Abstract
There is increasing evidence that ischemic brain injury is modulated by peripheral signaling. Peripheral organ ischemia can induce brain inflammation and injury. We therefore hypothesized that brain injury sustained after cardiac arrest (CA) is influenced by peripheral organ ischemia and that peripheral organ protection can reduce brain injury after CA and cardiopulmonary resuscitation (CPR). Male C57Bl/6 mice were subjected to CA/CPR. Brain temperature was maintained at 37.5°C±0.0°C in all animals. Body temperature was maintained at 35.1°C±0.1°C (normothermia) or 28.8°C±1.5°C (extracranial hypothermia [ExHy]) during CA. Body temperature after resuscitation was maintained at 35°C in all animals. Behavioral testing was performed at 1, 3, 5, and 7 days after CA/CPR. Either 3 or 7 days after CA/CPR, blood was analyzed for serum urea nitrogen, creatinine, alanine aminotransferase, aspartate aminotransferase, and interleukin-1β; mice were euthanized; and brains were sectioned. CA/CPR caused peripheral organ and brain injury. ExHy animals experienced transient reduction in brain temperature after resuscitation (2.1°C±0.5°C for 4 minutes). Surprisingly, ExHy did not change peripheral organ damage. In contrast, hippocampal injury was reduced at 3 days after CA/CPR in ExHy animals (22.4%±6.2% vs. 45.7%±9.1%, p=0.04, n=15/group). This study has two main findings. Hypothermia limited to CA does not reduce peripheral organ injury. This unexpected finding suggests that after brief ischemia, such as during CA/CPR, signaling or events after reperfusion may be more injurious than those during the ischemic period. Second, peripheral organ hypothermia during CA reduces hippocampal injury independent of peripheral organ protection. While it is possible that this protection is due to subtle differences in brain temperature during early reperfusion, we speculate that additional mechanisms may be involved. Our findings add to the growing understanding of brain-body cross-talk by suggesting that peripheral interventions can protect the brain even if peripheral organ injury is not altered.
Introduction
Cardiac arrest (CA) is the cause of nearly 500,000 deaths/year in the United States alone (Zheng et al., 2001). Decades of research have led to a single significant post-resuscitation therapy, therapeutic hypothermia (TH), which improves neurologic outcome and the implementation of which appears to be improving meaningful survival after CA (Bernard et al., 2002; Hypothermia After Cardiac Arrest Study Group et al., 2002; Girotra et al., 2012). Although there is extensive evidence that hypothermia is effective in reducing injury after CA and other ischemic insults in animal models (Leonov et al., 1990), the mechanism(s) by which hypothermia interrupts the process is complex and incompletely understood (Yenari and Han, 2012). Unfortunately, since TH can be difficult to implement it may be underutilized (Laver et al., 2006; Merchant et al., 2006), suggesting that other, more accessible therapies acting through similar mechanisms might improve outcomes further. Understanding the mechanisms by which hypothermia alters outcome is, therefore, essential to further improvement in meaningful survival after CA.
Emerging data suggest that ischemic injury to one organ can induce injury in other organs. For example, experimental acute kidney injury (AKI) causes cardiac dysfunction and induces apoptotic cell death in cardiac muscle and lungs (Hassoun et al., 2007, 2009; White et al., 2012). Similarly, both lung and bowel ischemia/reperfusion induce acute liver injury (Horie and Ishii, 2001; Esme et al., 2006). Within the brain, AKI causes microglial activation and cell death within the hippocampal CA1 (Liu et al., 2008). Peripheral injury as experienced during surgery similarly causes an inflammatory response that activates microglia in the hippocampus and affects behavior (Cibelli et al., 2010; Terrando et al., 2010). Liver ischemia/reperfusion alters neuronal phenotypes and induces Fos expression in multiple regions (Bundzikova et al., 2011). Taken together, these data suggest that interventions which mitigate the effect of ischemic injury to one organ system might improve outcome in another system by interrupting organ cross-talk.
The purpose of the present study was to determine whether brain injury after CA is exacerbated by cross-talk from injured extracranial organs and whether protection of peripheral organs can thus reduce brain injury. We specifically hypothesized that intra-arrest hypothermia of extracranial organs would reduce extracranial organ injury and that this extracranial protection would subsequently reduce intracranial (i.e., brain) organ injury. To test this hypothesis, we employed a well-characterized mouse model of CA and cardiopulmonary resuscitation (CPR) in which temperature of the brain and other organs can be independently controlled, and in which we have previously demonstrated both neurologic and peripheral organ injury (Hutchens et al., 2007, 2010, 2011; Allen et al., 2011).
Materials and Methods
Animals and experimental groups
This study was conducted in accordance with the National Institutes of Health guidelines for the care and use of animals in research, and all animal protocols were approved by the Oregon Health and Science University Institutional Animal Care and Use Committee. Six- to eight-week-old, 20–25 g male C57Bl/6 mice (Charles River Laboratories, Boston, MA) were subjected to CA/CPR after being randomly assigned to treatment groups—normothermia versus extracranial hypothermia (ExHy) in all experimental protocols.
