Abstract
Background:
The key determinants of daytime drowsiness in sleep disordered breathing (SDB) are unclear. Hypercapnia has not been examined as a potential contributor due to the lack of reliable measurement during sleep. To overcome this limitation, we studied predominantly hypercapnic SDB patients to investigate the role of hypercapnia on EEG activation and daytime sleepiness.
Methods:
We measured overnight polysomnography (PSG), arterial blood gases, and Epworth Sleepiness Scale in 55 severe SDB patients with obesity hypoventilation syndrome or overlap syndrome (COPD+ obstructive sleep apnea) before and ∼3 months after positive airway pressure (PAP) treatment. Quantitative EEG analyses were performed, and the Delta/ Alpha ratio was used as an indicator of EEG activation.
Results:
After the PAP treatment, these patients showed a significant decrease in their waking pCO2, daytime sleepiness, as well as all key breathing/oxygenation parameters during sleep. Overnight Delta/Alpha ratio of EEG was significantly reduced. There is a significant cross-correlation between a reduced wake pCO2, a faster (more activated) sleep EEG (reduced Delta/Alpha ratio) and reduced daytime sleepiness (all p < 0.05) with PAP treatment. Multiple regression analyses showed the degree of change in hypercapnia to be the only significant predictor for both ESS and Delta/ Alpha ratio.
Conclusions:
Hypercapnia is a key correlate of EEG activation and daytime sleepiness in hypercapnic SDB patients. The relationship between hypercapnia and sleepiness may be mediated by reduced neuro-electrical brain activity.
Commentary:
A commentary on this article appears in this issue on page 523.
Citation:
Wang D, Piper AJ, Yee BJ, Wong KK, Kim JW, D'Rozario A, Rowsell L, Dijk DJ, Grunstein RR. Hypercapnia is a key correlate of EEG activation and daytime sleepiness in hypercapnic sleep disordered breathing patients. J Clin Sleep Med 2014;10(5):517-522.
Keywords: CO2, O2, EEG Spectra, daytime drowsiness, hypersomnolence, hypoxia, cortical depression.
The key determinants of general sleep disordered breathing (SDB) related daytime drowsiness are unclear. Many candidate factors such as intermittent hypoxia, apnea-hypopnea index (AHI), sleep fragmentation, BMI, sleep time, and metabolic factors have been studied, but none show a strong correlation with daytime sleepiness.1–9 Among those factors, relatively stronger evidence supports a role for oxygen desaturation index (ODI) or intermittent hypoxia in daytime sleepiness.1,2,6 However, supplemental O2 does not improve hypersomnolence in obstructive sleep apnea (OSA) patients despite improving oxygenation.10–12 Similarly, O2 therapy does not improve neurocognitive or psychosocial performances in hypoxemic chronic obstructive pulmonary disease (COPD).13,14 In contrast, some human experimental studies suggest that hypercapnia can cause impaired mental and psychomotor function.15–18 While SDB is characterized by recurrent episodes of both hypoxia and hypercapnia, the relationship of hypercapnia to daytime sleepiness in this patient group has not been investigated.1–5 This omission is likely due to the lack of clinical equipment to reliably measure continuous pCO2 during the overnight PSG. However, in hypercapnic SDB patients, particularly those with obesity hypoventilation syndrome (OHS) and overlap syndrome (COPD+OSA), a wide range of pCO2 can be accurately measured during awake with arterial blood gas (ABG). Therefore this subtype of SDB patients could be an ideal patient group to investigate the relationship between hypoxia, hypercapnia, and daytime sleepiness.
BRIEF SUMMARY
Current Knowledge/Study Rationale: The key determinants of daytime drowsiness in sleep disordered breathing (SDB) are unclear. hypercapnia has not been examined as a potential contributor due to the lack of reliable measurement during sleep.
Study Impact: Our study identified that hypercapnia is a key correlate of SDB -related daytime sleepiness in severe SDB population, and there is a significant cross-correlation between the changes of hypercapnia, EEG spectral activity, and daytime sleepiness.
Dose-dependent anesthesia-like effects of CO2 have been previously reported.19 In fact, CO2 has long been used as a stunning agent to produce unconsciousness during porcine slaughter, while hypoxia does not produce this effect.20 A clinical study found that untreated OSA patients have slower EEG than normal subjects, which is not related to hypoxia. CPAP treatment corrected the EEG slowing together with reduced daytime sleepiness.21 Recently we observed that respiratory failure patients have a paradoxically high percentage of slow wave sleep; awake pCO2 measured by ABG is the best predictor variable for the EEG change.22 Furthermore, progressive hypercapnia but not hypoxia decreases EEG activation measured by an increased EEG Delta/Alpha (D/A) ratio.23
Given these findings, we hypothesized that in hypercapnic SDB patients, the decrease in hypercapnia, but not hypoxia, is a key correlate for the improvement in daytime sleepiness, and may cross-correlate with changes in EEG spectral power— such as D/A ratio—reflecting changes in EEG activation.21,24–26 We conducted an observational study using PAP treatment to test these hypotheses.
METHODS
The clinical study was conducted at the clinical sleep laboratory of Royal Prince Alfred Hospital (RPAH), a major teaching hospital of the University of Sydney. The study protocol was originally designed to test clinical outcomes of OHS patients receiving CPAP/BPAP treatment over a 3-month period. Data of the present study come from post hoc analyses of the original study. The study protocol was approved by Sydney South West Area Health Service Ethics Review Committee (Protocol Numbers: X03-0022). All participants provided written informed consent. The Australian & New Zealand Clinical Trial Registry number is ACTRN12605000096651.
