Abstract
Study Objective:
Recent investigations suggest that motor skill learning is impaired in patients with obstructive sleep apnea (OSA) syndrome; however, it is not fully understood at what stages of learning this impairment occurs. The current study aimed to compare motor learning and memory across both daytime acquisition and overnight consolidation.
Methods:
Twelve OSA patients and twelve control participants, matched for age and education, were recruited and completed the Karolinska Sleepiness Scale and the sequential finger-tapping task (SFTT), a motor skill learning task, both before and after polysomnographic recorded sleep.
Results:
During the evening acquisition phase both groups showed significant and equitable improvement in the number of correctly typed sequences across trials. On retesting the following morning, the control patients showed significantly greater improvement overnight (15.35%) compared to OSA patients (1.78%). The post sleep improvement in controls, but lacking in OSA patients, was typical of a sleep dependent enhancement effect. The magnitude of improvement overnight for either group was not significantly correlated with any of the recorded sleep variables.
Conclusions:
These results suggest daytime/practice related acquisition of motor skill is largely intact in OSA patients; however, marked impairment in the consolidation phase is evident following a sleep period. This particular pattern of dysfunction may remain unnoticed following single-day learning/memory assessments.
Citation:
Landry S, Anderson C, Andrewartha P, Sasse A, Conduit R. The impact of obstructive sleep apnea on motor skill acquisition and consolidation. J Clin Sleep Med 2014;10(5):491-496.
Keywords: obstructive sleep apnea, OSA, memory, learning, motor skills, sleep-dependent consolidation
Obstructive sleep apnea (OSA) has been associated with impairments in vigilance, executive function, and memory.1–3 Several large-scale studies suggest that OSA patients are only mildly affected by this impairment4–6; however, memory complaints are common among OSA patients.7 As such these studies may not accurately gauge the full extent of memory dysfunction in these cases. Typical tests of memory function assess immediate and delayed recall to assess short-term and long-term memory, respectively. Long-term memory assessments are sensitive to the consolidation of memory. Therefore when assessing this performance in a sleep disordered group it may be important that retesting delay span a sleep period, and hence assess memory consolidation processes proposed to occur during sleep.8,9
The role of sleep in the process of memory consolidation is well established. A prototypical example of this is the explicit motor-sequence learning paradigm.10–13 Here a procedural motor skill learning task, which involves the repetition of an explicitly known sequence of finger movements, shows “offline” improvements in speed and accuracy (in the absence of further practice).This is observed only if this interval includes a period of sleep. This sleep-dependent task improvement is proposed to reflect enhanced consolidation processes occurring within sleep.10
Considering the disrupting nature of OSA on sleep continuity, quality, and structure,14 little is known about the effect OSA has on sleep-dependent motor skill consolidation. Kloepfer and colleagues investigated OSA related procedural memory impairment using the mirror tracing task.15 OSA patients demonstrated less trial by trial improvement (i.e., less practice-related improvement), resulting in significantly flatter learning curves over the pre-sleep learning trials. Specific analysis of the performance improvement over the sleep period was inconclusive, as controls did not show expected sleep related improvements, but conversely the OSA patients did.
BRIEF SUMMARY
Current Knowledge/Study Rationale: A growing literature suggests intervening sleep plays a vital role in the consolidation of motor skill learning. Previous studies investigating the potential harmful effect of untreated Obstructive Sleep Apnea (OSA) on this process have as yet produced conflicting results.
Study Impact: OSA patients, compared to controls, did not show expected motor skill improvements following sleep. Future assessments of learning and memory dysfunction in OSA populations may need to use a retention interval that spans a sleep period to fully gauge the extent of impairment.
An investigation using OSA to examine the effect of fragmented sleep on overnight memory consolidation compared patients with OSA with matched control participants on age, gender, and subjective/objective sleepiness outcomes.16 While the motor sequence learning task showed typical sleep related improvement in the controls, the OSA group did not show overnight improvement. Additionally no evidence of practice-based impairment was found in the evening practice trials. While the study design allowed for group differences based only on apnea-hypopnea index (AHI), oxygen nadir, and arousal index, the matching of sleepiness outcomes resulted in a reasonably younger (mean age of 31.9 ± 1.7) group of OSA patients with typically milder OSA (mean AHI of 17.1 ± 2.6) paired with sleepier than normal controls (mean ESS score of 9.9 ± 1.6). As a result, this may not accurately represent the clinical characteristics of typical patients who have carried moderate-severe OSA for longer periods of time, with more extensive deficits in daytime function and motor learning acquisition. Considering the conflicting nature of previous reports, the present study further examined motor skill acquisition and consolidation using a clinically representative group of OSA patients.