CA and CPR with controlled intra- and extracranial temperature
We performed CA/CPR as previously described with the addition of independent control of intra- and extracranial temperature (Hutchens et al., 2007, 2010, 2011; Allen et al., 2011). Briefly, we subjected mice to 8 minutes CA, using active control with warming via a separate temperature control system to keep the brain normothermic during CA for all animals. In one group (ExHy), we used active cooling to achieve rectal hypothermia, maintaining intracranial normothermia. In the control group (normothermia), both intracranial and extracranial temperatures were maintained within the normal range throughout CA/CPR and early recovery. We compared histologic and functional central nervous system outcome as well as serum indicators of renal and hepatic function and inflammation. General anesthesia was induced with 4% isoflurane in 2:1 air:oxygen mixture, and then maintained with 1–2% isoflurane. Animals were then placed in the supine position under a warming lamp. A rectal temperature probe was inserted. In the preliminary temperature monitoring protocol, temporalis muscle, intraprenchymal brain, and auricular canal temperature probes were inserted at this point; in the primary protocol, only the auricular canal probe was placed at this time. The heating lamp was controlled by a proportional-integral-derivative algorithm-controlled temperature controller (Digi-Sense; Cole Parmer, Vernon Hills, IL), set to 37.0°C, and a rectal temperature of 37.0°C±0.5°C was maintained. A PE-10 catheter was placed in the right jugular vein. After tracheal intubation with a 22G teflon catheter (InSyte-W; BD, Franklin, NJ), a coil of polyethylene tubing connected to a temperature-controlled water bath and pump was secured around the head to control brain temperature. The water bath temperature was maintained at 41.0°C using a temperature-controlled heating element. Subcutaneous electrocardiography (EKG) electrodes were placed. CA was then induced with an intravenous injection of 40 μL of 0.5 M potassium chloride and confirmed by the presence of asystole on the EKG. The endotracheal tube was disconnected. During CA, the auricular canal temperature was maintained at 37.5°C±0.0°C by intermittent actuation of the pump connected to the head coil. In the ExHy group, the heating lamp was turned off, and a rectal temperature of 28.8°C±1.5°C was achieved and maintained using isopropyl alcohol wipes of the thorax and abdomen. In the normothermia group, a rectal temperature of 35.1°C±0.1°C was maintained using an insulated blanket and the automated temperature controller with heating lamp. 7.5 minutes after onset of CA, the endotracheal tube was reconnected and mechanical ventilation was restarted with 100% oxygen. Eight minutes after the onset of CA, chest compressions were initiated at a rate of 300/min. Epinephrine, 5–16 μg in 0.9% sodium chloride solution (0.5–1.0 mL) was administered intravenously. Return of spontaneous circulation (ROSC) was confirmed by EKG and visible cardiac contractions on the chest wall. After ROSC, the rectal temperature target was 35.0°C±0.5°C in both groups, and the heating lamp and head coil were reactivated to achieve this goal. The trachea was extubated when spontaneous respiratory rate was >60 breaths/min. Animals were then placed in a recovery cage, which was placed on a warming pad controlled at 37°C.
Temperature monitoring and intracranial thermal effects of extracranial hypothermic arrest
In this preliminary protocol, animals (n=4 normothermic, n=5 ExHy) were subjected to CA/CPR as described earlier; however, temperature was simultaneously electronically monitored within the auricular canal, the temporalis muscle, the brain parenchyma, and the rectum. Parenychymal brain temperature was monitored by insertion of a temperature probe through a needle hole placed 2 mm deep within the left parietal region. Mice in this cohort were killed immediately after recovering from CA/CPR, and no further outcomes were measured.
Sham procedure
Animals in the sham procedure group underwent all procedures described earlier in CA and CPR with Controlled Intra- and Extracranial Temperature section, with the exception of induction of CA and subsequent administration of cardiac compressions and epinephrine. Sham animals were sacrificed 3 days later and outcomes were measured identically to treatment animals.
Measurement of histopathologic brain injury
Three (n=15/treatment) or 7 days (n=11/treatment) after CA/CPR, mice were deeply anesthetized with 4% isoflurane. Blood was drawn for renal and hepatic functional outcomes and serum cytokine levels, and the mice were then transcardially perfused with 0.9% saline followed by 4% paraformaldehyde. The brain was removed and paraffin embedded, and 6 μm coronal sections were cut through the dorsal hippocampus. These were stained with hematoxylin and eosin for analysis of neuronal death. The hippocampal CA1 region was analyzed at three levels, 100 μm apart, starting −1.5 mm from the bregma. Nonviable neurons were identified by bright pink eosinophilic cytoplasm and a dark pyknotic nucleus. Viable and nonviable neurons were counted in three microscopic fields per section, and the percentage of nonviable neurons was averaged for three sections per animal as previously described (Allen et al., 2011).
Measurement of functional central nervous system injury
Each animal was scored for neurologic deficit every other day starting 24 hours after CA/CPR using a scoring system that graded consciousness, interaction, eye appearance, breathing, food/water intake, nest building, gait, ability to hold on to a wire, and overall activity each on a 0–2 to 0–5 point scale as previously described (Allen et al., 2011). The resulting neuroscore had a range of 0–23, with 0 indicating fully normal function.
Measurement of renal and hepatic injury
At the time of euthanasia, blood was drawn from the apex of the left ventricle and placed in lithium heparin tubes. Samples were then analyzed for urea nitrogen, creatinine, alanine aminotransferase (ALT), and aspartate aminotransferase (AST) using an enzyme-coupled point of care analyzer (Abaxis Medical Diagnostics, Union City, CA).
Measurement of systemic inflammatory response (IL-1β)
At the time of euthanasia, blood was drawn from the apex of the left ventricle and collected in serum separation tubes. Serum was stored at −80°C until analysis. Interleukin (IL)-1β concentration was measured using 50 μL serum in a mouse IL-1β ELISA (eBioscience, Inc., San Diego, CA), according to the manufacturer's instructions.
Statistical analysis
Statistical analyses were performed using GraphPad Prism 6.0 (GraphPad, LaJolla, CA). All data are presented as mean±SEM. Two-group comparisons were performed using t-tests with correction for unequal variance, as appropriate, and two-tailed p-values were reported. A nonparametric test, the Mann–Whitney, was employed for nonparametric data. Time-dependent between-group comparisons were assessed using two-factor analysis of variance followed by the Holm–Sidak test for multiple comparisons. Twenty-four hours of survival was assessed using chi-square analysis. Correlation was assessed using Spearman's test. Statistical significance was inferred from p<0.05.
Results
Auricular canal temperature most closely approximates brain parenchymal temperature during CA/CPR
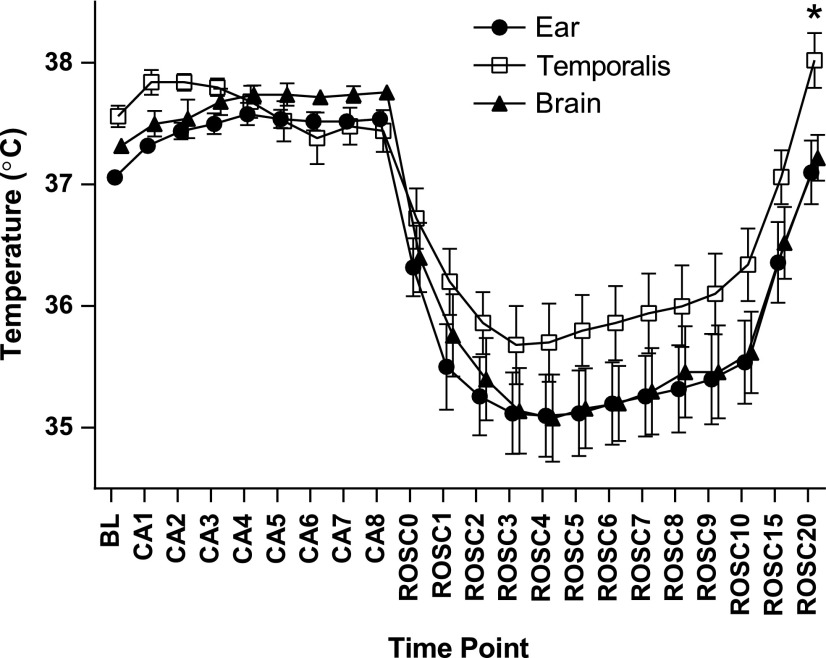
Overall, the interaction between site and measured temperature was highly significant (p<0.0001), with temporalis temperature overestimating brain parenchymal temperature by as much as 0.9°C±0.4°C. As shown in Figure 1, the discrepancy between temporalis and brain parenchymal temperature reached a maximum 20 minutes after ROSC, while the difference between auricular canal and brain parenchymal temperature was negligible and nonsignificant at all time points. Therefore, in the experiments that followed, auricular temperature was used to monitor brain parenchymal temperature.