Patients and Procedure
Study patients were consecutive patients recruited from the Sleep and Respiratory Failure Clinics of RPAH. We sampled late afternoon awake ABG in predominantly hypercapnic patients with severe SDB. Fifty-five OSA patients with either OHS or overlap syndrome (COPD+OSA) underwent 2 overnight PSGs before and after ∼3 months continuous/bilevel positive airway pressure (PAP) treatment. The recruited patients had daytime hypercapnia (arterial pCO2 > 45 mm Hg) and/or frequent hypoventilation with significant oxygen desaturations during the initial diagnostic PSG studies. Hypoventilation was defined by an awake daytime pCO2 > 45 mm Hg or during sleep a sustained fall in SpO2 > 4% from baseline values accompanied by a rise in PtcCO2 > 8 mm Hg. COPD was defined as FEV1/FVC ratio < 70%, with severity based on percent of predicted FEV1 (GOLD criteria, www.goldcopd.com). We included both OHS and overlap syndrome patients, considering that the effect of hypercapnia/hypoxia may share a common mechanism in affecting EEG and daytime sleepiness. Quantitative EEG spectral analyses were performed on the overnight PSG recordings. A score for the Epworth Sleepiness Scale (ESS), a widely used measure of subjective daytime sleepiness, was calculated in each patient.27
PSG
Overnight standard in-laboratory PSG was performed (between 22:00 and 07:00) using Compumedics E series (Compumedics; Victoria, Australia) or Alice 4 & 5 (Respironics, USA) diagnostic sleep systems. Each PSG included 4 channels of electroencephalogram (EEG) (C3/A2, C4/A1, O1/ A2, O2/A1), 2 channels of electrooculogram, chin electromyogram, anterior tibial electromyogram, electrocardiogram (ECG), body position, nasal pressure, chest and abdomen movements, and SpO2. PSG recordings were scored by experienced sleep technologists using standard criteria.28–30 Respiratory events were scored according to Chicago criteria,29 but no respiratory effort-related arousal events were marked. Sleep arousals were scored according to the American Sleep Disorder Association task force criteria.30 AHI was calculated by dividing the total number of apneas and hypopneas by the total sleep time (hours). ODI was calculated by dividing the total number of ≥ 3% SpO2 dips by the total sleep time (hours).
EEG Spectral Analyses
We converted all EEG recordings to European Data Format for the spectral analyses. We analyzed the EEG power spectra for each 5-sec segment. All EEG study sampling rates were > 200 Hz. A standard fast Fourier transform with a rectangular weighting window was performed twice: first, to the largest power of 2 data points smaller than the total number of data points, selected from the beginning of the segment, and second, to the same number of data points selected from the end. This double fast Fourier transform method weights middle data points. Delta, theta, alpha, and beta bands were defined as the frequency ranges 0.5-4.5, 4.5-8, 8-12, and 12-32 Hz, respectively, appropriate for typical adults. The EEGs were then further examined by an automatic algorithm which excluded EEG segments showing excessive delta power using a standard 2 sigma rule (i.e., median + 2 standard deviations). For our statistical analysis, we focused primarily on the EEG recorded at C3/A2. However, when the C3 channel was contaminated by excessive artifact, we used C4/A1 as alternative channel.
Statistical Analyses
Descriptive data were expressed as mean ± SD, unless otherwise stated. Pair-wise comparisons were tested by paired t-test or Wilcoxon signed-rank test depending on the normality of data distribution. Unpaired t-tests and Mann-Whitney U tests were used for between group comparisons where appropriate. Associations were tested by either Pearson or Spearman tests also based on normality of distribution. Among the EEG spectral measures, Delta/Alpha ratio was the primary outcome of interest. ESS is the other primary outcome of interest. Stepwise multiple linear regression analyses were used to identify factors contributing to the variance of ESS and D/A ratio respectively. A p-value < 0.05 was considered as significant. Analyses were performed using SPSS 17 (SPSS, Chicago, USA).
RESULTS
From the 55 patients tested, we obtained satisfactory data in 41 patients (30M, 11F, aged 54.6 ± 12.8 years). Data were excluded due to either an unsatisfactory EEG quality for spectral analyses or failure to take blood for ABG in any phase of the study. Thirty-two EEG spectra data were analyzed from C3/A2 channel, and 9 were analyzed from C4/A1 channel. Of these 41 patients, 26 had OHS and 15 had overlap syndrome. Twenty-five patients received BPAP treatment and 16 received CPAP treatment. The treatment option was allocated randomly as a part of the original study protocol in comparing the therapeutic outcomes of CPAP and BPAP. Three patients were using O2 supplementation during taking blood samples for ABG and during the initial diagnostic PSG. No patient had central apnea index > 5/h either in baseline or after PAP treatment.
