Abstract
Objective: The study aimed to evaluate the ability of optical coherence tomography (OCT) to guide and identify pulp exposure using an erbium: yttrium–aluminum–garnet (Er:YAG) laser. Background data: The Er:YAG laser has been proven to be effective in ablating dental hard tissue and offers advantages, as there is none of the vibration and noise you get with conventional methods, but it has limitations in relation to the tactile feedback that would aid in identification of entry into the pulp chamber. Based on depth-resolved optical reflectivity, OCT technology has been developed to provide high-resolution, cross-sectional images of the internal structure of biological tissues. Materials and methods: The pulp chambers of 20 human mandibular incisors were examined, and the average thickness of hard tissue covering the pulp chamber was assessed using micro-computed tomography (micro-CT) images. An Er:YAG laser was used to gradually penetrate the hard tissue over the pulp chamber under microscopic guidance. The preparation was constantly imaged using a swept-source OCT at 10 sec intervals until a pulp chamber exposure was identified using the technology. The pulp exposure was re-examined under the microscope and compared with micro-CT images for verification. Results: The pulp exposures of 20 incisors were all verified microscopically and with micro-CT images. The thickness of hard tissue penetrated by the laser ranged from 0.44 to 1.69 mm. Conclusions: Swept-source OCT is a useful tool for identifying pulp exposure during access opening with the Er: YAG laser.
Introduction
Gaining access to the pulp chamber is the first step toward achieving a proper pathway to the root canal system during nonsurgical root canal treatment.1
In general, high-speed handpieces are widely used to acess to the pulp chamber. However, the vibration and noise associated with the cutting of dental hard tissue cause discomfort and anxiety in both pediatric and adult patients.2–5 Several types of lasers have been investigated as alternatives to mechanical preparation of dental hard structures. The erbium: yttrium–aluminum–garnet (Er:YAG) laser emits light of a wavelength (2940 nm) that is strongly absorbed by water, resulting in rapid and expansive vaporization of water contained in dental hard tissues. In the study of Dommisch et al.,6 the Er:YAG laser was found to perform carious tissue removal with effectiveness equal to that of rotary instruments. Mazeki et al.7 found that it was useful to use an Er:YAG laser to prepare root canal orifices. These findings have promoted the application of the Er:YAG laser in creating access to the pulp chamber. Er:YAG lasers employ a delicate probe that involves none of the vibration and noise of the conventional methods.8 It has the potential to provide a useful operative field when used with or without water spray under the dental microscope.
Because a laser does not provide operators with the tactile feedback of a rotary instrument when it cuts dental hard tissue,6,8,9 visualization of the tooth surface during laser ablation would aid in identification of entry into the pulp chamber. Traditionally, radiography has been used to image the shape and position of the pulp space encompassed by hard tissue, but it only provides two-dimensional (2D) images of three-dimensional (3D) objects. Supplementary and angled intraoral radiographs are often required, particularly in the case of teeth with inclination and rotation, or with a narrow pulp cavity produced by secondary and tertiary dentin, which can be the result of aging, pathology, and occlusion. These situations increase the ionizing radiation exposure to patients, which is of special concern to pediatric and pregnant patients.10–13
Optical coherence tomography (OCT) is a new noninvasive imaging technique that enables cross-sectional imaging of biological structures at a submicron scale. Based on the principle of low-coherence interferometry, OCT uses a near-infrared light source to scan the area of interest, and processes the scattered and reflected or transmitted signals, including depth-resolved information from within the specimen, to create 2D or 3D high-resolution images.10,14 In dentistry, Baumgartner et al. has demonstrated OCT images of sound and decayed dentin. Fonseca et al.15 and Braz et al.16 visualized the pulp space under different thicknesses of dentin and pulp-dentin complex. Bakhsh et al.10 successfully defined resin–dentin interface gaps on OCT images.
One subtype of Fourier domain OCT, sweep-source OCT (SS-OCT), offers improved imaging speed compared with earlier OCT systems.10,14 The aim of this study was to evaluate the ability of OCT to guide and identify pulp exposure using an Er: YAG laser.
Materials and Methods
The study was approved by the Ethics Committee of Tokyo Medical and Dental University (No. 923).
Twenty extracted human mandibular incisors without caries or prior root canal treatment were selected from the tooth pool of the Section of Pulp Biology and Endodontics, Tokyo Medical and Dental University Hospital. After any calculus was removed, all teeth were stored in distilled water containing a few crystals of thymol at room temperature.