FIG. 1.
Auricular canal temperature is not different from brain parenchymal temperature. Temperature measured is plotted by minute within the cardiac arrest (CA)/cardiopulmonary resuscitation (CPR) sequence (baseline=BL, arrest=CA, return of spontaneous circulation=ROSC; time point in minutes follows the phase abbreviation, e.g., ROSC10=10 minutes after return of spontaneous circulation). Temperature was monitored within the auricular canal (“Ear”), within the brain parenchyma (“Brain”), and within the temporalis (“Temporalis”) muscle during CA/CPR and recovery. There was no significant difference between auricular and brain temperature at any time point. Temporalis temperature overestimated brain temperature, statistically significant at the ROSC20 time point (mean±SEM, n=9).
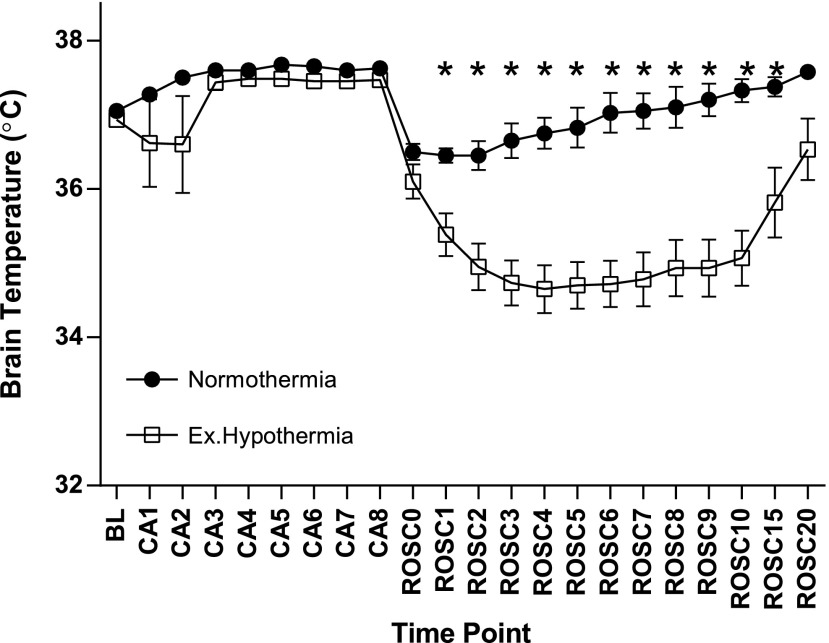
Intra-arrest ExHy induces transient post-arrest intracranial hypothermia
In the preliminary, brain parenchymal temperature monitoring protocol, body cavity temperature control resulted in ExHy (mean rectal temperature immediately after ROSC: 35.1°C±0.1°C vs. 28.8°C±1.5°C, gradient 6.4°C±1.7°C). Although the head and body were actively, independently temperature controlled, active resuscitation coincided with a transient fall in brain parenchymal temperature during the recovery period (at the point of maximal gradient, mean brain parenchymal temperature was 34.7°C±0.3°C in ExHy-treated animals as compared with normothermic controls' 36.8°C±0.2°C, 4 minutes after ROSC, p<0.0001). This difference, illustrated in Figure 2, resolved after 15 minutes of the recovery period.
FIG. 2.
Extracranial hypothermia induces transient brain parenchymal cooling. Temperature was measured within the brain parenchyma with a temperature probe placed 2 mm deep within the left parietal region and is plotted by minute within the CA/CPR sequence (baseline=BL, arrest=CA, return of spontaneous circulation=ROSC, time point in minutes follows the phase abbreviation, e.g., ROSC10=10 minutes after return of spontaneous circulation). Mice treated with extracranial hypothermia experienced a transient drop in brain parenchymal temperature despite external warming of the cranium (maximal 2.1°C±0.33°C at 6 minutes post-ROSC) (*p<0.05, mean±SEM, n=9).