METHODS
Participants
Twenty-four participants (mean age 52 ± 6.26: range: 40 y and 64 y: male n = 18) were recruited. The OSA group comprised 12 participants recruited from a hospital-based sleep disorders unit (Victorian Rehabilitation Centre, Melbourne). All patients underwent a night of clinical polysomnographic (PSG) recording as part of a standard diagnostic testing for OSA. No patients were previously diagnosed or exposed to treatment prior to experimental procedures. Twelve age-matched (± 2 years) and education-matched control participants were recruited from university campus posters and local newspaper advertisements. Education was assessed categorically on highest level attained with the following categories; high school non-graduate, high school graduate, certificate, diploma or bachelor's degree, and postgraduate diploma or degree. All participants were excluded if they (a) had known brain injuries, (b) were currently taking medications or drugs, (c) had a medical condition likely to affect performance on the tests, (d) were diagnosed with a neurological disorder, or (e) performed shift work. Control participants were also excluded if they had been diagnosed with a sleep disorder or if overnight PSG recording revealed an AHI > 5. All participants gave informed written consent before taking part in the current experiment. Ethics approval was obtained from the by the Monash University Standing Committee on Ethics in Research Involving Humans (SCERH, Approval # 2005-327).
Procedure
OSA participants were administered the sequential finger tapping task (SFTT) between 21:30-22:30 (pre-sleep training) prior to a standard diagnostic sleep study at the Victorian Rehabilitation Centre sleep laboratory. Morning administration of the task was conducted between 06:30 and 07:00 (post-sleep testing). Control participants underwent the same procedure at the Monash University sleep laboratory. To reduce the impact of sleep inertia, all participants were required to be awake ≥ 30 min before morning retest procedures could begin. No adaptation night was utilized, as both groups completed the same laboratory procedures.
Pre-sleep training on the SFTT involved twelve 30-sec trials, each separated by a rest period of 30 seconds. Post-sleep testing involved completion of the same 12 trials with the same intervening rest periods. The number of correctly typed sequences and the number of errors were calculated for each trial. Participants completed the Epworth Sleepiness Scale (ESS)17 before commencement of the evening test session to evaluate trait levels of daytime sleepiness. Participants also completed the Karolinska Sleepiness Scale (KSS)18 prior to both the evening pre-sleep training and the morning post-sleep training trials.
Design
This study utilized a two-way within-between groups design to examine whether OSA patients (compared to matched controls) showed impaired motor skill learning over both evening training and overnight consolidation periods. The SFTT was used to compare motor skill improvements over multiple trials, both before and after recorded sleep.
Sleep Recording
PSG data were collected using an S-Series 16 channel Polygraph with W-Series Sleep/Replay display and analysis software (Compumedics Pty, Ltd. Melbourne, Australia). Gold-plated electrodes (Model F-E5GH; Grass Instruments Co., CA), were applied to the scalp using a standard PSG recording montage: C3/A2 and C4/A1, EOG left and right outer canthi each referenced to Fp1 and EMG at the left and right masseter jaw musculature. EEG traces were calibrated at 50 μV = 1 cm with impedances < 5 kΩ. Nasal and oral air flow were measured using a piezo-ceramic thermistor placed on the philtrum, and nasal cannula fed into the outer nostrils. Respiratory effort was measured via thoracic and abdominal belt sensors. Body position was recorded using a position sensor, affixed to the center of the thoracic belt. Leg movements were measured via piezo-ceramic sensors taped to the dorsa of both feet. Oximetry was measured with an oximeter placed over a fingertip.
Sleep staging, hypopneas, and obstructive and central apneas were scored according to the 2007 American Academy of Sleep Medicine (AASM) criteria.19 Length of sleep and the proportion of stages N1, N2, N3, and REM sleep were calculated, as well as the total number of apneas and hypopneas per hour of sleep in order to calculate the apnea hypopnea index (AHI). Sleep spindles were visually scored for all N2 epochs. These were defined by activity in the 12-16 Hz frequency band lasting > 0.5 seconds with a typical amplitude-fusiform morphology. Spindle density (ratio) was calculated by the number of spindles in N2 divided by the length (in minutes) of N2 sleep.