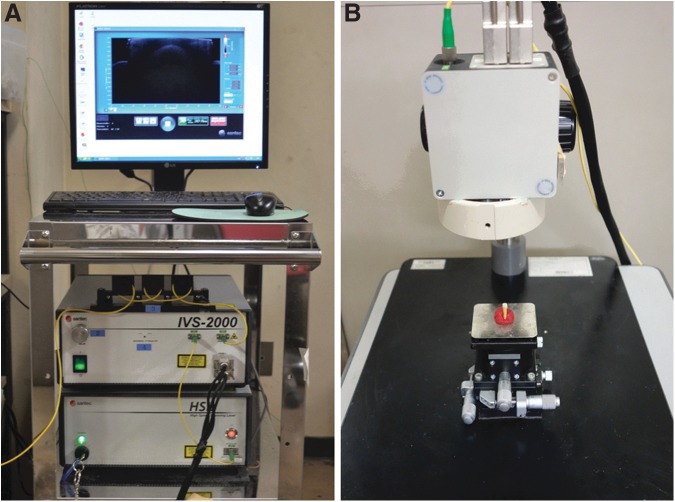
The SS-OCT (OCT-2000®, Santec, Komaki, Japan) (Fig. 1) used in this study applies a laser light source that scans at a 20 kHz sweep rate across the wavelengths from 1260 to 1360 nm, with the central wavelength at 1319 nm. The light power is<10 mW, within the limits of the American National Standard Institute. The axial resolution of the SS-OCT system is 11 μm in air and 7 μm in biological tissue, assuming a refractive index of ∼1.5. A lateral resolution of 17 μm is determined by the objective lens in the probe. The light source is projected onto the sample's surface and scanned across the area of interest in two dimensions. Backscattered light from the sample is analyzed using a Fourier transform algorithm to reveal the depth information of the sample. A series of 2D scan sections can be used to generate a 240×240×400 pixel (4×4×3.2 mm) 3D image within 4 sec.
FIG. 1.
(A) Optical coherence tomography (OCT) system including Mach–Zehnder interferometer, laser source, data processing computer, and image display. (B) Sample stage with manual adjustment unit and laser projecting arm.
Each extracted tooth was scanned with micro-computed tomography (micro-CT) (inspeXio SMX100CT®, Shimadzu, Kyoto, Japan), to calculate the distance between the lingual surface of the tooth and the pulp chamber (Fig. 2A). Subsequently, layers of the hard tissue on the lingual surface were ground off to make the pulp chamber visible on the SS-OCT images. The tooth was then scanned again with micro-CT. The intended entry point of the access to the pulp chamber was marked on the lingual surface where the residual hard tissue was the thinnest, and the corresponding thickness was calculated (Fig. 2B). All the measurements were repeated three times and the average value was recorded.
FIG. 2.
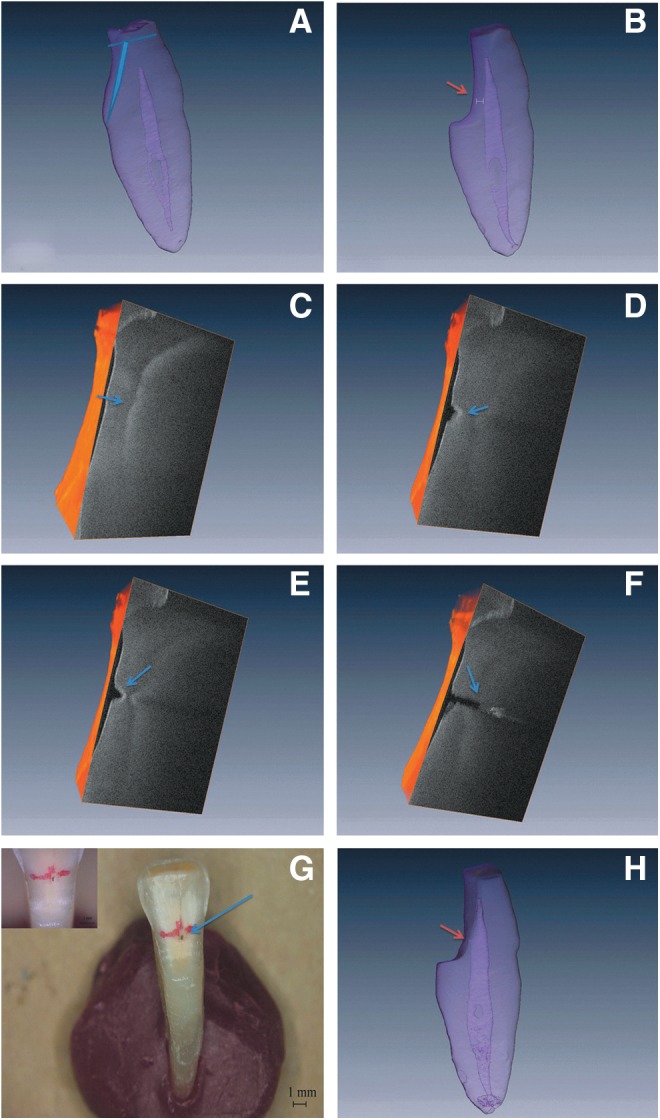
(A) The blue lines indicate the removal of enamel and part of the dentin on the lingual surface of the tooth based on measurements using micro-computed tomography-three dimensional (micro-CT 3D) images. (B) Entry point for the access cavity (orange arrow) where the SS-OCT assessments were performed. The thickness of residual dentin (white double arrow) was determined on micro-CT 3D images. (C) Cross-sectional contour of the intact pulp chamber on the OCT 3D image (blue arrow). (D and E) Cross-sectional appearance of the developing laser burst crater visualized with OCT 3D images (blue arrow). (F) Cross-sectional appearance of pulp exposure on the OCT 3D image (blue arrow). (G) Pulp exposure confirmed under the microscope (blue arrow). (H) Pulp exposure (orange arrow) was confirmed on the micro-CT 3D image.