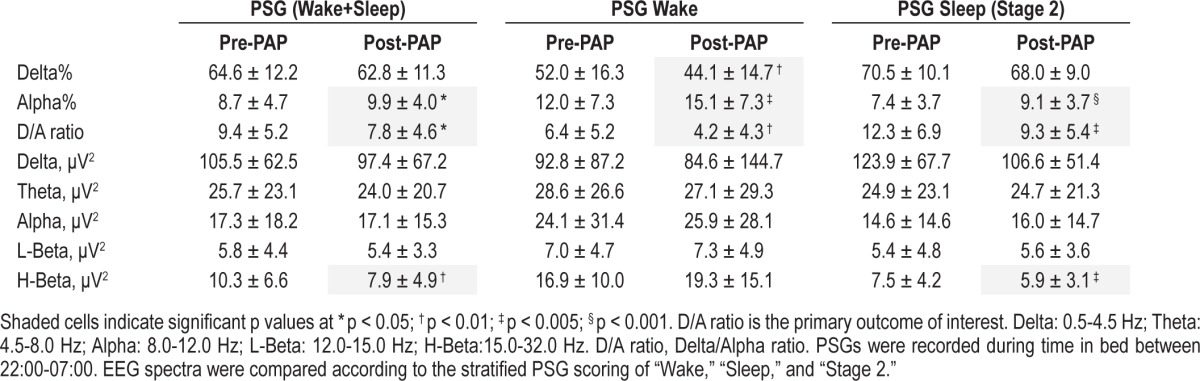
As shown in Table 1, after ∼3 months PAP treatment, patients showed a significant decrease in their waking pCO2, daytime sleepiness, as well as all key breathing/oxygenation parameters during sleep. BMI also decreased slightly. Similar to our previous report,31 no difference was found between the options of CPAP and BPAP in improving awake pCO2 (p = 0.27) and SpO2 nadir during sleep (p = 0.62) in the present study. EEG spectral analyses showed that the D/A ratio of EEG was significantly reduced during both sleeping and waking periods, indicating a generally faster, more activated EEG spectral profile following treatment (Table 2). There was also a reduction in High Beta power (15-32 Hz) after the PAP treatment (Table 2). This reduction was correlated with the decrease in arousal index (r = 0.33, p < 0.05).
Table 1.
Sleep and pCO2 data pre- and ~3 months post-PAP in 41 patients
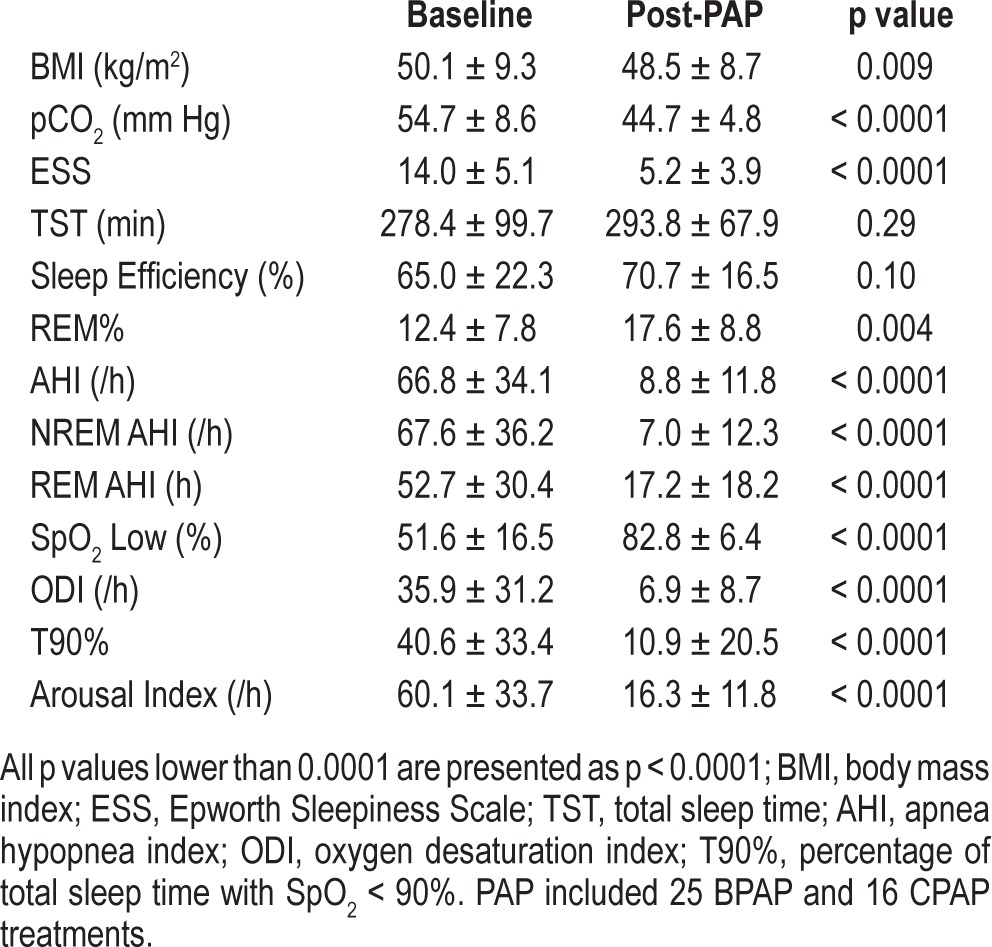
Table 2.
Quantitative EEG spectral analyses data pre- and ~3 months post-PAP in 41 patients
A cross-correlation was observed between a reduced wake pCO2, a faster (more activated) EEG (reduced D/A ratio) and reduced daytime sleepiness (Table 3, Figure 1). The cross-correlation pattern was similar between OHS and overlap syndrome subtypes of patients as shown in Figure 1. Specifically, there was a consistent pattern of positive correlations between the change of %Delta and ESS and pCO2; and negative correlations between the change of %Alpha and ESS and pCO2 (Table 3). The ESS change also correlated significantly with waking pCO2 change (r = 0.42, p = 0.007; Figure 1). This pattern of the cross-correlations existed in both waking and sleeping periods of the PSG recordings (Table 3).