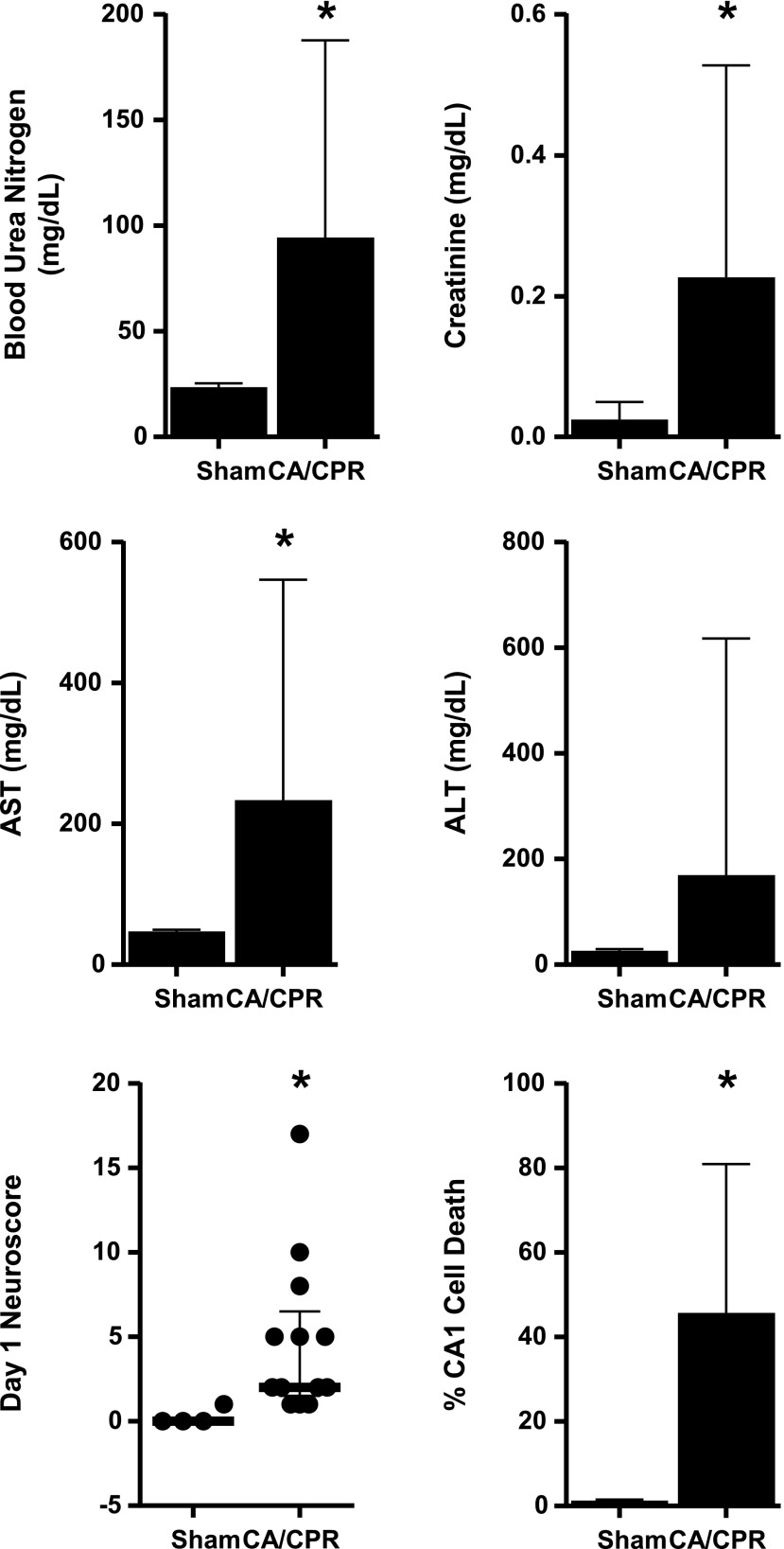
CA/CPR induces multiorgan injury
As seen in Figure 3, serum functional markers, neurobehavioral deficits, and brain histology indicated multiorgan injury 3 days after CA/CPR, compared with sham procedure [blood urea nitrogen (BUN) 94±24 (CA/CPR, n=15) vs. 24±2 mg/dL (sham, n=4), serum creatinine (sCr) 0.2±0.1 vs. 0.0±0.0 mg/dL, AST 234±81 vs. 47±5 mg/dL, neuroscore 6.3±1.8 vs. 0.0±0, CA1 death 46.4%±16.6% vs. 1.3%±0.2%, mean±SEM, p<0.05]. ALT (169±115 vs. 26±7 mg/dL) was not significantly different 3 days after CA/CPR compared with sham procedure. Serum IL-1β was undetectable (<8 pg/mL) in all samples.
FIG. 3.
CA/CPR induces multiorgan injury. Blood urea nitrogen (BUN), serum creatinine, aspartate aminotransferase (AST), alanine aminotransferase (ALT), and hippocampal CA1 cell death were measured in animals subjected to sham procedure or CA/CPR 3 days following the procedure. Neurobehavioral scoring was performed at 24 hours after procedures (*p<0.05, mean±SEM [BUN, sCr, AST, ALT, % CA1 Cell death] or median±interquartile range [neuroscore] presented n=4 sham and 15 CA/CPR).
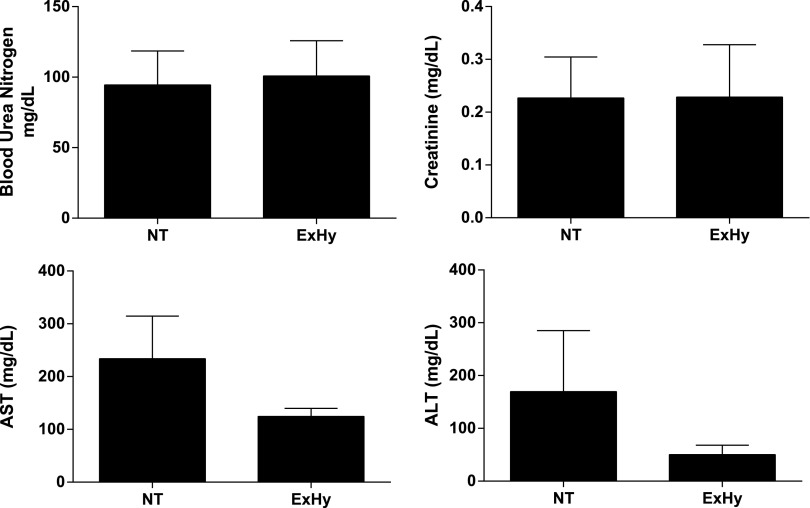
CA/CPR-induced renal and hepatic injury is not ameliorated by intra-arrest ExHy
As seen in Table 1, resuscitation requirements were not altered by treatment group assignment. Figure 4 illustrates that BUN (94±24 vs. 101±25 mg/dL, p=0.85, n=15/g), sCr (0.23±0.08 vs. 0.23±0.10 mg/dL, p=0.99, n=15/g), AST (234±81 vs. 125±15 mg/dL, p=0.21, n=15/g), and ALT (170±116 vs. 50±18 mg/dL, p=0.33, n=15/g) were not significantly different between normothermic and hypothermic animals at 3 days after CA/CPR. Similarly, there was no difference between groups at 7 days after CA/CPR (data not shown).
Table 1.
Cardiac Arrest/Cardiopulmonary Resuscitation Data
| Normothermia | Extracranial hypothermia | p | |
|---|---|---|---|
| Time to ROSC (s) | 113.5±6.4 | 128.1±8.6 | 0.11 |
| Epinephrine dose (μg/g body weight) | 0.5±0.0 | 0.5±0.0 | 0.13 |
| Baseline pre-arrest auricular canal temperature (BL, °C) | 37.0±0.0 | 37.1±0.0 | 0.5 |
| Intra-CA auricular canal temperature (CA5, °C) | 37.7±0.1 | 37.4±0.0 | <0.01 |
| Auricular canal temperature 5 min after ROSC (ROSC5, °C) | 36.4±0.2 | 34.5±0.2 | <0.01 |
| Auricular canal temperature 20 min after ROSC (ROSC20, °C) | 35.8±0.6 | 36.3.3±0.2 | 0.4 |
| ROSC mean rectal temperature (ROSC0, °C) | 35.1±0.1 | 28.8±1.5 | <0.01 |
| 24 h Survival (%) | 75.8 | 78.3 | 0.55 |
CA, cardiac arrest; ROSC, return of spontaneous circulation.