An Er:YAG laser system (Erwin advErl, J. Morita MFG, Kyoto, Japan) with a flat contact probe tip (ϕ=400 mm; C400F, J. Morita MFG, Kyoto, Japan) was used to prepare the access cavities under microscopic guidance (OPMI 99; Carl Zeiss, Jena, Germany). During the preparation, laser energy was transmitted through the laser tip placed perpendicular to the marked entrance site at a rate of 100 mJ, 10 pps with water spray at 5 mL/min for a 10 sec duration. After each 10 sec treatment, SS-OCT was used immediately to scan the laser crater with the light beam projected at ∼90 degrees to the lingual surface of the tooth. The treatments were repeated until pulp exposure was revealed on an SS-OCT image. The pulp exposure was verified using a #10 K file (Zipperer, VDW GmbH, Munich, Germany) under a microscope (×12), and also with micro-CT.
Imaging software (Amira5.3®, Visage Imaging, Richmond, Austria) was used to evaluate the data obtained from the micro-CT and SS-OCT.
Results
The SS-OCT images showed different gray scale levels clearly distinguishing the pulp chamber from the dental hard tissue (Fig. 2C–F). The pulp chamber surface featured a convex contour with a lighter gray level (higher scattered intensity), and the hard tissue appeared darker (lower scattered intensity) (Fig. 2C). Figures 2D and E show gradual eradication of the hard tissue, forming a narrow crater, and Fig. 2F shows pulp exposure, which was confirmed visually and by micro-CT (Fig. 2G and H).
The thickness of the hard tissue penetrated by the laser ranged from 0.44 to 1.69 mm, with an average of 0.83mm.
Discussion
In this study, a laser energy of 100 mJ, 10 pps was used, based on prior studies.8 To control the formation of the laser crater for evaluation of the ability of the SS-OCT system to visualize the process, the laser irradiation was performed under microscopic guidance and each irradiation was set at 10 sec. As shown in Fig. 2C, the intact pulp chamber was distinctly visualized on the SS-OCT image, which is in agreement with the findings of previous studies.15,16 A series of SS-OCT images depicting the depth increments of the developing laser crater and its position relative to the pulp chamber is shown in Fig. 2D and E. Figure 2F shows an image of the breached convex surface of the hard tissue, which was verified visually and with 3D micro-CT as a pulp exposure (Fig. 2G and H). These results showed that the SS-OCT system was able to provide accurate guidance for the laser preparation of an access cavity.
Because laser irradiation is reported to sometimes cause submicroscopic pulp exposure,17 our results suggest that the SS-OCT technique might be also useful to guard against accidental pulp exposure in laser removal of deep carious tissue. As the SS-OCT system uses a tuned laser light source projected through a handheld probe, it may be feasible to integrate an Er:YAG laser device with an SS-OCT system in conjunction with a dental microscope, to achieve sophisticated optical treatments in the future.
Although the SS-OCT system provides micron-resolution images of structures below the tissue surface, the shallow penetration depth is a limit of this technique. The maximum imaging depth is approximately 2–3 mm in most tissues.18 In this in vitro study, layers of hard tissue on the lingual surface of the teeth were removed to visualize the pulp chamber well, and the thickness of residual hard tissue varied from 0.44 to 1.69 mm. Although this depth is shallow compared with other dental imaging techniques, including cone beam computed tomography, the technique has unique advantages of providing almost real-time images with fine resolution.
Conclusions
Under the limitation of current study, the SS-OCT system was demonstrated to be an effective tool to indicate pulp exposure during laser access cavity preparation. A combined application of Er:YAG laser and SS-OCT guidance will be a promising clinical technique.