Table 3.
Cross-correlation between the changes of EEG spectra, ESS, and pCO2
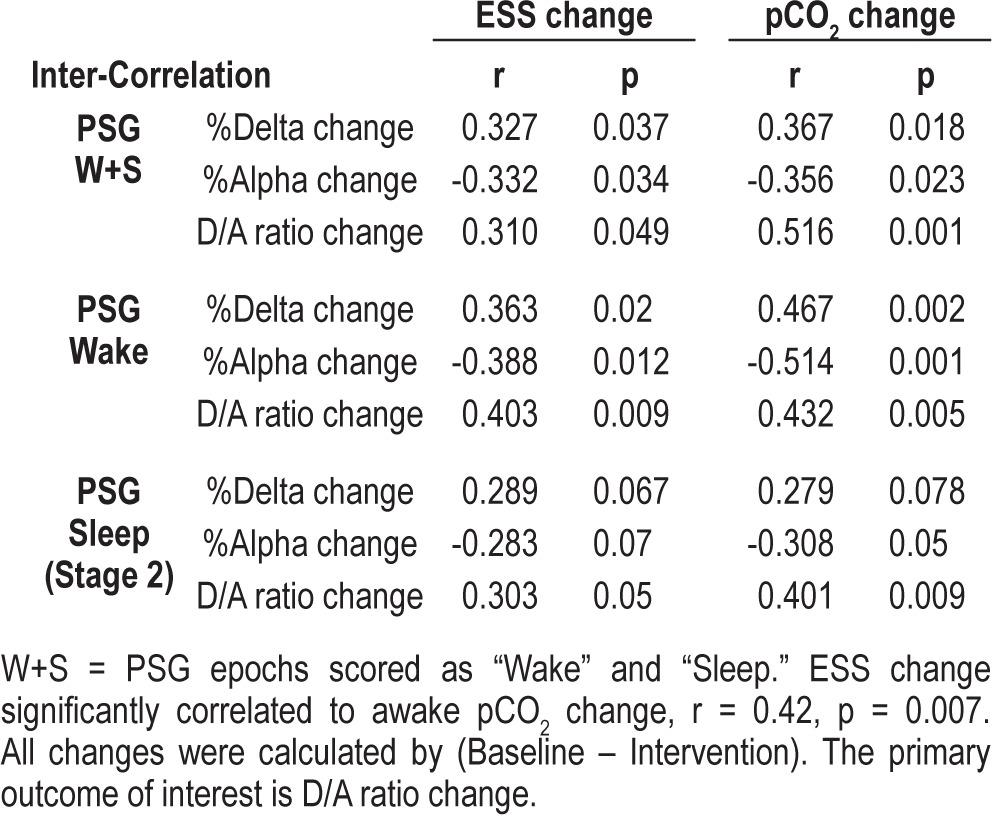
Figure 1. Inter-correlations between the changes of pCO2, EEG Delta/Alpha ratio and ESS before and after PAP treatment.
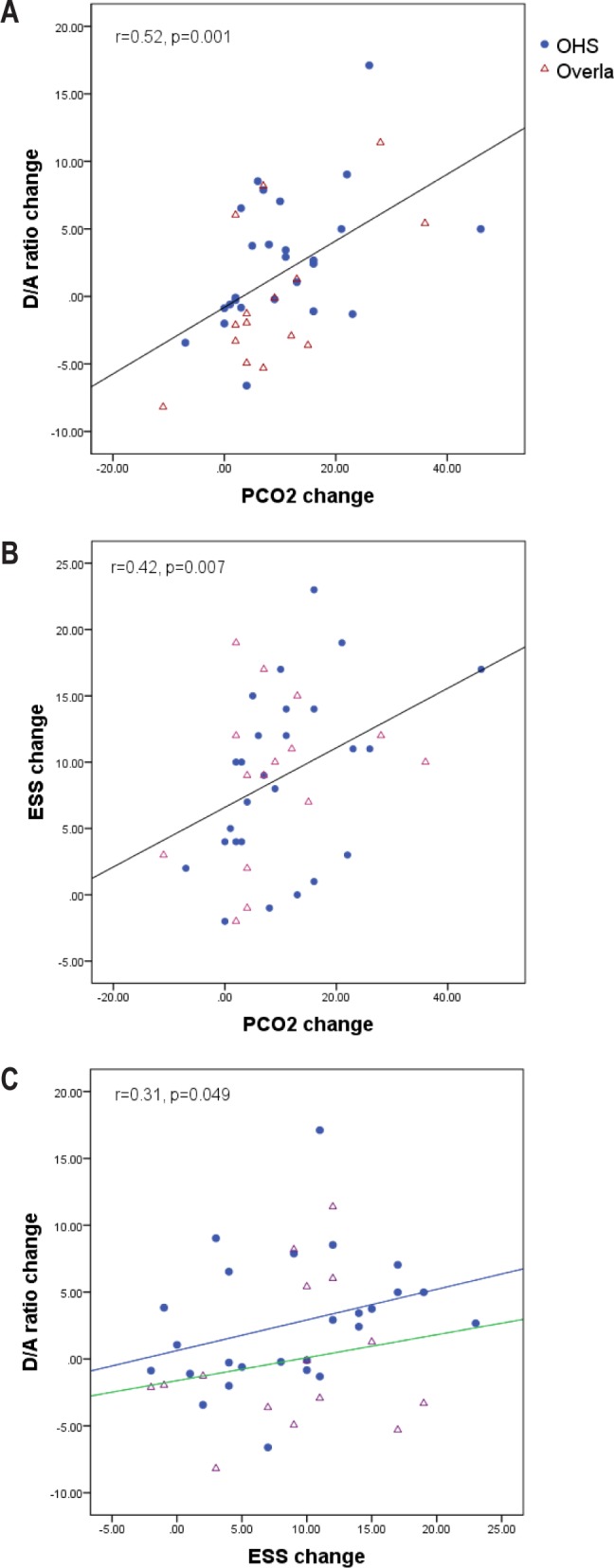
The 3 panels show consistent pattern of relationships between OHS and overlap subtypes. Panel C shows that even though the overall correlation between the changes of ESS and D/A ratio is relevantly loose (p = 0.049), the OHS and overlap subgroups show a similar pattern of relationship.
