Abstract
Animals exposed to anthropogenic disturbance make trade-offs between perceived risk and the cost of leaving disturbed areas. Impact assessments tend to focus on overt behavioural responses leading to displacement, but trade-offs may also impact individual energy budgets through reduced foraging performance. Previous studies found no evidence for broad-scale displacement of harbour porpoises exposed to impulse noise from a 10 day two-dimensional seismic survey. Here, we used an array of passive acoustic loggers coupled with calibrated noise measurements to test whether the seismic survey influenced the activity patterns of porpoises remaining in the area. We showed that the probability of recording a buzz declined by 15% in the ensonified area and was positively related to distance from the source vessel. We also estimated received levels at the hydrophones and characterized the noise response curve. Our results demonstrate how environmental impact assessments can be developed to assess more subtle effects of noise disturbance on activity patterns and foraging efficiency.
Keywords: activity budget, anthropogenic disturbance, environmental impact assessment, foraging efficiency
1. Introduction
Human disturbance may not lead to acute, easily measurable behavioural responses in exposed wildlife [1,2]. Less overt effects can arise from trade-offs that animals make [3], such as deciding to remain in disturbed areas and tolerating higher exposure levels where prey are abundant [4]. However, these trade-offs may also influence activity budgets, potentially affecting fecundity and survival [1,5] or having biologically significant consequences at a population level [6–9].
There is particular concern over the potential impacts of underwater anthropogenic noise on marine mammals [10,11]. Aversive behaviour towards intense noise sources such as pile driving (e.g. [12]) and seismic surveys [13] may not necessarily lead to broad-scale displacement [14]. However, it is not known whether there are more subtle changes in the activity budgets of animals remaining in exposed areas.
Patterns of echolocation clicks can be used to characterize the activity of both bats [15] and odontocetes [16–19]. Here, we use an array of passive acoustic loggers coupled with calibrated noise measurements to assess the effects of a seismic survey on the activity pattern of harbour porpoises (Phocoena phocoena).
2. Material and methods
The study was conducted around a commercial two-dimensional seismic survey in northeast Scotland. The survey vessel used a 470 cu inch airgun array with a shot point interval of 5–6 s to survey a 200 km2 area between 1 and 11 September 2011 (see the electronic supplementary material). Full details are provided in [14].
Patterns of porpoise echolocation clicks were characterized at 22 sites in and around the seismic survey area throughout August and September 2011 (figure 1). Data from V.1 continuous porpoise detectors (CPODs) (www.chelonia.co.uk) had previously been used to characterize the occurrence of porpoises [14]. While the sensitivity of CPODs at different sites could vary between different units or different local conditions, our sampling design and mixed modelling approach were designed to account for this (see the electronic supplementary material).
Figure 1.
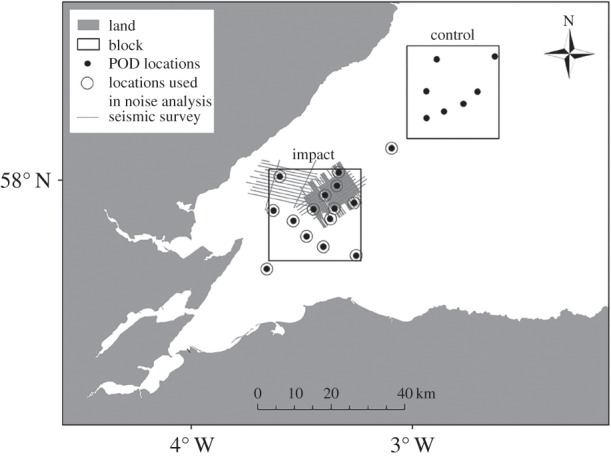
Map of the study area showing the seismic survey lines and the locations of CPOD deployments.
We calculated the inter-click intervals (ICIs) from the time series of detected clicks. A Gaussian mixture-model was fitted to log-transformed ICIs to identify different patterns of echolocation clicks [16]. Each ICI was classified as either a regular ICI (regular clicking for navigation and prey searching), a buzz ICI (buzzes associated with attempted prey captures or social communication), or an inter-train ICI (pauses between click trains) [20,21] (see the electronic supplementary material).