FIG. 4.
Renal and hepatic function at 3 days post-CA/CPR is not altered by extracranial hypothermia. BUN and serum creatinine, AST, and ALT were measured at 3 days after CA/CPR and were not different between treatment groups (mean±SEM, n=15/g).
Intra-arrest ExHy ameliorates brain injury
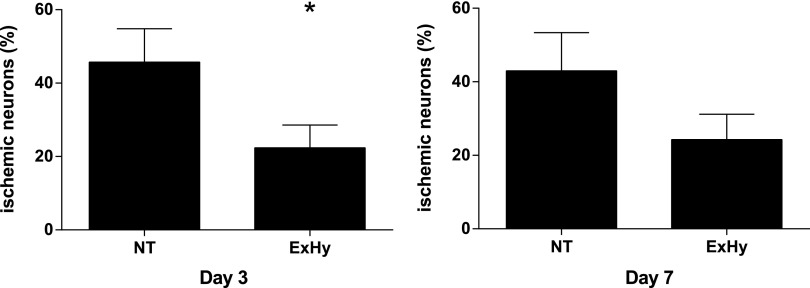
Animals treated with ExHy demonstrated significantly fewer ischemic, dead, or dying neurons in the hippocampal CA1 region at 3 days after CA/CPR (22.4%±6.2% vs. 45.7%±9.1%, p<0.05, n=15/group). The effect was still evident at 7 days after CA/CPR, although the difference was no longer statistically significant (24.3±6.0 vs. 43.0±10.4, p=0.15, n=11/group), Figure 5. Gross behavior, as assessed by the neuroscore, was not different between treatment groups (day 1 hypothermia 5±0 vs. normothermia 4±1, n=22/group, day 3 3±1 vs. 5±1, n=22/group, day 7 1±0 vs. 1±0, n=11/group). There was no correlation between hepatic or renal injury and either histopathologic brain injury or behavioral deficits in any group at any time point.
FIG. 5.
Neurohistological outcome of CA/CPR at 3 and 7 days post-arrest. Animals subjected to CA/CPR with extracranial hypothermia suffered significantly less neuronal death than those subjected to whole-body normothermic arrest as measured at 3 days post-CA/CPR. This effect was no longer significant at 7 days post-CA/CPR (*p=0.04, mean±SEM, n=15 for day 3 results, left. n=11/g for day 7, p>0.05).
Discussion
The main finding of this investigation is that ExHy during CA, without reduction in brain temperature, protects the brain after CA and CPR. This occurred despite the lack of a demonstrable reduction in extracranial end-organ function at 3 days after CA/CPR. Our findings add to the field, as they illustrate the importance of extracranial organs for brain protection and injury after CA.
The protection from ischemia/reperfusion injury provided by whole-body hypothermia has been widely described in decades of active research, and use of post-insult, TH has improved the outcomes of CA patients in recent years (Arrich et al., 2012; Girotra et al., 2012). However, TH remains underutilized (Laver et al., 2006) and dose and timing are controversial (Kuboyama et al., 1993; Italian Cooling Experience (ICE) Study Group, 2012; Yu and Liu, 2013). Accordingly, the mechanism of neuroprotection from hypothermic interventions is unclear, not because of lack of research, but at least, in part, because of the myriad of potential candidate mechanisms. Although a comprehensive review of the voluminous literature on the mechanisms of hypothermic protection is beyond the scope of this discussion, putative mechanisms have included reduction in cerebral metabolic demand (Williams and Spencer, 1958), interruption of apoptotic pathways (Xu et al., 2002), altered mitochondrial stress (Yenari et al., 2002), alteration of transporter kinetics (Lipski et al., 2006), and reduction of immune/inflammatory mechanisms (Meybohm et al., 2010), among others.
The traditional assumption is that brain hypothermia is the key to protection mediated by TH after CA. This notion was supported by early experimental studies which showed that selective post-injury cooling of the head protects the brain as effectively as whole body cooling. However, these studies used global (Corbett et al., 1997) or focal (Maier et al., 1998) ischemia models that interrupted blood flow to the brain only, avoiding whole body ischemia. In contrast, CA injures the entire body and affects all organ systems, resulting in a “postresuscitation disease” that has been likened to sepsis (Adrie et al., 2004).
Ischemic injury to peripheral organs, including kidneys (Liu et al., 2008), lungs (Holland et al., 2003; Rincon et al., 2012), and gut (Zhou et al., 2012), can activate microglia, induce neuronal death, and worsen brain injury. Hypothermia reduces injury to these extra-cranial organs (Chang et al., 2008; Santora et al., 2010) and reduces injurious organ cross-talk and resulting remote organ injury (Santora et al., 2010). The reduction of hippocampal injury in the animals treated with extra-cranial hypothermia in our study may be explained by a similar suppression of systemic organ cross-talk. It was surprising, however, that we did not find sustained organ protection of either liver or kidneys by hypothermia. Hypothermia-induced protection from ischemia is clearly an established concept. Others have shown that intra-ischemic hypothermia protects peripheral organs in models of isolated organ ischemia, including kidneys (Zager et al., 1989; Delbridge et al., 2007), liver (Behrends et al., 2006; Kuboki et al., 2007), lung (Shoji et al., 2005), and gut (Hassoun et al., 2002). This protection is mostly attributed to maintained levels of high-energy phosphates in hypothermic organs and to a lesser degree to a reduction of inflammation and infiltration of immune cells (Stefanutti et al., 2008). Studies of isolated organ ischemia use prolonged ischemia times that cause more severe injury than our CA model. It is, therefore, possible that energy stores in liver and kidneys are sufficient to prevent significant ischemic necrosis during the shorter period of ischemia in our CA model, thus limiting potential protection by hypothermia. This is supported by the finding that in our 8 minute model of CA/CPR, IL-1β was undetectable in all animals; while in a 10 minute model of CA, Burne-Taney et al. (2003) found increased levels of IL-1β 24 hours after CA/CPR. An alternative explanation is simply that the extensive liver and kidney injury we and others (Li et al., 2012) have demonstrated in this model at 24 hours post-arrest is partially resolved by the time point of investigation we used in this study. This would also be consistent with the findings of Burne-Taney et al. (2003), who found that changes in sCr were largely resolved at 72 hours after CA/CPR. Curiously, a large clinical multi-center trial of TH after CA also did not confirm that hypothermia improves renal function after CA (Zeiner et al., 2004).
