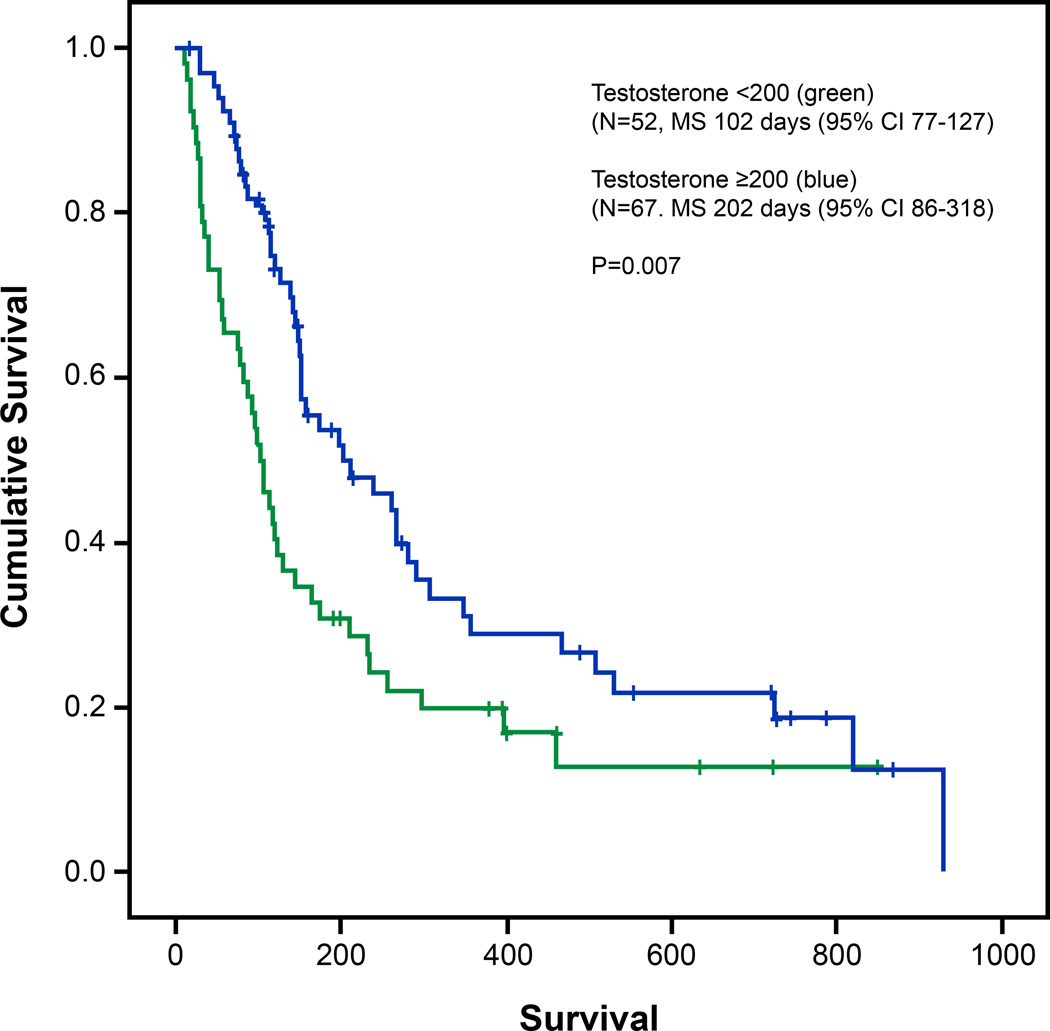
The median survival of our patient population was 151 days (95% CI; 116–186 days). Survival of male cancer patients with testosterone levels <200 mg/dL were significantly decreased compared to patients with levels ≥200 mg/dL, (102 vs 202 with p=0.007, Figure 1). For patients with the lowest tertile values for TT and BT, there was a significant association for worse survival, while only a trend was noted for FT values. Table 5 Of note, patients in the middle tertile of TT values had the best survival; however, for FT and BT, patient survival improved as values increased.