Acknowledgments
This work was supported by a Research Grant for Longevity Sciences (23-20) from the Ministry of Health, Labour and Welfare and a Grant-in-Aid for Scientific Research (B) (No. 25293385-00) from the Japan Society for the Promotion of Science.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Vertucci F.J., and Haddix J.E. (2011). Tooth morphology and access cavity preparation, in: Pathways of the Pulp. Cohen S., Hargreaves K. M. (eds.) St. Louis: Elsevier Mosby, pp. 136–137 [Google Scholar]
- 2.De Moor R.J., and Delme K.I. (2009). Laser-assisted cavity preparation and adhesion to erbium-lased tooth structure: part 1. Laser-assisted cavity preparation. J. Adhes. Dent. 11, 427–438 [DOI] [PubMed] [Google Scholar]
- 3.Delme K., Meire M., De Bruyne M., Nammour S., and De Moor R. (2009). Cavity preparation using an Er:YAG laser in the adult dentition [in French]. Rev. Belge. Med. Dent. (1984). 64, 71–80 [PubMed] [Google Scholar]
- 4.Keller U., Hibst R., Geurtsen W., et al. (1998). Erbium:YAG laser application in caries therapy. Evaluation of patient perception and acceptance. J. Dent. 26, 649–656 [DOI] [PubMed] [Google Scholar]
- 5.Liu J.F., Lai Y.L., Shu W.Y., and Lee S.Y. (2006). Acceptance and efficiency of Er:YAG laser for cavity preparation in children. Photomed. Laser Surg. 24, 489–493 [DOI] [PubMed] [Google Scholar]
- 6.Dommisch H., Peus K., Kneist S., et al. (2008). Fluorescence-controlled Er:YAG laser for caries removal in permanent teeth: a randomized clinical trial. Eur. J. Oral Sci. 116, 170–176 [DOI] [PubMed] [Google Scholar]
- 7.Mazeki K., Kimura Y., Yokoyama K., and Matsumoto K. (2003). Preparation of root canal orifices by Er:YAG laser irradiation: in vitro and clinical observations. J. Clin. Laser Med. Surg. 21, 85–91 [DOI] [PubMed] [Google Scholar]
- 8.Aoki A., Ishikawa I., Yamada T., et al. (1998). Comparison between Er:YAG laser and conventional technique for root caries treatment in vitro. J. Dent. Res. 77, 1404–1414 [DOI] [PubMed] [Google Scholar]
- 9.Schwass D.R., Leichter J.W., Purton D.G., and Swain M.V. (2012). Evaluating the efficiency of caries removal using an Er:YAG laser driven by fluorescence feedback control. Arch. Oral Biol. 58, 603–610 [DOI] [PubMed] [Google Scholar]
- 10.Bakhsh T.A., Sadr A., Shimada Y., Tagami J., and Sumi Y. (2011). Non-invasive quantification of resin-dentin interfacial gaps using optical coherence tomography: validation against confocal microscopy. Dent. Mater. 27, 915–925 [DOI] [PubMed] [Google Scholar]
- 11.Neelakantan P., Subbarao C., and Subbarao C.V. (2010). Comparative evaluation of modified canal staining and clearing technique, cone-beam computed tomography, peripheral quantitative computed tomography, spiral computed tomography, and plain and contrast medium-enhanced digital radiography in studying root canal morphology. J. Endod. 36, 1547–1551 [DOI] [PubMed] [Google Scholar]
- 12.Patel S. (2009). New dimensions in endodontic imaging: Part 2. Cone beam computed tomography. Int. Endod. J. 42, 463–475 [DOI] [PubMed] [Google Scholar]
- 13.Vertucci F.J. (2005). Root canal morphology and its relationship to endodontic procedures. Endod. Topics 10, 3–29 [Google Scholar]
- 14.Hariri I., Sadr A., Shimada Y., Tagami J., and Sumi Y. (2012). Effects of structural orientation of enamel and dentine on light attenuation and local refractive index: an optical coherence tomography study. J. Dent. 40, 387–396 [DOI] [PubMed] [Google Scholar]
- 15.Fonseca D.D., Kyotoku B.B., Maia A.M., and Gomes A.S. (2009). In vitro imaging of remaining dentin and pulp chamber by optical coherence tomography: comparison between 850 and 1280 nm. J. Biomed. Opt. 14, 024009. [DOI] [PubMed] [Google Scholar]
- 16.Braz A.K., Kyotoku B.B., and Gomes A.S. (2009). In vitro tomographic image of human pulp-dentin complex: optical coherence tomography and histology. J. Endod. 35, 1218–1221 [DOI] [PubMed] [Google Scholar]
- 17.Giachetti L., Scaminaci Russo D., Scarpelli F., and Vitale M. (2004). SEM analysis of dentin treated with the Er:YAG laser: a pilot study of the consequences resulting from laser use on adhesion mechanisms. J. Clin. Laser Med. Surg. 22, 35–41 [DOI] [PubMed] [Google Scholar]
- 18.Fujimoto J.G. (2003). Optical coherence tomography for ultrahigh resolution in vivo imaging. Nat. Biotechnol. 21, 1361–1367 [DOI] [PubMed] [Google Scholar]