The Sequential Finger Tapping Task (SFTT)
Motor skill learning was measured using the sequential finger tapping task (SFTT).10 This task requires the participant to type a 5-digit sequence, specifically 4 1 3 2 4, using a standard computer keyboard with their non-dominant hand. A visual cue of the sequence pattern was displayed at the top of the screen during the trial (4 1 3 2 4). Participants were required to reproduce the sequence as quickly and as accurately as possible during a 30-sec trial period. This trial period was repeated 12 times (see below). No accuracy feedback was provided, although a white dot was displayed in response to a key press forming a row from left to right. SFTT performance was measured by the number of correctly typed sequences per 30-sec trial. The task is by design a learning task and thus the change in performance (improvement) over time or training is the primary measure.
Statistical Analysis
Two specific areas of SFTT task improvement were investigated: practice-related improvement and overnight consolidation-related improvement. Practice related improvement specifically related to the increase in the number of correctly typed sequences between the first pre-sleep practice trial (trial E1, baseline performance) and the average of the final 3 pre-sleep practice trials (E10-E12). Overnight consolidation related improvement was specifically measured by the increase in correct sequences between the final 3 pre-sleep practice trials (E10-E12) and the first 3 post-sleep testing trials (M1-M3) conducted in the morning.
Two-way ANOVAs were used to assess overall task performance between groups as well as the change in performance (i.e., improvement) over practice and overnight periods within groups. Specific values are represented as means ± standard error of the mean (SEM). A p-value < 0.05 was considered statistically significant; effect sizes were calculated by partial eta squared such that 0.0099 = small effect, 0.0588 = moderate effect, 0.1379 = strong effect.20
RESULTS
Demographic and Sleep Variables
Data for age, gender, sleep quality, and subjective sleepiness for both OSA patients and control participants can be seen in Table 1. There were no differences for age or sex between the OSA patients and controls. Trait levels of subjective sleepiness (ESS) were significantly higher for OSA patients than controls. While OSA patients were significantly sleepier prior to the pre-sleep training trials, in the evening both groups scored similarly on the morning retest. See Table 1.
Table 1.
Statistics for demographic, sleep and subjective sleepiness ratings. Means ± SEM and inferential statistics are shown.
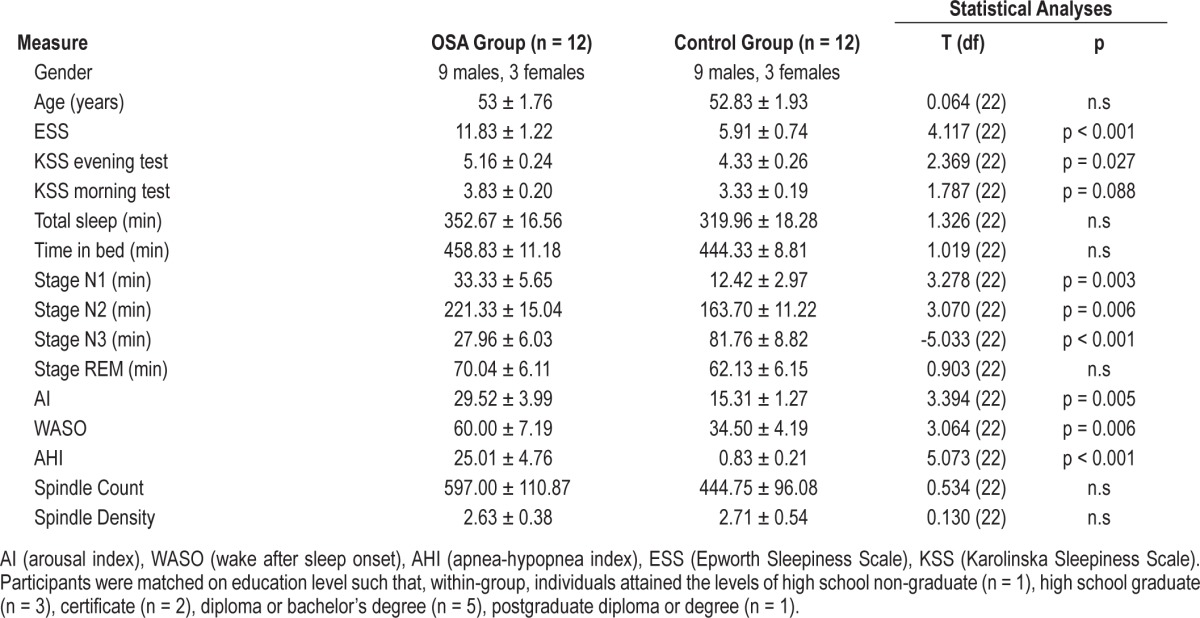
There were large clinical differences between the groups: OSA patients had a significantly higher AHI than control participants, and subsequently higher arousal indexes (AI) and greater wake after sleep onset (WASO). For sleep architecture, while there was no difference in total sleep time between groups, OSA patients spent more time in stage N1 and N2 sleep, and significantly less time in N3. There were no significant differences in spindle count or density between OSA patients and controls. See Table 1.