Fig. 1.
Survival analyses (Kaplan-Meier) with comparisons of curves by the log rank test. Survival of male patients with testosterone levels <200mg/dL (green) was decreased compared with patients with levels ≥200mg/dL (blue)
Table 5.
Total, Free, and Bioavailable Testosterone Association with Survival in Male Patients with Advanced Cancer
| Testosterone Test (ng/dL) |
Tertile1 Low Median Survival Days (Q1-Q3) |
Tertile2 Middle Median Survival Days (Q1-Q3) |
Tertile3 High Median Survival Days (Q1-Q3) |
p-value |
|---|---|---|---|---|
| Total Testosterone | 92 (66.3–117.7) | 240 (122.7–357.3) | 174 (112.0–235.0) | 0.002 |
| Free Testosterone | 95 (47.0–143.0) | 150 (11.00–189.0) | 210 (71.90–348.0) | 0.089 |
| Bio-available Testosterone | 86 (49.6–122.4) | 174 (71.50–276.5) | 280 (151.2–408.8) | 0.016 |
Variables that were significant with P-values <0.10 in univariate analysis included cancer diagnosis, ECOG performance status, TT, FT, BT, CRP, white blood cell count, hemoglobin, albumin and corrected calcium, while a patient’s age and race were not significant. For the multivariate analysis, the groups were dichotomized to the following: TT (≤200 vs. >200 ng/dL), FT (≤5 vs. >5 ng/dL), BT (≤40 vs. >40 ng/dL), CRP (≤22.6 vs. >22.6 mg/L), WBC count (≤6.1 vs. >6.1 K/UL), hemoglobin (≤11.3 vs. >11.3 g/dL), hypoalbuminemia (≤3.8 vs. >3.8 mg/dL) and corrected calcium (≤10.2 vs. >10.2 mg/dL). Decreased survival was associated with only low TT (HR 1.66; p=0.03), deteriorating ECOG performance status (HR 1.55; p=0.004), elevated CRP (HR 3.28; p<0.001), and decreased albumin (HR 2.52; p<0.001). Table 4.
Table 4.
Multivariate survival analysis
| HR | 95% CI | p-value | |
|---|---|---|---|
| ECOG Performance Status | 1.55 | 1.15–2.10 | 0.004 |
| Total Testosterone ≤ 200 (ng/dL) | 1.66 | 1.04–2.64 | 0.034 |
| CRP > 22.6 (mg/L) | 3.28 | 1.87–5.74 | <0.001 |
| Albumin ≤ 3.8 (mg/dL) | 2.52 | 1.54–4.11 | <0.001 |
ECOG – Eastern Cooperative Oncology Group; CRP – High-sensitivity C-reactive protein
Secondary to the heterogenous patient population, gastrointestinal, head and neck, and lung cancer patients were also analyzed separately. In 48 male patients with gastrointestinal cancer, patients with low TT ≤ 200ng/dL had median survival of 80 days (95% CI 31–105) versus 157 days (95% CI 139–266), p=0.004. In 30 lung cancer patients, a median survival of 115 days (95% CI 58–231) for hypogonadic men versus 508 days (95% CI 72–930) for patients with TT >200ng/dL, p=0.03. In 18 patients with head and neck malignancies, the median survival for hypogonadic males was 144 days (95% CI 12-∞) versus 240 days (86-∞) which was not significant, p=0.27, most likely secondary to the small sample size.