Following [14], we used data from CPODs in 25 km by 25 km control and impact areas (figure 1; see the electronic supplementary material) to assess the effect of seismic surveys on the occurrence of buzz ICIs when porpoises were present (i.e. in hours in which at least one ICI was detected). The presence of buzz ICIs was modelled as a function of seismic period (before or during) and experimental block (control or impact). Crucially, we also evaluated the interaction between these covariates to test whether seismic surveys influenced buzzing activity. We fitted a binary mixed-effects model in R 13.01 [22], with location included as a random effect. Model selection was based on Akaike's information criterion and the significance of retained covariates evaluated using Wald's tests.
The presence of buzz ICIs for each minute in which at least one ICI was detected was then modelled as a function of distance from the seismic vessel. Following previous studies [12,14] on likely spatial scales of porpoise responses to noise, we used data from within 25 km of the vessel (see the electronic supplementary material). A binary generalized linear model was fitted using generalized estimating equations (GEE-GLM) [23] (see the electronic supplementary material). We used QICu for model selection [24], and Wald's tests to assess significance [25].
Calibrated noise measurements were made at 15 sites between 1.6 and 61.8 km from the seismic vessel [14]. Airgun noise was characterized in terms of broadband sound exposure level for single pulses in dB re 1 µPa2s (using the region of the waveform that contained the central 90% of the pulse's energy; hereafter sound exposure level (SEL)). Peak-to-peak source levels were estimated to be 242–253 dB re 1 µPa at 1 m. We tested whether there was a relationship between buzz occurrence and estimated noise levels at the corresponding CPOD location using a bootstrapping procedure (see the electronic supplementary material).
3. Results
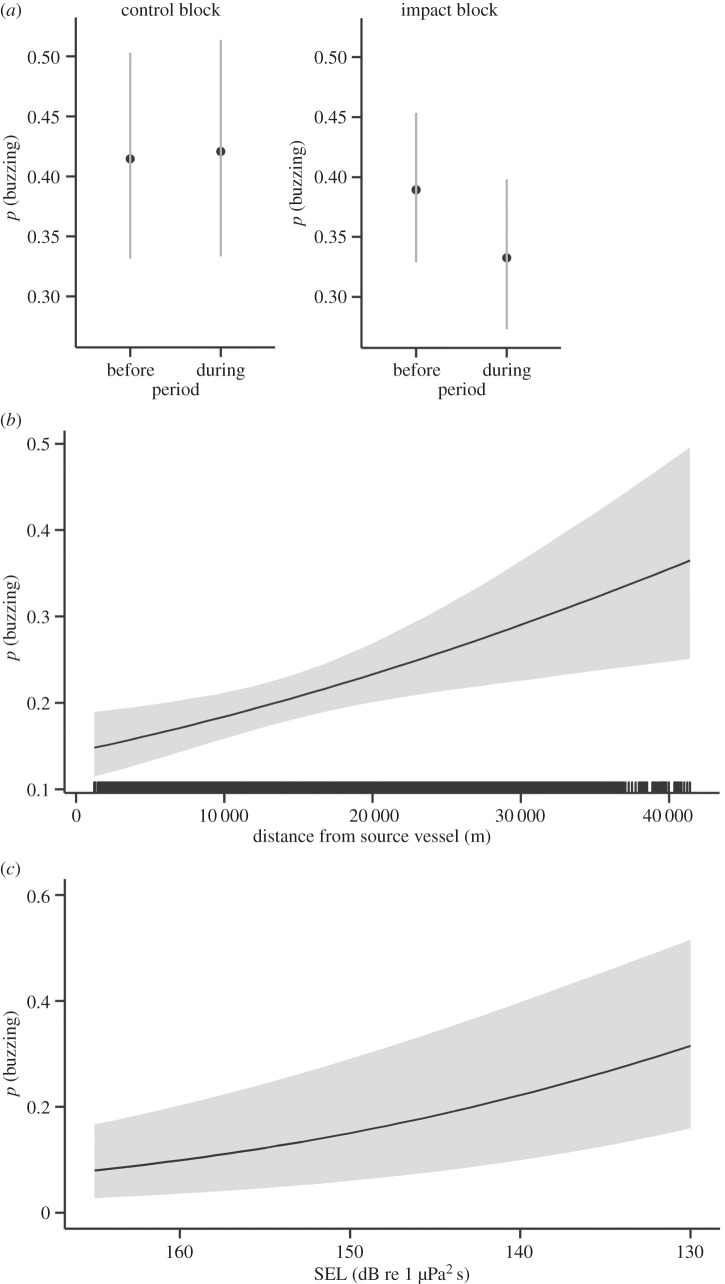
There was a significant interaction between experimental block and seismic period (Wald's test: χ21 = 7.2, p = 0.007), indicating that the seismic survey had a differential effect in the control and impact block. This resulted in a 15% reduction in the occurrence of buzz ICIs in the impact block during the survey (figure 2). Wide confidence intervals (CIs) highlight high levels of natural variability in detection of buzzes.