Pre-Sleep Practice-Related Improvement
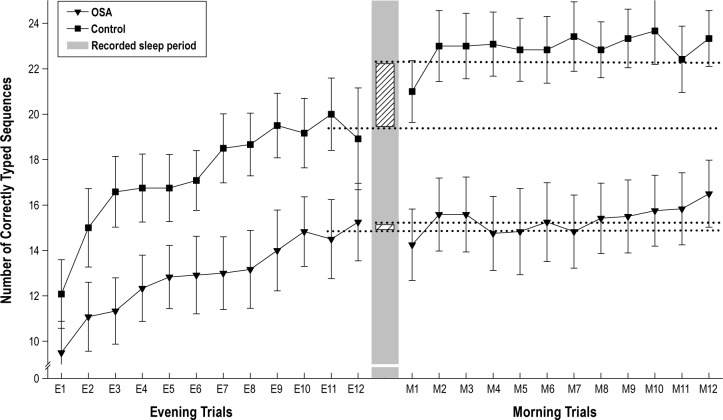
Figure 1 shows SFTT performance speed across all pre-sleep practice trials as well as the post-sleep testing trials. A mixed 2 × 2 ANOVA examining group differences in performance and improvement over the pre-sleep practice trials (E1 to E10-12) revealed a significant improvement across the evening practice sessions (F1,22 = 81.14, p < 0.001, partial η2 = 0.787).
Figure 1. Mean number of correctly typed sequences for each pre-sleep practice trial (E1-12) and post-sleep testing trial (M1-12) for both OSA patients (n = 12, triangles) and control participants (n = 12, squares).
The separating gray bar donates the recorded sleep period. Lined bars represent the overnight consolidation related improvement which was greater for control participants (2.97 ± 0.73 sequences, 15.35%) than OSA patients (0.27 ± 0.45 sequences, 1.78%). Dotted lines represent, for each group, the mean values for the last 3 pre-sleep practice trials (E10-E12) and the first 3 post-sleep testing trials (M1-M3).
However, there was no main group effect (p = 0.108) or interaction (p = 0.186). Task improvement (increases in number of correctly typed sequences) did not significantly differ between OSA patients (5.36 ± 0.74 sequences/30 sec, 56.42%) and controls (7.27 ± 1.19 sequences/30 sec, 60.48%; p = 0.188), suggesting comparable learning between groups across the pre-sleep practice trials.
Overnight Consolidation-Related Improvement
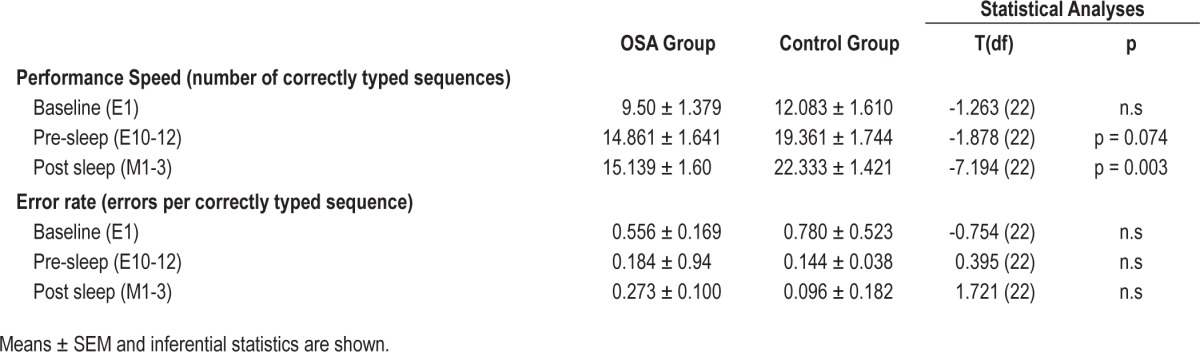
A further mixed 2 × 2 ANOVA exploring task improvement across the overnight transition (E10-12 to M1-3) showed a significant interaction (F1,22 = 9.884, p = 0.005, partial η2 = 0.310), with the control group showing a greater improvement: 15.35% (2.97 ± 0.73 sequences/30 sec) after sleep, compared to the OSA group who showed 1.78% improvement (0.27 ± 0.45 sequences/30 sec). Main effects of overnight task improvement (F1,22 = 14.381, p = 0.001, partial η2 = 0.395) and group (F1,22 = 6.871, p = 0.016, partial η2 = 0.238) were significant. Table 2 shows means and SEM data for baseline, pre-sleep, and post-sleep performance trials.