Relationship between Free and Total Testosterone
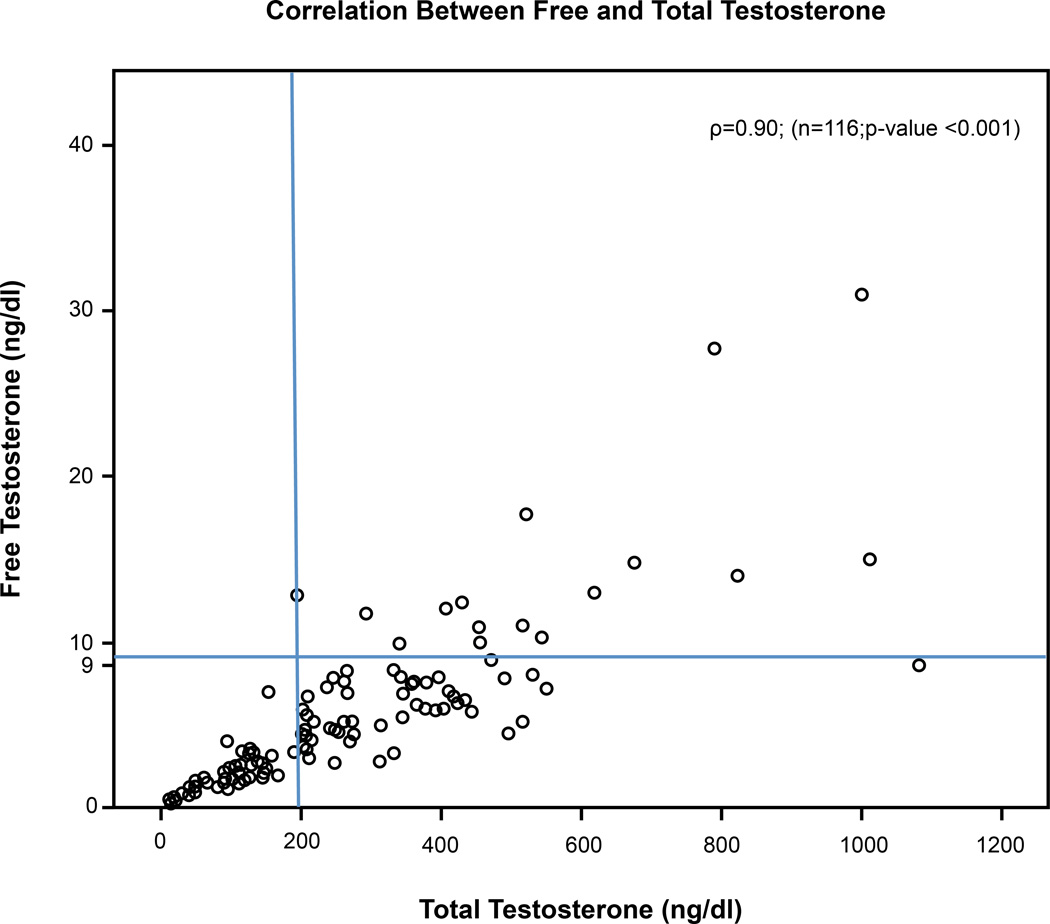
Figure 2 plots FT versus TT (in ng/mL); normal FT is ≥9 ng/mL and normal TT is ≥200 ng/dL. TT, when compared with FT, was noted to be 49% sensitive and 94% specific for determining hypogonadism in male patients with advanced cancer. A value of TT < 200 ng/dL had a positive predictive value (PPV) of 98%, negative predictive value (NPV) of 23%, and accuracy of 55%. A receiver operating characteristic (ROC) curve developed using the methodology of Mithat Gonen resulted in an area under the curve (AUC) of only 0.6 with no indication of the best cutoff value associated with increased symptoms or worse survival.
Fig. 2.
Normal values for tree testosterone (≥9ng/dL); Normal values for total testosterone (≥200ng/dL)
Discussion
Various lab criteria have been used to define hypogonadism. [14–16] For young male patients, studies have used 2.5 standard deviations below the mean TT values of about 319 ng/dL as the cutoff point for initiation of therapy. The American Association of Clinical Endocrinologists define the cutoff value for low TT between 200–400 ng/dL, and patients with symptoms are candidates for replacement therapy but no specific recommendations have been established. [17]
Our study confirms the high frequency of hypogonadism in male cancer patients who were seen in consultation for symptoms of cancer-related fatigue and/or anorexia-cachexia. Roughly half, 44%, of male cancer patients were hypogonadal based on a strict criteria for hypogonadism of TT < 200ng/dL, and a higher frequency of patients, 86%, were noted to be hypogonadal when using FT value with a cutoff value below 9ng/dL and 89% of patients when BT level below 61ng/dL.