Figure 2.
Modelling results. (a) Changes in the probability of feeding buzz occurrence in control and impact blocks, before and during the survey. (b) Estimated relationship between the probability of buzz occurrence and distance from the source vessel. (c) Estimated relationship between the probability of buzz occurrence and unweighted SEL.
The probability of occurrence of buzzes increased significantly with distance from source (Wald's test: χ21 = 9.6, p = 0.002), ranging from approximately 0.15 at 0 km (95% CI: 0.11–0.19) to 0.35 at 40 km (95% CI: 0.25–0.48) (figure 2).
Noise measurements allowed us to characterize this relationship in terms of estimated received noise levels (figure 2). This probability increased from 0.07 (95% CI: 0.03–0.17) to 0.31 (95% CI: 0.16–0.52) for SEL that varied from 165 to 130 dB re 1 μPa2s (figure 2).
4. Discussion
Short-term responses to this seismic survey did not result in broad-scale displacement, suggesting that impact assessments should focus on sub-lethal effects within affected sites [14]. Our results indicate that porpoises remaining in the impact area reduced their buzzing activity by 15% during the seismic survey. Moreover, the probability of detecting buzz ICIs when porpoises were present increased with distance from the source vessel, suggesting that the likelihood of buzzing was dependent upon received noise intensity. The baseline probability of occurrence of buzzes was around 0.4 in the impact block before the survey, although with high natural variability. This probability declined to 0.1–0.2 at estimated received SEL of 150–165 dB re 1 μPa2s (figure 2). Such changes were unlikely to result from the presence of seismic noise, as this was mostly below 400 Hz [14], while most energy in porpoise clicks is within 110–150 kHz [26].
Porpoises may use high-repetition click trains for prey capture or for social communication [27]. Thus, observed changes in buzzing occurrence could reflect disruption of either foraging or social activities. Despite this uncertainty, these data provide a worst-case indication of the extent to which foraging was disrupted by noise exposure. These effects may result from prey reactions to noise [28], leading to reduced porpoise foraging rates. Alternatively, foraging effort may change if porpoises adjust time budgets or diving behaviour to avoid noise. Irrespective of the proximate mechanism(s) through which seismic surveys reduced buzzing rates, this clearly has the potential to affect the energy balance of exposed animals.
High metabolic rates [29] mean that porpoises have limited ability to cope with prolonged starvation [30]. Our results provide an estimate of the noise levels at which porpoise activity patterns are disrupted, and an indication of the scale of potential reductions in foraging activity [6]. Porpoise occurrence and activity is typically characterized by large seasonal and diel variability [19,31,32], as also reflected in our results (figure 2). Further studies are now required to explore the environmental conditions that drive this variation, and develop energetic models to assess whether this scale of disturbance has long-term consequences for individual energy budgets [33].
Acknowledgements
We thank colleagues at Kongsberg Maritime, Moray First Marine, Gardline Geosurvey and the Scottish Fishermen's Federation for essential support in the field, and those at DECC, PA Resources and Caithness Petroleum Ltd. for ensuring that this research was effectively integrated into their regulatory and commercial activities. The project benefitted at all stages from a scientific steering group and broad stakeholder group with statutory and NGO representation. We thank them, and Tim Barton, Keith Needham, Alex Douglas and the many other colleagues who provided support during both fieldwork and data analysis. Thanks to David Lusseau and two anonymous reviewers for their useful comments and suggestions.
The commercial seismic surveys studied in this paper were licensed by the Department of Energy and Climate Change (DECC) and followed UK guidelines to reduce potential impacts on marine mammals.
Data accessibility
The dataset used in this paper is available in the Dryad data repository: doi:10.5061/dryad.1847s
Funding statement
Data collection was funded through a partnership project that was coordinated by the UK Department of Energy and Climate Change (DECC) and jointly funded by DECC, Scottish Government, Oil and Gas UK Ltd. and COWRIE. E.P. was supported through the MASTS pooling initiative (the Marine Alliance for Science and Technology for Scotland). MASTS is funded by the Scottish Funding Council (grant reference no. HR09011) and contributing institutions. None of the analysis nor write up of the paper involved or was influenced by the sponsors of the research.