Table 2.
SFTT performance speed and error rate between groups at baseline, pre-sleep, and post-sleep.
Correlational analysis examined whether the lack of overnight improvement for OSA patients was due to lower performance prior to sleep. No significant correlation between performance prior to sleep (average of E10-12) and improvement overnight was found for the OSA group (p = 0.474). In contrast, for the control group, a significant negative correlation (r = -0.611, p = 0.035, r2 = 0.373) suggested lower task performance prior to sleep was associated with greater improvement overnight.
Subjective sleepiness was not related to pre-sleep performance or post-sleep improvement. KSS evening scores were not associated with evening SFTT performance (average of trials E10-12, OSA, p = 0.253; controls, p = 0.511) or overnight improvement (OSA, p = 0.699; controls, p = 0.846). Similarly, KSS morning scores were not associated with morning performance (average of trials M1-3, OSA, p = 0.984; controls, p = 0.838) or overnight improvement (OSA, p = 0.715; controls, p = 0.303).
Error Analysis
SFTT error rate was calculated as the number of errors per correctly typed sequence. This was subjected to the same ANOVA analysis as task speed (see above). There were no significant changes in error rate over the pre-sleep practice trials, overnight, or between groups. No significant interactions were found (p ≥ 0.071). Means and SEM for the 3 trial points of comparison are depicted in Table 2.
Sleep Stage Analysis
Due to the sleep architectural differences between OSA patients and control participants (Table 1), correlational analysis was performed separately within groups. The amount of overnight improvement (in the number of sequences correct) was not significantly correlated with any sleep (stage length, TST, AI, WASO, etc.) or respiratory variable for the OSA patients (p > 0.05). While for the control participants there was a negative correlation between the extent of overnight improvement and WASO, after type 1 error correction from multiple comparisons this association became nonsignificant (α < 0.01; r = -0.645, n = 12, p = 0.02, r2 = 0.42). There was no such significant association found for arousal index (AI) or apnea hypopnea index (AHI) and overnight improvement.
Spindle Analysis
For OSA patients, there was a positive correlation between spindle density and overnight task improvement, although this did not reach statistical significance (r = 0.550, p = 0.064). This was similarly found for spindle count (r = 0.502, p = 0.096). For controls these correlations were by comparison weak, negative and statistically insignificant (density: r = -0.146, p = 0.651; count: r = -0.141, p = 0.662).
DISCUSSION
OSA patients and age/education matched control participants had similar performance gains in the number of correct typed sequences across a pre-sleep evening practice phase on the sequential finger tapping task (SFTT). Despite OSA patients' higher subjective sleepiness ratings prior to this session, the group was unimpaired in the practice-related aspect of motor skill acquisition. However, a different pattern of results was evident after an intervening period of overnight sleep: control participants showed a 15.35% improvement in task performance, compared to OSA patients who showed only a marginal improvement (1.78%).