In a previous study by our group, we reported a high frequency of hypogonadism (74%) based on TT levels in male cancer patients with cachexia, [8] and slightly lower frequency (64%) based on measurements of FT, [1] compared to the current study. Other groups have recently reported hypogonadism, based on calculated BT <70ng/dl, to be more common in cancer patients with cachexia, 73%, than in cancer patients without weight loss, 53%, and non-cancer control group, 45%. [18] The variation in frequency of hypogonadism may be due to differences in patient population being studied, the assay used to measure TT, FT, BT (or the use of calculated BT), and the variability in the lower limit of testosterone which is used by researchers to define hypogonadism.
Testosterone is present in two forms in the blood, a “free” (1–4%) unbound state or bound, the majority ~98%, either tightly (60%) to sex hormone binding globulin (SHBG, also known as sex steroid binding globulin (SSBG)) or loosely to albumin (38%). BT is the sum of FT plus testosterone loosely bound to albumin which is able to readily enter cells and considered to be biologically active. Testosterone bound to SHBG is considered inactive. SHBG is often altered in illness and, in patients with cancer, is increased resulting in falsely elevated levels of TT. [2, 6] Unlike our previous studies, we measured not only serum TT, but also FT and BT levels. As compared to FT, TT had high specificity but moderate sensitivity and may not be adequate by itself to screen for hypogonadism in male patients with cancer. For male cancer patients with a TT < 200 ng/dL, the PPV was high confirming hypogonadism; however, for TT ≥ 200, the NPV was not adequate to rule out gonadal dysfunction. In addition, future studies with a larger sample size are needed to determine a ROC curve for testosterone values to determine appropriate threshold for increased symptom burden and survival outcomes.
Both serum TT and FT were associated with clinical symptoms including fatigue, anxiety, decreased well-being, and dyspnea. BT was also significantly associated with symptom of fatigue and uniquely with poor appetite but not anxiety, sense of well-being, or dyspnea. Previous studies by our group have reported a significant association among low testosterone in male cancer patients with symptoms such as increased fatigue, poor libido, depression, and insomnia [1, 8]. In a recent study, TT was significantly associated with clinical symptom of poor libido while the calculated BT did not show a similar association, and the authors argue to test for both TT and cBT. [18] In our study, both TT and FT had similar correlations with clinical symptom burden while the association among BT and clinical symptoms of anxiety, sense of well-being, and dyspnea were less significant and more research is needed to determine the best clinically meaningful test to determine gonadal dysfunction in male patients with cancer.
Similar to previous studies, the use of opioids had a direct correlation with the frequency of hypogonadism in our patient population. In addition, we have previously reported that in 61 advanced male patients who had been tested for 25 hydroxy vitamin D and BT, there was a trend for positive association for vitamin D and testosterone. [19] In the current study with a larger sample size, no significant association among TT, FT, or BT with vitamin D was noted.
TT, FT, and BT values were all inversely correlated with serum hs-CRP, weight loss, and directly with performance status. In addition, an exploratory multivariate analysis, only TT, and not FT or BT, was associated independently with poor prognosis along with elevated CRP, poor ECOG performance status, and decreased albumin. Other groups have also shown low testosterone associated with signs of chronic inflammation, weight loss, and poor performance status. [18] Low testosterone has been reported to be associated with all-cause mortality in men with type 2 diabetes, [20] increased cardiovascular mortality, [21] and poor survival in elderly males. [22] To our knowledge, this is the first study to confirm that low testosterone in advanced cancer patients is associated with poor survival. When patients were divided into tertiles based on TT, FT, and BT values, a significant associated with poor survival was noted with TT and BT values, while patients with FT only a trend for poor prognosis was noted. In the multivariate analysis, only TT was significant for worse survival in male cancer patients. It is plausible that TT is a better marker for overall gonadal function and more sensitive to suppression by chronic inflammation or cytokine mediated immune response. Since these findings are preliminary, future studies with a larger sample size are needed.