References
- 1.Frid A, Dill L. 2002. Human-caused disturbance stimuli as a form of predation risk. Conserv. Ecol. 6, 11. [Google Scholar]
- 2.Gill JA, Norris K, Sutherland WJ. 2001. Why behavioural responses may not reflect the population consequences of human disturbance. Biol. Conserv. 97, 265–268. ( 10.1016/S0006-3207(00)00002-1) [DOI] [Google Scholar]
- 3.Beale CM, Monaghan P. 2004. Behavioural responses to human disturbance: a matter of choice? Anim. Behav. 68, 1065–1069. ( 10.1016/j.anbehav.2004.07.002) [DOI] [Google Scholar]
- 4.Bejder L, Samuels A, Whitehead H, Finn H, Allen S. 2009. Impact assessment research: use and misuse of habituation, sensitisation and tolerance in describing wildlife responses to anthropogenic stimuli. Mar. Ecol. Prog. Ser. 395, 177–185. ( 10.3354/meps07979) [DOI] [Google Scholar]
- 5.McClung MR, Seddon PJ, Massaro M, Setiawan AN. 2004. Nature-based tourism impacts on yellow-eyed penguins Megadyptes antipodes: does unregulated visitor access affect fledging weight and juvenile survival? Biol. Conserv. 119, 279–285. ( 10.1016/j.biocon.2003.11.012) [DOI] [Google Scholar]
- 6.New LF, et al. 2013. Modeling the biological significance of behavioral change in coastal bottlenose dolphins in response to disturbance. Funct. Ecol. 27, 314–322. ( 10.1111/1365-2435.12052) [DOI] [Google Scholar]
- 7.Duchesne M, Côté SD, Barrette C. 2000. Responses of woodland caribou to winter ecotourism in the Charlevoix Biosphere Reserve, Canada. Biol. Conserv. 96, 311–317. ( 10.1016/S0006-3207(00)00082-3) [DOI] [Google Scholar]
- 8.Lusseau D. 2003. Effects of tour boats on the behavior of bottlenose dolphins: using Markov chains to model anthropogenic impacts. Conserv. Biol. 17, 1785–1793. ( 10.1111/j.1523-1739.2003.00054.x) [DOI] [Google Scholar]
- 9.Blumstein DT. 2006. Developing an evolutionary ecology of fear: how life history and natural history traits affect disturbance tolerance in birds. Anim. Behav. 71, 389–399. ( 10.1016/j.anbehav.2005.05.010) [DOI] [Google Scholar]
- 10.Nowacek DP, Thorne LH, Johnston DW, Tyack PL. 2007. Responses of cetaceans to anthropogenic noise. Mammal Rev. 37, 81–115. ( 10.1111/j.1365-2907.2007.00104.x) [DOI] [Google Scholar]
- 11.Weilgart LS. 2007. The impacts of anthropogenic ocean noise on cetaceans and implications for management. Can. J. Zool. 85, 1091–1116. ( 10.1139/Z07-101) [DOI] [Google Scholar]
- 12.Brandt M, Diederichs A, Betke K, Nehls G. 2011. Responses of harbour porpoises to pile driving at the Horns Rev II offshore wind farm in the Danish North Sea. Mar. Ecol. Prog. Ser. 421, 205–216. ( 10.3354/meps08888) [DOI] [Google Scholar]
- 13.Stone C, Tasker M. 2006. The effects of seismic airguns on cetaceans in UK waters. J. Cetacean. Res. Manag. 8, 255–263. [Google Scholar]
- 14.Thompson PM, Brookes KL, Graham IM, Barton TR, Needham K, Bradbury G, Merchant ND. 2013. Short-term disturbance by a commercial two dimensional seismic survey does not lead to long-term displacement of harbour porpoises. Proc. R. Soc. B 280 ( 10.1098/rspb.2013.2001) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Schnitzler H-U, Moss CF, Denzinger A. 2003. From spatial orientation to food acquisition in echolocating bats. Trends Ecol. Evol. 18, 386–394. ( 10.1016/S0169-5347(03)00185-X) [DOI] [Google Scholar]
- 16.Pirotta E, Thompson PM, Miller PI, Brookes KL, Cheney B, Barton TR, Graham IM, Lusseau D. 2014. Scale-dependent foraging ecology of a marine top predator modelled using passive acoustic data. Funct. Ecol. 28, 206–217. ( 10.1111/1365-2435.12146) [DOI] [Google Scholar]