Reperfusion with cold core blood induced a slight reduction in brain parenchymal temperature for 14 minutes after ROSC in animals treated with ExHy, despite active independent temperature control (as seen in Fig. 2). Hypothermia can cause regional cerebral vasodilation in larger animal models (Gelman et al., 1996), which could potentially have contributed to reduced injury in the ExHy group. However, the magnitude of the observed reduction of brain temperature was small (maximally 2.1°C) and transient. The temperature reduction required in other studies to produce cerebral vasodilation after resuscitation from CA and induce neuroprotection was much more severe and lasted longer [temperature as low as 28°C for 45 minutes after ROSC (Gelman et al., 1996) or as low as 20°C for 30 minutes after ROSC (Horn et al., 1991)]. Similarly, a reduction of brain temperature below 34°C for >1–2 hours was required to induce neuroprotection of hippocampal CA1 in small animal studies of hypothermia after CA (Nakajima et al., 1997; Takata et al., 2005; Che et al., 2011). It, therefore, appears unlikely that the brief reduction in brain temperature after ROSC fully explains the brain protection observed in our study; however, an alternative explanation of our data remains that a very brief, a very slight reduction in brain temperature immediately after reperfusion without further temperature management could be neuroprotective.
Our study has several limitations. No direct conclusions regarding the use of TH, which is generally in the moderate range, and applied after ischemia/reperfusion, should be drawn from our study, in which we manipulated extracranial temperature only during CA and not after. Another possible limitation is the lack of histological analysis of organ injury (kidney or liver). However, our previous studies have demonstrated a very strong correlation between kidney injury measured by FluoroJade B histology and serum levels of creatinine and BUN (Hutchens et al., 2010, 2012). Our current study cannot rule out a role of inflammation in organ cross-talk after CA, as we were unable to detect sustained elevation of plasma cytokines at 3 days after the insult. Further investigations at earlier time points after CA are needed to determine whether blunting of the inflammatory response by ExHy may contribute to neuroprotection. Finally, it is possible that macrophysiologic differences due to intra-arrest thermal management might have contributed to the observed effect. Systemic hypothermia causes systemic vasoconstriction, which could potentially affect cardiac function. Since both groups required similar doses of epinephrine and similar duration of CPR to achieve ROSC, and also had similar survival rates, it is unlikely that intra-arrest ExHy had profound effects on cardiac function. We did not directly measure cardiac output or blood pressure in this small rodent model.
A growing and important literature supports local cooling of the brain to prevent injury after neurologic insult in animals and humans (Clark and Colbourne, 2007; Fingas et al., 2007; Esposito et al., 2014). To our knowledge, we are the first to differentially manipulate brain and body temperature, holding brain temperature constant, during CA in order to evaluate the site of hypothermic protection. Using this model, we found that cooling the body while holding the brain normothermic is also neuroprotective. We believe that the ability to produce ExHy may provide an experimental platform for identification of protective signals originating outside of the CNS.
Conclusions
Our findings suggest that interventions targeting peripheral organs can influence the development of neurologic injury after ischemia reperfusion. Our data add to the growing understanding of cross-talk between different organ systems and emphasize that brain injury after CA cannot be seen in isolation. Future studies will identify the specific mediators utilized by different organ sources in this cross-talk, with the hope that interrupting or altering the cross-talk may lead to better brain-saving therapies. Our findings further emphasize the importance of using models of actual CA to test potential clinical treatments, rather than extrapolating from studies using selective brain ischemia.
Acknowledgments
This work was supported by the U.S. National Institutes of Health grants DK090754-01 (M.P.H.), NS067051-02 (I.P.K.), and NS058792-05 (P.S.H.).
Disclosure Statement
The authors declare that no competing financial interests exist.
References
- Adrie C, Laurent I, Monchi M, Cariou A, Dhainaou JF, Spaulding C. Postresuscitation disease after cardiac arrest: a sepsis-like syndrome? Curr Opin Crit Care 2004;10:208–212 [DOI] [PubMed] [Google Scholar]
- Allen D, Nakayama S, Kuroiwa M, Nakano T, Palmateer J, Kosaka Y, Ballesteros C, Watanabe M, Bond CT, Lujan R, Maylie J, Adelman JP, Herson PS. SK2 channels are neuroprotective for ischemia-induced neuronal cell death. J Cereb Blood Flow Metab 2011;31:2302–2312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arrich J, Holzer M, Havel C, Mullner M, Herkner H. Hypothermia for neuroprotection in adults after cardiopulmonary resuscitation. Cochrane Database Syst Rev 2012;9:CD004128. [DOI] [PubMed] [Google Scholar]
- Behrends M, Hirose R, Serkova NJ, Coatney JL, Bedolli M, Yardi J, Park YH, Niemann CU. Mild hypothermia reduces the inflammatory response and hepatic ischemia/reperfusion injury in rats. Liver Int 2006;26:734–741 [DOI] [PubMed] [Google Scholar]
- Bernard SA, Gray TW, Buist MD, Jones BM, Silvester W, Gutteridge G, Smith K. Treatment of comatose survivors of out-of-hospital cardiac arrest with induced hypothermia. N Engl J Med 2002;346:557–563 [DOI] [PubMed] [Google Scholar]