Inflammatory cytokines may lead to endocrine dysfunction which can contribute to symptoms of fatigue, loss of weight, and poor quality of life. The eugonadal sick syndrome hypothesizes that gonadal dysfunction is a normal response to illness. In the short term, gonadal dysfunction may provide a survival advantage by diverting energy to fight off infection or repair a wound. However, in chronic illness such as cancer, gonadal dysfunction resulting in low testosterone may result in a symptom burden which diminishes the quality of life of patients over a sustained period of time. Whether or not gonadal dysfunction is an epiphenomenon associated with cancer patients being critically and chronically ill or if hypogonadism directly contributes to the symptom burden experienced by male cancer patients is still unclear.
Clinical practice guidelines by the Endocrine Society address replacement of testosterone in chronic illnesses such as HIV but not in patients with cancer. [17] A preliminary study conducted by our group examining the benefits of testosterone replacement therapy with the primary outcome of fatigue in hypogonadic male cancer patients remained inconclusive but did show a trend towards improved sexual desire, significant improvements in performance status, and fatigue (FACIT-Fatigue subscale) with prolonged use, ≥72 days. [23] A limitation of the study included the small sample size and the modest increase in serum levels of testosterone after replacement. Other studies examining testosterone replacement in non-cancer patients have reported improvements in fatigue and quality of life. [24]
Further research is required in order to determine the effectiveness of testosterone replacement in hypogonadal men with advanced cancer. In male patients with advanced cancer and a poor prognosis, clinical studies are needed examining the symptomatic benefits of testosterone replacement on outcomes including libido, fatigue, and quality of life. In cancer patients with a prognosis of greater than 6 months, studies examining the benefits of testosterone replacement on lean body mass, bone health, need for blood transfusions, performance status, and overall survival are needed with close monitoring for potential complications.
A limitation of our current study is the lack of assessments for sexual concerns or decreased libido. Diminished libido has been previously observed in male cancer patients with low testosterone [1] and is noted to be a clinical symptom that is understudied and undertreated in patients with advanced cancer. [17] Screening for sexual concerns in advanced cancer patients should be integrated into clinical practice. Also, other symptoms unique to deficiency of testosterone include the loss of mental acuity and menopausal-type hot flushes, which often occurs during acute onset of hypogonadism, may have not be captured without routine screening.
In addition to limitations in comprehensive symptom assessment, diurnal fluctuations in testosterone posed a challenge for accurately determining hypogonadism, since levels peak at 8am and reach a nadir 12 hours later. [25] Patients were instructed to obtain laboratory values between 7am–11am. If patients had altered sleep patterns, patient were instructed to obtain laboratory work-up within 2 hour upon awakening. Non-compliance with instructions in chronically-ill patients may be a factor resulting in testosterone values which are falsely low. Also, prior to testosterone replacement, it is advised to repeat testing to confirm hypogonadism in clinical practice. [17]
Conclusion
The frequency of hypogonadism in male patients with advanced cancer was high and associated with symptoms of fatigue, anxiety, and worse sense of well-being. Systemic inflammation as measured by CRP, history of weight loss, and the use of strong opioids were significantly associated with decreased testosterone in male cancer patients. Low testosterone is associated with a decreased survival independent of CRP, albumin, and performance status. Whether or not inadequate gonadal function is an epiphenomenon associated with critical illness or a potentially treatable complication of cancer, or its treatment, is unclear and more research is needed.