The intact practice related improvement in the OSA group is consistent with previous investigations of procedural memory in OSA patients utilizing single-day testing procedures.21,22 In contrast, studies that have utilized the mirror tracing task6,15 have shown significantly flatter learning curves over practice periods, reflecting daytime practice-based learning impairments in OSA patients. However as a complex motor learning task, mirror tracing may be more susceptible to impairment in OSA populations, particularly as it relies on motor coordination and drawing, a domain that appears more substantially impaired than motor speed in OSAS.1
Despite rate of improvement over the practice sessions being comparable between groups, there was a visible trend (Figure 1) of poorer (slower) performance in the OSA patients, characterized by fewer typed sequences overall. Although this is in contrast to Djonlagic et al.16 who found no evidence of any such performance impairment, the OSA group in their study was on average 20 years younger and with had milder OSA (mean AHI of 17.1 vs 25.01) compared to our patient group. While our differences may have also been due to higher evening sleepiness as reflected by higher KSS ratings in the OSA group, we found no significant associations between KSS and motor task performance. Although age and prior education were controlled for, prior experience with touch typing was unknown and uncontrolled, and therefore may have contributed to variations in participant performance. Most importantly, however, these group differences in performance were statistically insignificant (p > 0.1).
As expected, the OSA patients showed a marginal increase in performance after sleep, whereas the control group showed significantly greater improvement. The magnitude of this improvement in the control group was typical of the sleep related memory enhancement effect as observed in previous experiments.10,11 The lack of significant overnight improvement in OSA patients is consistent with Djonlagic et al.16 despite the differences in patient severity between these two studies. It is then possible that the threshold for this impairment might be sufficiently low to manifest in patients of mild OSA severity, where daytime cognitive impairments may not be noticeable and the negative health effects of untreated OSA may be minor.
Improvements in overnight performance on the SFTT have been specifically related to N2 sleep.10,12 As OSA patients in the current study show significantly longer N2 sleep, it could be argued that OSA patients should have shown greater task improvement overnight. More specifically, sleep spindles have been associated with sleep dependent improvements on motor tasks, including the SFTT.12,23 Despite this, we found no significant differences in spindle count or density between patients and controls, and no significant associations between sleep spindles and overnight task improvement within either group. However we did find significantly reduced N3 sleep in OSA patients compared to controls. Although N3 sleep has been primarily associated with declarative task improvements,24 it is possible that the reduced N3 time, such as that found in OSA patients, may disrupt typical sleep related consolidation processes and in part account for the currently observed deficit in overnight consolidation.
A key limitation of the current study was that performance improvement overnight was not compared to improvement over an equal period of waking. Therefore it has not specifically demonstrated that the consolidation related improvement shown in controls, and lacking in OSA patients, is dependent on sleep. However, as the overnight period was dominated by sleep in both groups and the key difference between groups was the diagnosis of a sleep disorder, it is likely that sleep played a large role in the observed consolidation process. Despite this, the amount of overnight improvement in OSA group was not significantly correlated with any of the subjective sleepiness ratings, AHI, sleep architecture, or fragmentary sleep measures.
An alternative explanation for this pattern of results is that the reduced motor performance post-sleep evidenced by OSA patients could have been affected by variations in biological timing of tests between groups. That is, OSA patients' performance may have been preferentially sensitive to circadian variations in motor performance, or more significantly, OSA patients may have been subject to an extended period of sleep inertia. However, as KSS ratings in the morning were comparable between groups, this is unlikely to account for the post-sleep reduction in performance. Future studies might implement a measure of circadian phase, as well as an added objective measure of performance such as the PVT or the learning of a novel motor sequence to further address these concerns.
Another possibility is that the reduced pre-sleep performance speed itself may have affected the level of overnight improvement. This impairment would then reflect deficient encoding prior to sleep rather than sleep related consolidation. Further analyses suggest this is unlikely. Firstly, OSA patients' pre-sleep performance was not significantly correlated with the extent of overnight improvements. Secondly, in controls this correlation was significant but negative, meaning that the poorer performing participants prior to sleep tended to show the greatest improvements following sleep. This result is not unanticipated, as more complex and difficult finger sequences show greater benefit following sleep.25
The present study found OSA patients do not demonstrate expected sleep dependent motor improvements following sleep. This suggests that the consolidation aspect of motor learning is specifically impaired in OSA patients. Similarly impaired overnight consolidation has been established in other patient groups with sleep disorders such as insomnia and narcolepsy, as well as psychological disorders in which sleep disruption is common, such as major depression and schizophrenia.26–31 This may suggest fragmentary/disrupted sleep structure as a primary determinant of this impairment. The question remains then of what minimal level of sleep disruption is necessary to impair this consolidation process. This concerns not only the extent but also the specific form of sleep disruption. Further research into the specifics of this threshold will help elucidate the boundaries of what constitutes healthy and unhealthy sleep for enhanced memory consolidation in healthy and sleep disordered patient groups.