- Bundzikova J, Pirnik Z, Lackovicova L, Mravec B, Kiss A. Activation of different neuronal phenotypes in the rat brain induced by liver ischemia-reperfusion injury: dual Fos/Neuropeptide immunohistochemistry. Cell Mol Neurobiol 2011;31:293–301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burne-Taney MJ, Kofler J, Yokota N, Weisfeldt M, Traystman RJ, Rabb H. Acute renal failure after whole body ischemia is characterized by inflammation and T cell-mediated injury. Am J Physiol Renal Physiol 2003;285:F87–F94 [DOI] [PubMed] [Google Scholar]
- Chang H, Huang KL, Li MH, Hsu CW, Tsai SH, Chu SJ. Manipulations of core temperatures in ischemia-reperfusion lung injury in rabbits. Pulm Pharmacol Ther 2008;21:285–291 [DOI] [PubMed] [Google Scholar]
- Che D, Li L, Kopil CM, Liu Z, Guo W, Neumar RW. Impact of therapeutic hypothermia onset and duration on survival, neurologic function, and neurodegeneration after cardiac arrest. Crit Care Med 2011;39:1423–1430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, Takata M, Lever IJ, Nanchahal J, Fanselow MS, Maze M. Role of interleukin-1beta in postoperative cognitive dysfunction. Ann Neurol 2010;68:360–368 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark DL, Colbourne F. A simple method to induce focal brain hypothermia in rats. J Cereb Blood Flow Metab 2007;27:115–122 [DOI] [PubMed] [Google Scholar]
- Corbett D, Nurse S, Colbourne F. Hypothermic neuroprotection. A global ischemia study using 18- to 20-month-old gerbils. Stroke 1997;28:2238–2242; discussion 2243 [DOI] [PubMed] [Google Scholar]
- Delbridge MS, Shrestha BM, Raftery AT, El Nahas AM, Haylor J. FTY720 reduces extracellular matrix expansion associated with ischemia-reperfusion induced injury. Transplant Proc 2007;39:2992–2996 [DOI] [PubMed] [Google Scholar]
- Esme H, Fidan H, Koken T, Solak O. Effect of lung ischemia–reperfusion on oxidative stress parameters of remote tissues. Eur J Cardiothorac Surg 2006;29:294–298 [DOI] [PubMed] [Google Scholar]
- Esposito E, Ebner M, Ziemann U, Poli S. In cold blood: intraarteral cold infusions for selective brain cooling in stroke. J Cereb Blood Flow Metab 2014. DOI: 10.1038/jcbfm.2014.29 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fingas M, Clark DL, Colbourne F. The effects of selective brain hypothermia on intracerebral hemorrhage in rats. Exp Neurol 2007;208:277–284 [DOI] [PubMed] [Google Scholar]
- Gelman B, Schleien CL, Lohe A, Kuluz JW. Selective brain cooling in infant piglets after cardiac arrest and resuscitation. Crit Care Med 1996;24:1009–1017 [DOI] [PubMed] [Google Scholar]
- Girotra S, Nallamothu BK, Spertus JA, Li Y, Krumholz HM, Chan PS. Trends in survival after in-hospital cardiac arrest. N Engl J Med 2012;367:1912–1920 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hassoun HT, Grigoryev DN, Lie ML, Liu M, Cheadle C, Tuder RM, Rabb H. Ischemic acute kidney injury induces a distant organ functional and genomic response distinguishable from bilateral nephrectomy. Am J Physiol Renal Physiol 2007;293:F30–F40 [DOI] [PubMed] [Google Scholar]
- Hassoun HT, Kozar RA, Kone BC, Safi HJ, Moore FA. Intraischemic hypothermia differentially modulates oxidative stress proteins during mesenteric ischemia/reperfusion. Surgery 2002;132:369–376 [DOI] [PubMed] [Google Scholar]
- Hassoun HT, Lie ML, Grigoryev DN, Liu M, Tuder RM, Rabb H. Kidney ischemia-reperfusion injury induces caspase-dependent pulmonary apoptosis. Am J Physiol Renal Physiol 2009;297:F125–F137 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland MC, Mackersie RC, Morabito D, Campbell AR, Kivett VA, Patel R, Erickson VR, Pittet JF. The development of acute lung injury is associated with worse neurologic outcome in patients with severe traumatic brain injury. J Trauma 2003;55:106–111 [DOI] [PubMed] [Google Scholar]
- Horie Y, Ishii H. Liver dysfunction elicited by gut ischemia–reperfusion. Pathophysiology 2001;8:11–20 [DOI] [PubMed] [Google Scholar]
- Horn M, Schlote W, Henrich HA. Global cerebral ischemia and subsequent selective hypothermia. A neuropathological and morphometrical study on ischemic neuronal damage in cat. Acta Neuropathol 1991;81:443–449 [DOI] [PubMed] [Google Scholar]
- Hutchens MP, Fujiyoshi T, Komers R, Herson PS, Anderson S. Estrogen protects renal endothelial barrier function from ischemia-reperfusion in vitro and in vivo. Am J Physiol Renal Physiol 2012;303:F377–F385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens MP, Nakano T, Dunlap J, Traystman RJ, Hurn PD, Alkayed NJ. Soluble epoxide hydrolase gene deletion reduces survival after cardiac arrest and cardiopulmonary resuscitation. Resuscitation 2007;76:89–94 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens MP, Nakano T, Kosaka Y, Dunlap J, Zhang W, Herson PS, Murphy SJ, Anderson S, Hurn PD. Estrogen is renoprotective via a non-receptor dependent mechanism after cardiac arrest in vivo. Anesthesiology 2010;112:395–405 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutchens MP, Traystman RJ, Fujiyoshi T, Nakayama S, Herson PS. Normothermic cardiac arrest and cardiopulmonary resuscitation: a mouse model of ischemia-reperfusion injury. J Vis Exp 2011; 3116 DOI: 10.3791/3116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hypothermia After Cardiac Arrest Study Group. Mild therapeutic hypothermia to improve the neurologic outcome after cardiac arrest. N Engl J Med 2002;346:549–556 [DOI] [PubMed] [Google Scholar]
- Italian Cooling Experience (ICE) Study Group. Early- versus late-initiation of therapeutic hypothermia after cardiac arrest: preliminary observations from the experience of 17 Italian intensive care units. Resuscitation 2012;83:823–828 [DOI] [PubMed] [Google Scholar]
- Kuboki S, Schuster R, Blanchard J, Pritts TA, Wong HR, Lentsch AB. Role of heat shock protein 70 in hepatic ischemia-reperfusion injury in mice. Am J Physiol Gastrointest Liver Physiol 2007;292:G1141–G1149 [DOI] [PubMed] [Google Scholar]
- Kuboyama K, Safar P, Radovsky A, Tisherman SA, Stezoski SW, Alexander H. Delay in cooling negates the beneficial effect of mild resuscitative cerebral hypothermia after cardiac arrest in dogs: a prospective, randomized study. Crit Care Med 1993;21:1348–1358 [DOI] [PubMed] [Google Scholar]