Given that many related parameters such as hypoxia, hypercapnia, arousal index, and BMI were improved after the PAP treatment (Table 1), we conducted multiple linear regression analyses, using the changes in ESS and D/A ratio as dependent variables respectively. Using the change of ESS as the dependent variable, the only significant predictor was pCO2 change, explaining 15% of the variance of ESS (t = 2.44, p = 0.02). The change in ODI was the second best predictor but was not statistically significant, explaining only 7% of the variance in ESS. Other variables such as the changes in REM%, AHI, SpO2 nadir, BMI, D/A ratio, arousal index, and sleep efficiency, were not significantly associated with the ESS. In regression analyses using the change of D/A ratio as the dependent variable, pCO2 change was again the only significant predictor, explaining 27% of the variance in D/A ratio (t = 3.51, p = 0.001). SpO2 nadir was the second best predictor (not significant), explaining only 4% of variance of D/A ratio. Other nonsignificant predictor variables were the changes of BMI, arousal index, AHI, sleep efficiency, and ODI.
DISCUSSION
We have demonstrated that hypercapnia but not hypoxia is a key correlate of both daytime sleepiness and EEG activation in patients with hypercapnic SDB. The decrease in hypercapnia was the best predictor of both reduction in daytime sleepiness and increase in EEG activation (reduced D/A ratio). We speculate that hypercapnia may cause daytime sleepiness through a reduction in brain activation in a hypercapnic SDB population.
SDB is usually associated with cyclic patterns of hypoxia and hypercapnia. In the present study, we demonstrated a significant cross-correlation between a reduced waking pCO2, a faster, more activated EEG spectral profile, and a reduced reported daytime sleepiness. Using multiple linear regression modeling, we demonstrated that hypercapnia accounted for more than double the variance of ESS compared to ODI (a measure of intermittent hypoxia frequency), and nearly 7 times the variance in EEG spectral (D/A ratio) compared to hypoxic severity. By contrast, hypoxia was not a significant predictor for the variance of ESS, although it is still a better predictor than AHI, arousal index, BMI, and sleep efficiency.
We are not aware of any previous study showing hypercapnia as covarying with hypersomnolence in any subtypes of SDB.1–7 In the largest PAP intervention trial in sleep apnea, measures of hypoxia were only weakly associated with ESS, explaining < 2% of the variance. The degree of hypercapnia in their study patients was unknown.6 In another large study of determinants of sleepiness in 2,882 OSA patients,32 there was no real difference in hypoxia between those patients with or without sleepiness (only 1% difference). Interestingly, OSA patients with excess sleepiness had increased slow wave sleep compared to the non-sleepy patients, supporting a correlation between daytime sleepiness and a slower EEG spectral activity32; pCO2 was not measured in this study.32 Importantly, if hypoxia is a key factor in sleepiness, then hypersomnolence should be alleviated by giving supplemental O2. However, this prediction is not supported by the few relevant studies.10–13
Our notion that hypercapnia affects cerebral neural activity is supported by a number of experimental animal and human studies. Hypercapnia, acute or chronic, leads to the slowing of EEG in eels, rats, rabbits, dogs, and monkeys.33–37 In human experimental studies, hypercapnia led to slower EEG spectral activity with decreased alpha and beta activity38–42 and increased delta activity.39 A recent study tested the effects of mild hypercapnia (5% CO2) on magnetoencephalogram, event-related potentials, auditory pattern recognition, and visual semantic tasks in seven healthy volunteers.41 Hypercapnia attenuated evoked and spontaneous magnetoencephalogram spectral activity. In addition, comparable decreases were observed in early sensory components in both auditory and visual modalities as well as cognitive components related to memory and language, and the depressant effects were distributed across all cortical regions.41 Similarly, a few experimental studies reported dose-response relationship between higher CO2 tensions and impaired cognitive and psychomotor performance.15–17 In addition, breathing of CO2 was reported to attenuate sensory and affective components of experimental ischemic pain and produce a dose-dependent elevation of heat pain threshold.19 In this context, 80% CO2 is commonly used as a porcine stunning agent to produce unconsciousness before slaughtering; hypoxia does not produce a similar anesthetic effect.20,43 Given these data, exposure to sustained hypercapnia or possibly even brief bursts of intermittent hypercapnia in sleep disordered breathing may result in drowsiness secondary to reduced brain neuroelectrical activation and overall depression of cortical activity.