Acknowledgments
Eduardo Bruera is supported in part by a National Institutes of Health grant #s RO1NR010162-01A1, RO1CA122292-01, RO1CA124481-01, and the MD Anderson Cancer center support grant #CA016672. Egidio Del Fabbro is supported in part by an American Cancer Society grant# PEP-08-299-01-PC1
Footnotes
Funding Sources for Manuscript: None
References
- 1.Strasser F, Palmer JL, Schover LR, et al. The impact of hypogonadism and autonomic dysfunction on fatigue, emotional function, and sexual desire in male patients with advanced cancer: a pilot study. Cancer. 2006;107:2949–2957. doi: 10.1002/cncr.22339. [DOI] [PubMed] [Google Scholar]
- 2.Burney BO, Hayes TG, Smiechowska J, et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97(5):E700–E709. doi: 10.1210/jc.2011-2387. [DOI] [PubMed] [Google Scholar]
- 3.Chlebowski RT, Heber D. Hypogonadism in male patients with metastatic cancer prior to chemotherapy. Cancer Res. 1982;42(56):2495–2498. [PubMed] [Google Scholar]
- 4.Gerl A, Muhlbayer D, Hansmann G, Mraz W, Hiddemann W. The impact of chemotherapy on Ledydig cell function in long term survivors of germ cell tumors. Cancer. 2001;91(7):1297–1303. doi: 10.1002/1097-0142(20010401)91:7<1297::aid-cncr1132>3.0.co;2-z. [DOI] [PubMed] [Google Scholar]
- 5.Dev R, Del Fabbro E, Bruera E. Association between megestrol acetate treatment and symptomatic adrenal insufficiency with hypogonadism in male patients with cancer. 2007;110(6):1173–1177. doi: 10.1002/cncr.22924. [DOI] [PubMed] [Google Scholar]
- 6.Garcia JM, Li H, Mann D, Epner D, et al. Hypogonadism in male patients with cancer. Cancer. 2006;106(12):2583–2591. doi: 10.1002/cncr.21889. [DOI] [PubMed] [Google Scholar]
- 7.Rajagopal A, Vassilopoulou-Sellin R, Palmer JL, Kaur G, Bruera E. Symptomatic hypogonadism in male survivors of cancer with chronic exposure to opioids. Cancer. 2004;100(4):851–858. doi: 10.1002/cncr.20028. [DOI] [PubMed] [Google Scholar]
- 8.Del Fabbro E, Hui D, Nooruddin ZI, et al. Associations among hypogonadism, C-reactive protein, symptom burden, and survival in male cancer patients with cachexia: a preliminary report. J Pain Symptom Manage. 2010;39(6):1016–1024. doi: 10.1016/j.jpainsymman.2009.09.021. [DOI] [PubMed] [Google Scholar]
- 9.Bruera e, Kuehn N, Miller MJ, Selmser P, Macmillan K. The Edmonton Symptom Assessment System (ESAS): a simple method for the assessment of palliative care patients. J Palliat Care. 1991;7(2):6–9. [PubMed] [Google Scholar]
- 10.Wang C, Catlin DH, Demers LM, Starcevic B, Swerdloff RS. Measurement of total testosterone in adult men: comparison of current laboratory methods versus liquid chromatograph-tandem mass spectrometry. J Clin Endocrinol Metab. 2004;89(2):534–543. doi: 10.1210/jc.2003-031287. [DOI] [PubMed] [Google Scholar]
- 11.Vermeulen A, Stoica T, Verdonck L. The apparent free testosterone concentration, an index of androgenicity. J Clin Endocrinol Metab. 1971;33(5):759–767. doi: 10.1210/jcem-33-5-759. [DOI] [PubMed] [Google Scholar]
- 12.Bammann BL, Coulam C, Jiang NS. Total and free testosterone during pregnancy. Am J Obstet Gynecol. 1980;137(3):293–298. doi: 10.1016/0002-9378(80)90912-6. [DOI] [PubMed] [Google Scholar]