- 17.Miller PJO, Johnson MP, Tyack PL. 2004. Sperm whale behaviour indicates the use of echolocation click buzzes ‘creaks’ in prey capture. Proc. R. Soc. Lond. B 271, 2239–2247. ( 10.1098/rspb.2004.2863) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Madsen PT, Johnson M, Aguilar Soto N, Zimmer WMX, Tyack PL. 2005. Biosonar performance of foraging beaked whales (Mesoplodon densirostris). J. Exp. Biol. 208, 181–194. ( 10.1242/jeb.01327) [DOI] [PubMed] [Google Scholar]
- 19.Carlström J. 2005. Diel variation in echolocation behavior of wild harbor porpoises. Mar. Mammal Sci. 21, 1–12. ( 10.1111/j.1748-7692.2005.tb01204.x) [DOI] [Google Scholar]
- 20.Thomas J, Moss C, Vater M. 2004. Echolocation in bats and dolphins. Chicago, IL: University of Chicago Press. [Google Scholar]
- 21.Madsen PT, Surlykke A. 2013. Functional convergence in bat and toothed whale biosonars. Physiology 28, 276–283. ( 10.1152/physiol.00008.2013) [DOI] [PubMed] [Google Scholar]
- 22.R Development Core Team 2013. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; See http://www.R-project.org/. [Google Scholar]
- 23.Liang KY, Zeger SL. 1986. Longitudinal data analysis using generalized linear models. Biometrika 73, 13–22. ( 10.1093/biomet/73.1.13) [DOI] [Google Scholar]
- 24.Pan W. 2001. Akaike's information criterion in generalized estimating equations. Biometrics 57, 120–125. ( 10.1111/j.0006-341X.2001.00120.x) [DOI] [PubMed] [Google Scholar]
- 25.Hardin JW, Hilbe JM. 2003. Generalized estimating equations, 3rd edn London, UK: Chapman and Hall/CRC Press. [Google Scholar]
- 26.Møhl B, Andersen S. 1973. Echolocation: high-frequency component in the click of the harbour porpoise (Phocoena ph. L.). J. Acoust. Soc. Am. 54, 1368 ( 10.1121/1.1914435) [DOI] [PubMed] [Google Scholar]
- 27.Clausen K, Wahlberg M, Beedholm K, DeRuiter S, Madsen PT. 2011. Click communication in harbour porpoises Phocoena phocoena. Bioacoustics 20, 1–28. ( 10.1080/09524622.2011.9753630) [DOI] [Google Scholar]
- 28.Popper AN, Fewtrell J, Smith ME, McCauley RD. 2003. Anthropogenic sound: effects on the behavior and physiology of fishes. Mar. Technol. Soc. J. 6, 35–40. ( 10.4031/002533203787537050) [DOI] [Google Scholar]
- 29.Lockyer C. 2007. All creatures great and smaller: a study in cetacean life history energetics. J. Mar. Biol. Assoc. UK 87, 1035–1045. ( 10.1017/S0025315407054720) [DOI] [Google Scholar]
- 30.Kastelein RA, Hardeman J, Boer H. 1997. Food consumption and body weight of harbour porpoises (Phocoena phocoena). In The biology of harbour porpoise (eds Read A, Wiepkema P, Nachtigall PE.), pp. 217–233. Woerden, The Netherlands: De Spil Publishers. [Google Scholar]
- 31.Verfuß UK, Honnef CG, Meding A, Dähne M, Mundry R, Benke H. 2007. Geographical and seasonal variation of harbour porpoise (Phocoena phocoena) presence in the German Baltic Sea revealed by passive acoustic monitoring. J. Mar. Biol. Assoc. UK 87, 165 ( 10.1017/S0025315407054938) [DOI] [Google Scholar]
- 32.Gallus A, Dähne M, Verfuß U, Bräger S, Adler S, Siebert U, Benke H. 2012. Use of static passive acoustic monitoring to assess the status of the ‘Critically Endangered’ Baltic harbour porpoise in German waters. Endang. Species Res. 18, 265–278. ( 10.3354/esr00448) [DOI] [Google Scholar]
- 33.Nabe-Nielsen J, Sibly RM, Tougaard J, Teilmann J, Sveegaard S. 2014. Effects of noise and by-catch on a Danish harbour porpoise population. Ecol. Model. 272, 242–251. ( 10.1016/j.ecolmodel.2013.09.025) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The dataset used in this paper is available in the Dryad data repository: doi:10.5061/dryad.1847s