In our studies, we used the Delta/Alpha ratio obtained from EEG spectral analyses as an objective marker of EEG activation that correlated to daytime sleepiness. An important consideration is that the D/A ratio can avoid the misinterpretation of an increased Delta power purely caused by a global, frequency independent, increase in EEG power. Ratios of slow and fast EEG frequency bands are commonly used in neurological studies to indicate activation of EEG.21,24–26 The D/A ratio has been previously identified as the best discriminator between wake, and stage 1, 2, and slow wave sleep,26 and the best brain biomarker correlating to the clinical outcomes of subacute ischemic stroke.24
In the present study we only examined severe SDB patients with awake hypercapnia, so our findings may not apply to SDB patients in general, especially mild-moderate OSA patients, although the mechanism may play a role. To study the less severe SDB patients requires a fully validated and accurate continuous measure of pCO2, fast enough to respond to the rapid changes due to respiratory events. Such a machine is currently not available. The commonly used transcutaneous pCO2 (PtcCO2) measurement are often affected by a drifting artifact, while a CO2 analyzer connected to nasal cannula cannot sample expired air during a prolonged apnea. We speculate that in milder SDB patients, the daytime hypersomnolence would be affected by the CO2 mechanisms of (1) cyclic hypercapnia episodes during apnea; (2) mild hypercapnia in daytime; and (3) SDB related chemoreflex changes, particularly those CO2 related factors such as CO2 threshold. Indeed, even in healthy individuals, average resting pCO2 may vary largely, ranging from 32 to 44 mm Hg.44
There are a number of limitations to the study. This is not a prospective study originally designed to serve the purpose. The data were retrieved from post hoc analyses of an interventional clinical study. Those patients were randomly allocated to BPAP or CPAP treatment, thus the PAP treatment they were receiving might not be fully tailored to individual clinical conditions. There was still some residual SDB after PAP treatment. Three patients were using O2 supplementation during blood sampling for ABG and during the initial diagnostic PSG, which could affect pCO2 and SpO2 results. In addition, most of our patients were taking different medications, and it is not possible to obtain a medication-free group of patients with severe SDB. Nevertheless, our study used patients as their own controls, so that the effect of this potential confounding factor was minimized. Furthermore, our studies relied on self-reported measures of sleepiness. A further study using multiple sleep latency test or maintenance of wakefulness test to quantify daytime sleepiness in addition to ESS and EEG spectral analyses would be more definitive. Moreover, the sampling rate of our two PSG systems was slightly different, being 256 Hz for Compumedics and 200 Hz for Alice. Nevertheless, as the primary outcome of interest of the present study is Delta/Alpha ratio (rather than an absolute spectral power) as an EEG activation marker, the potential confounding effect on our conclusion is therefore minimal. The last, it would be of interest to examine neurometabolic imaging during hypercapnia to clarify the relationship between hypercapnia, a slower EEG spectral profile and hypersomnolence.
In conclusion, we identified that in hypercapnic SDB population, hypercapnia is a key correlate of SDB-related daytime sleepiness, and there is a significant cross-correlation between the changes of hypercapnia, EEG spectral activity, and daytime sleepiness. We speculate that hypercapnia may cause drowsiness through a reduced brain neuroelectrical activation in this population. Whether similar mechanisms also apply to less severe SDB patients without daytime hypercapnia deserve further investigation.
DISCLOSURE STATEMENT
This was not an industry supported study. Dr. David Wang is supported by NHMRC Health Professional Research Fellowship (#571165), NHMRC Project Grant (#1043633), and Sydney Medical School Early Career Researcher/New Staff Award. Prof. Ronald Grunstein is supported by NHMRC Practitioner Fellowship. Dr. Jong-Won Kim is supported by NHMRC CRE in Respiratory and Sleep Medicine. The other authors have indicated no financial conflicts of interest.