- 13.Wheeler MJ. The determination of bio-available testosterone. Ann Clin Biochem. 1995;32(Pt 4):345–357. doi: 10.1177/000456329503200401. [DOI] [PubMed] [Google Scholar]
- 14.Vermeulen A. Androgen replacement therapy in the aging male-a critical evaluation. J Clin Endocrinol Metab. 2001;86(6):2380–2390. doi: 10.1210/jcem.86.6.7630. [DOI] [PubMed] [Google Scholar]
- 15.Harman SM, Metter EJ, Tobin JD, Pearson J, Blackman MR. Longitudinal effects of aging on serum total and free testosterone levels in healthy men: Baltimore Longitudinal Study of Aging. J Clin Endocrinol Metabl. 2001;86(2):724–731. doi: 10.1210/jcem.86.2.7219. [DOI] [PubMed] [Google Scholar]
- 16.Mulligan T, Frick MF, Zuraw QC, et al. Prevalence of hypogonadism in males at least 45 years: The HIM study. Int J Clin Pract. 2006;60(7):762–769. doi: 10.1111/j.1742-1241.2006.00992.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.American Association of Clinical Endocrinologists: medical guidelines for clinical practice for the evaluation and treatment of hypogonadism in adult male patients: 2002 update. Endcor Pract. 2002;8(6):440–456. [PubMed] [Google Scholar]
- 18.Burney BO, Hayes TG, Smiechowska J, et al. Low testosterone levels and increased inflammatory markers in patients with cancer and relationship with cachexia. J Clin Endocrinol Metab. 2012;97(5):E700–E709. doi: 10.1210/jc.2011-2387. [DOI] [PubMed] [Google Scholar]
- 19.Dev R, Del Fabbro E, Schwartz GG, et al. Preliminary report: vitamin D deficiency in advanced cancer patients with symptoms of fatigue or anorexia. Oncologist. 2011;16(11):1637–1641. doi: 10.1634/theoncologist.2011-0151. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Muraleedharan V, Marswh H, Kapoor D, Channer KS, Jones TH. Testosterone deficiency is associated with increased risk of mortality and testosterone replacement improves survival in men with type 2 diabetes. Eur J Endocrinol. 2013;169(6):725–733. doi: 10.1530/EJE-13-0321. [DOI] [PubMed] [Google Scholar]
- 21.Corona G, Rastrelli G, Monami M, et al. Hypogonadism as a risk factor for cardiovascular mortality in men: a meta-analytic study. Eur J Endocrinol. 2011;165(5):687–701. doi: 10.1530/EJE-11-0447. [DOI] [PubMed] [Google Scholar]
- 22.Tivesten A, Vandenput L, Labrie F, et al. Low serum testosterone and estradiol predict mortality in elderly men. J Clin Endocrinol Metab. 2009;94(7):2482–2488. doi: 10.1210/jc.2008-2650. [DOI] [PubMed] [Google Scholar]
- 23.Del Fabbro E, Garcia JM, Dev R, et al. Testosterone replacement for fatigue in hypogonadal ambulatory males with advanced cancer: a preliminary double-blind placebo-controlled trial. Support Care Cancer. 2013;21(9):2599–2607. doi: 10.1007/s00520-013-1832-5. [DOI] [PubMed] [Google Scholar]
- 24.Kapoor D, Clarke S, Stanworth R, Channer KS, Jones TH. The effect of testosterone replacement therapy on adipocytokines and C-reactive protein in hypogonadal men with type 2 diabetes. Eur J Endocrinol. 2007;156(5):595–602. doi: 10.1530/EJE-06-0737. [DOI] [PubMed] [Google Scholar]
- 25.Bremmer WJ, Vitiello MV, Prinz PN. Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab. 1983;56(6):1278–1281. doi: 10.1210/jcem-56-6-1278. [DOI] [PubMed] [Google Scholar]