DISCLOSURE STATEMENT
This was not an industry supported study. This research was entirely funded by university/faculty graduate research funding. The research was performed at Monash University and South Eastern Disordered Breathing Unit sleep laboratories, Victoria, Australia. The authors have indicated no financial conflicts of interest.
ACKNOWLEDGMENTS
The authors thank the staff and sleep technicians at the Victorian Rehabilitation Centre sleep laboratory, Suzanne Ftouni for her assistance with the graphical data, and Mathew Walker and Robert Stickgold for providing the sequential finger tapping task; as well as all patients and participants who took part in the present research.
REFERENCES
- 1.Beebe D, Groesz L, Wells C, Nichols A, McGee K. The neuropsychological effects of obstructive sleep apnea: a meta-analysis of norm-referenced and case-controlled data. Sleep. 2003;26:298–307. doi: 10.1093/sleep/26.3.298. [DOI] [PubMed] [Google Scholar]
- 2.Tsai JC. Neurological and neurobehavioral sequelae of obstructive sleep apnea. NeuroRehabilitation. 2010;26:85–94. doi: 10.3233/NRE-2010-0538. [DOI] [PubMed] [Google Scholar]
- 3.Wallace A, Bucks RS. Memory and obstructive sleep apnea: a meta analysis. Sleep. 2013;36:203–20. doi: 10.5665/sleep.2374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quan SF, Chan C, Dement W, et al. The association between obstructive sleep apnea and neurocognitive performance--the Apnea Positive Pressure Long-term Efficacy Study (APPLES) Sleep. 2011;34:303–14B. doi: 10.1093/sleep/34.3.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Quan SF, Wright R, Baldwin CM, et al. Obstructive sleep apnea-hypopnea and neurocognitive functioning in the Sleep Heart Health Study. Sleep Med. 2006;7:498–507. doi: 10.1016/j.sleep.2006.02.005. [DOI] [PubMed] [Google Scholar]
- 6.Naegele B, Launois SH, Mazza S, Feuerstein C, Pepin JL, Levy P. Which memory processes are affected in patients with obstructive sleep apnea? An evaluation of 3 types of memory. Sleep. 2006;29:533–44. doi: 10.1093/sleep/29.4.533. [DOI] [PubMed] [Google Scholar]
- 7.Skomro RP, Kryger MH. Clinical presentations of obstructive sleep apnea syndrome. Prog Cardiovasc Dis. 1999;41:331–40. doi: 10.1053/pcad.1999.0410331. [DOI] [PubMed] [Google Scholar]
- 8.Diekelmann S, Wilhelm I, Born J. The whats and whens of sleep-dependent memory consolidation. Sleep Med Rev. 2009;13:309–21. doi: 10.1016/j.smrv.2008.08.002. [DOI] [PubMed] [Google Scholar]
- 9.Mednick SC, Alaynick WA. Comparing models of sleep-dependent memory consolidation. J Exp Clin Med. 2010;2:156–64. [Google Scholar]
- 10.Walker MP, Brakefield T, Morgan A, Hobson JA, Stickgold R. Practice with sleep makes perfect: sleep-dependent motor skill learning. Neuron. 2002;35:205–11. doi: 10.1016/s0896-6273(02)00746-8. [DOI] [PubMed] [Google Scholar]
- 11.Walker MP, Brakefield T, Seidman J, Morgan A, Hobson JA, Stickgold R. Sleep and the time course of motor skill learning. Learn Mem. 2003;10:275–84. doi: 10.1101/lm.58503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nishida M, Walker M. Daytime naps, motor memory consolidation and regionally specific sleep spindles. PLoS One. 2007;2:e341–e41. doi: 10.1371/journal.pone.0000341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Korman M, Doyon J, Doljansky J, Carrier J, Dagan Y, Karni A. Daytime sleep condenses the time course of motor memory consolidation. Nat Neurosci. 2007;10:1206–13. doi: 10.1038/nn1959. [DOI] [PubMed] [Google Scholar]
- 14.American Academy of Sleep Medicine. Westchester, IL: American Academy of Sleep Medicine; 2001. International classification of sleep disorders, revised. [Google Scholar]