ABBREVIATIONS
- ABG
arterial blood gas
- AHI
apnea-hypopnea index
- BMI
body mass index
- COPD
chronic obstructive pulmonary disease
- CPAP
continuous positive airway pressure
- D/A ratio
Delta/Alpha ratio
- ECG
electrocardiogram
- EEG
electroencephalography
- ESS
Epworth Sleepiness Scale
- ODI
oxygen desaturation index
- OHS
obesity hypoventilation syndrome
- OSA
obstructive sleep apnoea
- PSG
polysomnography
- PtcCO2
transcutaneous PCO2
- REM
rapid eye movement sleep
- SDB
sleep disordered breathing
- SpO2
oxygen saturation
- T90
total sleep time with SpO2 < 90%
- TST
total sleep time
REFERENCES
- 1.Dempsey JA, Veasey SC, Morgan BJ, O'Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010;90:47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Engleman HM, Douglas NJ. Sleep. 4: Sleepiness, cognitive function, and quality of life in obstructive sleep apnoea/hypopnoea syndrome. Thorax. 2004;59:618–22. doi: 10.1136/thx.2003.015867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koehler U, Apelt S, Augsten M, et al. Daytime sleepiness in patients with obstructive sleep apnoea (OSA) - pathogenetic factors. Pneumologie. 2011;65:137–42. doi: 10.1055/s-0030-1255838. [DOI] [PubMed] [Google Scholar]
- 4.Kingshott RN, Vennelle M, Hoy CJ, Engleman HM, Deary IJ, Douglas NJ. Predictors of improvements in daytime function outcomes with CPAP therapy. Am J Respir Crit Care Med. 2000;161:866–71. doi: 10.1164/ajrccm.161.3.9905053. [DOI] [PubMed] [Google Scholar]
- 5.Roth T, Roehrs T, Rosenthal L. Hypersomnolence and neurocognitive performance in sleep apnea. Curr Opin Pulm Med. 1995;1:488–90. doi: 10.1097/00063198-199511000-00010. [DOI] [PubMed] [Google Scholar]
- 6.Quan SF, Chan CS, Dement WC, et al. The association between obstructive sleep apnea and neurocognitive performance--the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2011;34:303–14B. doi: 10.1093/sleep/34.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141:1601–10. doi: 10.1378/chest.11-2214. [DOI] [PubMed] [Google Scholar]
- 8.Vgontzas AN, Bixler EO, Chrousos GP. Metabolic disturbances in obesity versus sleep apnoea: the importance of visceral obesity and insulin resistance. J Intern Med. 2003;254:32–44. doi: 10.1046/j.1365-2796.2003.01177.x. [DOI] [PubMed] [Google Scholar]
- 9.Vgontzas AN, Papanicolaou DA, Bixler EO, Kales A, Tyson K, Chrousos GP. Elevation of plasma cytokines in disorders of excessive daytime sleepiness: role of sleep disturbance and obesity. J Clin Endocrinol Metab. 1997;82:1313–6. doi: 10.1210/jcem.82.5.3950. [DOI] [PubMed] [Google Scholar]
- 10.Gold AR, Schwartz AR, Bleecker ER, Smith PL. The effect of chronic nocturnal oxygen administration upon sleep apnea. Am Rev Respir Dis. 1986;134:925–9. doi: 10.1164/arrd.1986.134.5.925. [DOI] [PubMed] [Google Scholar]
- 11.Lim W, Bardwell WA, Loredo JS, et al. Neuropsychological effects of 2-week continuous positive airway pressure treatment and supplemental oxygen in patients with obstructive sleep apnea: a randomized placebo-controlled study. J Clin Sleep Med. 2007;3:380–6. [PMC free article] [PubMed] [Google Scholar]
- 12.Phillips BA, Schmitt FA, Berry DT, Lamb DG, Amin M, Cook YR. Treatment of obstructive sleep apnea. A preliminary report comparing nasal CPAP to nasal oxygen in patients with mild OSA. Chest. 1990;98:325–30. doi: 10.1378/chest.98.2.325. [DOI] [PubMed] [Google Scholar]
- 13.Pretto JJ, McDonald CF. Acute oxygen therapy does not improve cognitive and driving performance in hypoxaemic COPD. Respirology. 2008;13:1039–44. doi: 10.1111/j.1440-1843.2008.01392.x. [DOI] [PubMed] [Google Scholar]
- 14.Lahdensuo A, Ojanen M, Ahonen A, et al. Psychosocial effects of continuous oxygen therapy in hypoxaemic chronic obstructive pulmonary disease patients. Eur Respir J. 1989;2:977–80. [PubMed] [Google Scholar]
- 15.Fothergill DM, Hedges D, Morrison JB. Effects of CO2 and N2 partial pressures on cognitive and psychomotor performance. Undersea Biomed Res. 1991;18:1–19. [PubMed] [Google Scholar]
- 16.Henning RA, Sauter SL, Lanphier EH, Reddan WG. Behavioral effects of increased CO2 load in divers. Undersea Biomed Res. 1990;17:109–20. [PubMed] [Google Scholar]
- 17.Hesser CM, Fagraeus L, Adolfson J. Roles of nitrogen, oxygen, and carbon dioxide in compressed-air narcosis. Undersea Biomed Res. 1978;5:391–400. [PubMed] [Google Scholar]
- 18.Sayers JA, Smith RE, Holland RL, Keatinge WR. Effects of carbon dioxide on mental performance. J Appl Physiol. 1987;63:25–30. doi: 10.1152/jappl.1987.63.1.25. [DOI] [PubMed] [Google Scholar]
- 19.Gronroos M, Pertovaara A. A selective suppression of human pain sensitivity by carbon dioxide: central mechanisms implicated. Eur J Appl Physiol Occup Physiol. 1994;68:74–9. doi: 10.1007/BF00599245. [DOI] [PubMed] [Google Scholar]
- 20.Erhardt W, Ring C, Kraft H, et al. CO2-stunning of swine for slaughter from the anesthesiological viewpoint. Dtsch Tierarztl Wochenschr. 1989;96:92–9. [PubMed] [Google Scholar]
- 21.Morisson F, Decary A, Petit D, Lavigne G, Malo J, Montplaisir J. Daytime sleepiness and EEG spectral analysis in apneic patients before and after treatment with continuous positive airway pressure. Chest. 2001;119:45–52. doi: 10.1378/chest.119.1.45. [DOI] [PubMed] [Google Scholar]