- Laver SR, Padkin A, Atalla A, Nolan JP. Therapeutic hypothermia after cardiac arrest: a survey of practice in intensive care units in the united kingdom. Anaesthesia 2006;61:873–877 [DOI] [PubMed] [Google Scholar]
- Leonov Y, Sterz F, Safar P, Radovsky A, Oku K, Tisherman S, Sterz F. Mild cerebral hypothermia during and after cardiac arrest improves neurologic outcome in dogs. J Cereb Blood Flow Metab 1990;10:57–70 [DOI] [PubMed] [Google Scholar]
- Li X, Liu M, Bedja D, Thoburn C, Gabrielson K, Racusen L, Rabb H. Acute renal venous obstruction is more detrimental to the kidney than arterial occlusion: implication for murine models of acute kidney injury. Am J Physiol Renal Physiol 2012;302:F519–F525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lipski J, Park TI, Li D, Lee SC, Trevarton AJ, Chung KK, Freestone PS, Bai JZ. Involvement of TRP-like channels in the acute ischemic response of hippocampal CA1 neurons in brain slices. Brain Res 2006;1077:187–199 [DOI] [PubMed] [Google Scholar]
- Liu M, Liang Y, Chigurupati S, Lathia JD, Pletnikov M, Sun Z, Crow M, Ross CA, Mattson MP, Rabb H. Acute kidney injury leads to inflammation and functional changes in the brain. J Am Soc Nephrol 2008;19:1360–1370 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maier CM, Ahern K, Cheng ML, Lee JE, Yenari MA, Steinberg GK. Optimal depth and duration of mild hypothermia in a focal model of transient cerebral ischemia: effects on neurologic outcome, infarct size, apoptosis, and inflammation. Stroke 1998;29:2171–2180 [DOI] [PubMed] [Google Scholar]
- Merchant RM, Soar J, Skrifvars MB, Silfvast T, Edelson DP, Ahmad F, Huang KN, Khan M, Vanden Hoek TL, Becker LB, Abella BS. Therapeutic hypothermia utilization among physicians after resuscitation from cardiac arrest. Crit Care Med 2006;34:1935–1940 [DOI] [PubMed] [Google Scholar]
- Meybohm P, Gruenewald M, Zacharowski KD, Albrecht M, Lucius R, Fosel N, Hensler J, Zitta K, Bein B. Mild hypothermia alone or in combination with anesthetic post-conditioning reduces expression of inflammatory cytokines in the cerebral cortex of pigs after cardiopulmonary resuscitation. Crit Care 2010;14:R21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakajima Y, Fujimiya M, Maeda T, Mori A. Morphological investigation of the neuroprotective effects of graded hypothermia after diverse periods of global cerebral ischemia in gerbils. Brain Res 1997;765:113–121 [DOI] [PubMed] [Google Scholar]
- Rincon F, Ghosh S, Dey S, Maltenfort M, Vibbert M, Urtecho J, McBride W, Moussouttas M, Bell R, Ratliff JK, Jallo J. Impact of acute lung injury and acute respiratory distress syndrome after traumatic brain injury in the United States. Neurosurgery 2012;71:795–803 [DOI] [PubMed] [Google Scholar]
- Santora RJ, Lie ML, Grigoryev DN, Nasir O, Moore FA, Hassoun HT. Therapeutic distant organ effects of regional hypothermia during mesenteric ischemia-reperfusion injury. J Vasc Surg 2010;52:1003–1014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shoji T, Omasa M, Nakamura T, Yoshimura T, Yoshida H, Ikeyama K, Fukuse T, Wada H. Mild hypothermia ameliorates lung ischemia reperfusion injury in an ex vivo rat lung model. Eur Surg Res 2005;37:348–353 [DOI] [PubMed] [Google Scholar]
- Stefanutti G, Pierro A, Parkinson EJ, Smith VV, Eaton S. Moderate hypothermia as a rescue therapy against intestinal ischemia and reperfusion injury in the rat. Crit Care Med 2008;36:1564–1572 [DOI] [PubMed] [Google Scholar]
- Takata K, Takeda Y, Sato T, Nakatsuka H, Yokoyama M, Morita K. Effects of hypothermia for a short period on histologic outcome and extracellular glutamate concentration during and after cardiac arrest in rats. Crit Care Med 2005;33:1340–1345 [DOI] [PubMed] [Google Scholar]
- Terrando N, Monaco C, Ma D, Foxwell BM, Feldmann M, Maze M. Tumor necrosis factor-alpha triggers a cytokine cascade yielding postoperative cognitive decline. Proc Natl Acad Sci U S A 2010;107:20518–20522 [DOI] [PMC free article] [PubMed] [Google Scholar]
- White LE, Santora RJ, Cui Y, Moore FA, Hassoun HT. TNFR1-dependent pulmonary apoptosis during ischemic acute kidney injury. Am J Physiol Lung Cell Mol Physiol 2012;303:L449–L459 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams GR, Jr., Spencer FC. The clinical use of hypothermia following cardiac arrest. Ann Surg 1958;148:462–468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu L, Yenari MA, Steinberg GK, Giffard RG. Mild hypothermia reduces apoptosis of mouse neurons in vitro early in the cascade. J Cereb Blood Flow Metab 2002;22:21–28 [DOI] [PubMed] [Google Scholar]
- Yenari MA, Han HS. Neuroprotective mechanisms of hypothermia in brain ischaemia. Nat Rev Neurosci 2012;13:267–278 [DOI] [PubMed] [Google Scholar]
- Yenari MA, Iwayama S, Cheng D, Sun GH, Fujimura M, Morita-Fujimura Y, Chan PH, Steinberg GK. Mild hypothermia attenuates cytochrome c release but does not alter bcl-2 expression or caspase activation after experimental stroke. J Cereb Blood Flow Metab 2002;22:29–38 [DOI] [PubMed] [Google Scholar]
- Yu H, Liu J. The optimal timing of initiation of therapeutic hypothermia after cardiac arrest. Resuscitation 2013;84:e37. [DOI] [PubMed] [Google Scholar]
- Zager RA, Gmur DJ, Bredl CR, Eng MJ. Degree and time sequence of hypothermic protection against experimental ischemic acute renal failure. Circ Res 1989;65:1263–1269 [DOI] [PubMed] [Google Scholar]
- Zeiner A, Sunder-Plassmann G, Sterz F, Holzer M, Losert H, Laggner AN, Mullner M. The effect of mild therapeutic hypothermia on renal function after cardiopulmonary resuscitation in men. Resuscitation 2004;60:253–261 [DOI] [PubMed] [Google Scholar]
- Zheng Z, Croft JB, Giles WH, Mensah GA. Sudden cardiac death in the United States, 1989 to 1998. Circulation 2001;104:2158–2163 [DOI] [PubMed] [Google Scholar]
- Zhou J, Huang WQ, Li C, Wu GY, Li YS, Wen SH, Lei WL, Liu KX. Intestinal ischemia/reperfusion enhances microglial activation and induces cerebral injury and memory dysfunction in rats. Crit Care Med 2012;40:2438–2448 [DOI] [PubMed] [Google Scholar]