- 15.Kloepfer C, Riemann D, Nofzinger E, et al. Memory before and after sleep in patients with moderate obstructive sleep apnea. J Clin Sleep Med. 2009;5:540–8. [PMC free article] [PubMed] [Google Scholar]
- 16.Djonlagic I, Saboisky J, Carusona A, Stickgold R, Malhotra A. Increased sleep fragmentation leads to impaired off-Line consolidation of motor memories in humans. PLoS One. 2012;7:e34106. doi: 10.1371/journal.pone.0034106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Johns MW. A new method for measuring daytime sleepiness: The Epworth Sleepiness Scale. Sleep. 1991;14:540–5. doi: 10.1093/sleep/14.6.540. [DOI] [PubMed] [Google Scholar]
- 18.Akerstedt T, Gillberg M. Subjective and objective sleepiness in the active individual. Int J Neurosci. 1990;52:29–37. doi: 10.3109/00207459008994241. [DOI] [PubMed] [Google Scholar]
- 19.Iber C, Ancoli-Israel S, Chesson A, Quan SF. Westchester, IL: American Academy of Sleep Medicine; 2007. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. [Google Scholar]
- 20.Cohen J. Hillsdale, NJ: Lawrence Elbaum Associates; 1988. Statistical power analysis for the behavioral sciences. [Google Scholar]
- 21.Rouleau I, Decary A, Chicoine A-J, Montplaisir J. Procedural skill learning in obstructive sleep apnea syndrome. Sleep. 2002;25:401–11. [PubMed] [Google Scholar]
- 22.Nemeth D, Csábi E, Janacsek K, Várszegi M, Mari Z. Intact implicit probabilistic sequence learning in obstructive sleep apnea. J Sleep Res. 2011:396–401. doi: 10.1111/j.1365-2869.2011.00983.x. [DOI] [PubMed] [Google Scholar]
- 23.Fogel SM, Smith CT. Learning-dependent changes in sleep spindles and stage 2 sleep. J Sleep Res. 2006;15:250–5. doi: 10.1111/j.1365-2869.2006.00522.x. [DOI] [PubMed] [Google Scholar]
- 24.Diekelmann S, Biggel S, Rasch B, Born J. Offline consolidation of memory varies with time in slow wave sleep and can be accelerated by cuing memory reactivations. Neurobiol Learn Mem. 2012;98:103–11. doi: 10.1016/j.nlm.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Kuriyama K, Stickgold R, Walker MP. Sleep-dependent learning and motor-skill complexity. Learn Mem. 2004;11:705–13. doi: 10.1101/lm.76304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nissen C, Kloepfer C, Feige B, et al. Sleep-related memory consolidation in primary insomnia. J Sleep Res. 2011;20:129–36. doi: 10.1111/j.1365-2869.2010.00872.x. [DOI] [PubMed] [Google Scholar]
- 27.Backhaus J, Junghanns K, Born J, Hohaus K, Faasch F, Hohagen F. Impaired declarative memory consolidation during sleep in patients with primary insomnia: Influence of sleep architecture and nocturnal cortisol release. Biol Psychiatry. 2006;60:1324–30. doi: 10.1016/j.biopsych.2006.03.051. [DOI] [PubMed] [Google Scholar]
- 28.Cipolli C, Campana G, Campi C, et al. Sleep and time course of consolidation of visual discrimination skills in patients with narcolepsy-cataplexy. J Sleep Res. 2009;18:209–20. doi: 10.1111/j.1365-2869.2008.00712.x. [DOI] [PubMed] [Google Scholar]
- 29.Genzel L, Ali E, Dresler M, Steiger A, Tesfaye M. Sleep-dependent memory consolidation of a new task is inhibited in psychiatric patients. J Psychiatr Res. 2011;45:555–60. doi: 10.1016/j.jpsychires.2010.08.015. [DOI] [PubMed] [Google Scholar]
- 30.Dresler M, Kluge M, Genzel L, Schussler P, Steiger A. Impaired off-line memory consolidation in depression. Eur Neuropsychopharmacol. 2010;20:553–61. doi: 10.1016/j.euroneuro.2010.02.002. [DOI] [PubMed] [Google Scholar]
- 31.Wamsley EJ, Tucker MA, Shinn AK, et al. Reduced sleep spindles and spindle coherence in schizophrenia: mechanisms of impaired memory consolidation? Biol Psychiatry. 2012;71:154–61. doi: 10.1016/j.biopsych.2011.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]