- 22.Wang D, Piper AJ, Wong KK, et al. Slow wave sleep in patients with respiratory failure. Sleep Med. 2011;12:378–83. doi: 10.1016/j.sleep.2011.01.007. [DOI] [PubMed] [Google Scholar]
- 23.Wang D, Piper AJ, Yee BJ, et al. Hypercapnia: New insights into sleep-disordered breathing related daytime sleepiness. Sleep. 2013;36:173A. [Google Scholar]
- 24.Finnigan SP, Walsh M, Rose SE, Chalk JB. Quantitative EEG indices of sub-acute ischaemic stroke correlate with clinical outcomes. Clin Neurophysiol. 2007;118:2525–32. doi: 10.1016/j.clinph.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 25.Moraes Wdos S, Poyares DR, Guilleminault C, Ramos LR, Bertolucci PH, Tufik S. The effect of donepezil on sleep and REM sleep EEG in patients with Alzheimer disease: a double-blind placebo-controlled study. Sleep. 2006;29:199–205. doi: 10.1093/sleep/29.2.199. [DOI] [PubMed] [Google Scholar]
- 26.Susmakova K, Krakovska A. Classification of waking, sleep onset and deep sleep by single measures. Meas Sci Rev. 2007;7:34–8. [Google Scholar]
- 27.Johns MW. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 28.Rechtschaffen A, Kales A. Washington, DC: Public Health Services, U.S. Government Printing Office; 1968. A Manual of Standardized Terminology, Techniques and Scoring Systems for Sleep Stages of Human Subjects. [Google Scholar]
- 29.AASM Task Force. Sleep-related breathing disorders in adults: recommendations for syndrome definition and measurement technique in clinical research. Sleep. 1999;22:667–89. [PubMed] [Google Scholar]
- 30.American Sleep Disorders Association. EEG arousals: scoring rules and examples: a preliminary report from the Sleep Disorders Atlas Task Force of the American Sleep Disorders Association. Sleep. 1992;15:173–84. [PubMed] [Google Scholar]
- 31.Piper AJ, Wang D, Yee BJ, Barnes DJ, Grunstein RR. Randomised trial of CPAP vs bilevel support in the treatment of obesity hypoventilation syndrome without severe nocturnal desaturation. Thorax. 2008;63:395–401. doi: 10.1136/thx.2007.081315. [DOI] [PubMed] [Google Scholar]
- 32.Roure N, Gomez S, Mediano O, et al. Daytime sleepiness and polysomnography in obstructive sleep apnea patients. Sleep Med. 2008;9:727–31. doi: 10.1016/j.sleep.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 33.Barthelemy L, Mabin D, Belaud A, Peyraud C. [Electrical activity of the brain of the eel (Anguilla anguilla L.) subjected to hypoxia and hypercapnia] J Physiol (Paris) 1977;73:1035–44. [PubMed] [Google Scholar]
- 34.Forslid A, Ingvar M, Rosen I, Ingvar DH. Carbon dioxide narcosis: influence of short-term high concentration carbon dioxide inhalation on EEG and cortical evoked responses in the rat. Acta Physiol Scand. 1986;127:281–7. doi: 10.1111/j.1748-1716.1986.tb07907.x. [DOI] [PubMed] [Google Scholar]
- 35.Matakas F, Birkle J, Cervos-Navarro J. The effect of prolonged experimental hypercapnia on the brain. Acta Neuropathol. 1978;41:207–10. doi: 10.1007/BF00690437. [DOI] [PubMed] [Google Scholar]
- 36.Smith LJ, Greene SA, Moore MP, Keegan RD. Effects of altered arterial carbon dioxide tension on quantitative electroencephalography in halothane-anesthetized dogs. Am J Vet Res. 1994;55:467–71. [PubMed] [Google Scholar]
- 37.Zappe AC, Uludag K, Oeltermann A, Ugurbil K, Logothetis NK. The influence of moderate hypercapnia on neural activity in the anesthetized nonhuman primate. Cereb Cortex. 2008;18:2666–73. doi: 10.1093/cercor/bhn023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bloch-Salisbury E, Lansing R, Shea SA. Acute changes in carbon dioxide levels alter the electroencephalogram without affecting cognitive function. Psychophysiology. 2000;37:418–26. [PubMed] [Google Scholar]
- 39.Halpern P, Neufeld MY, Sade K, et al. Middle cerebral artery flow velocity decreases and electroencephalogram (EEG) changes occur as acute hypercapnia reverses. Intensive Care Med. 2003;29:1650–5. doi: 10.1007/s00134-003-1917-6. [DOI] [PubMed] [Google Scholar]
- 40.Kalkman CJ, Boezeman EH, Ribberink AA, Oosting J, Deen L, Bovill JG. Influence of changes in arterial carbon dioxide tension on the electroencephalogram and posterior tibial nerve somatosensory cortical evoked potentials during alfentanil/nitrous oxide anesthesia. Anesthesiology. 1991;75:68–74. doi: 10.1097/00000542-199107000-00012. [DOI] [PubMed] [Google Scholar]
- 41.Thesen T, Leontiev O, Song T, et al. Depression of cortical activity in humans by mild hypercapnia. Hum Brain Mapp. 2012;33:715–26. doi: 10.1002/hbm.21242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Woodbury DM, Karler R. The role of carbon dioxide in the nervous system. Anesthesiology. 1960;21:686–703. doi: 10.1097/00000542-196011000-00012. [DOI] [PubMed] [Google Scholar]
- 43.Hartung J, Nowak B, Waldmann KH, Ellerbrock S. CO2-stunning of slaughter pigs: effects on EEG, catecholamines and clinical reflexes. Dtsch Tierarztl Wochenschr. 2002;109:135–9. [PubMed] [Google Scholar]
- 44.Shea SA, Walter J, Murphy K, Guz A. Evidence for individuality of breathing patterns in resting healthy man. Respir Physiol. 1987;68:331–44. doi: 10.1016/s0034-5687(87)80018-x. [DOI] [PubMed] [Google Scholar]


