Abstract
Social norms that regulate reproductive and marital decisions generate impressive cross-cultural variation in the prevalence of kin marriages. In some societies, marriages among kin are the norm and this inbreeding creates intensive kinship networks concentrated within communities. In others, especially forager societies, most marriages are between more genealogically and geographically distant individuals, which generates a larger number of kin and affines of lesser relatedness in more extensive kinship networks spread out over multiple communities. Here, we investigate the fitness consequence of kin marriages across a sample of 46 small-scale societies (12 439 marriages). Results show that some non-forager societies (including horticulturalists, agriculturalists and pastoralists), but not foragers, have intensive kinship societies where fitness outcomes (measured as the number of surviving children in genealogies) peak at commonly high levels of spousal relatedness. By contrast, the extensive kinship systems of foragers have worse fitness outcomes at high levels of spousal relatedness. Overall, societies with greater levels of inbreeding showed a more positive relationship between fitness and spousal relatedness.
Keywords: human kinship, marriage patterns, fertility, subsistence, foragers, agriculture
1. Introduction
Human societies vary considerably in social norms that affect marriage and kinship. For example, the prevalence of marriages between first cousins varies from zero in many societies, especially foragers, to over 50% in some non-forager societies [1]. Bugos [2] defined intensive kinship systems as those societies that emphasize social norms of kin marriages and alliances that create dense, converging networks of coresident kin. There are a number of correlated cultural traits associated with intensive kinship systems that include more kin marriages [3], marriage arrangement [4], lineal fissions [5], endogamy [6], polygyny [1] and higher group relatedness [7].
In intensive kinship systems, fitness peaks have been observed for cousin (from close to distant) marriages for the Yanomamo [8], Ayoreo [2] and historical Iceland [9], with mixed evidence in India [10] and several other countries [11]. This is the expected optimality result given that marriage decisions reflect a trade-off between social benefits from marrying kin and the risks associated with inheriting deleterious recessive alleles (a ‘Goldilocks Zone’ of optimal mates). Kin marriages create alliances between lineages [12,13] to create shared interest among closely overlapping networks of kin and affines. When appropriate, close kin marriages can reduce the potential dilution of inherited family wealth such as land and livestock in non-forager societies [14]. The most intense manifestation of this is in close kin marriages of royal families [15]. Further, close kin marriages establish well-defined kin groups that may be well-suited for resource defence, and non-foragers have significantly higher rates of violence than forager societies [16].
On the other extreme are extensive human kinship systems associated with forager subsistence. Many foragers have fluid group membership [17] and frequent movement across the landscape [18,19] to exploit a diversity of unevenly distributed food resources [20]. In these societies, marriages are often between unrelated individuals or more genealogically distant kin living in far-flung communities [21]. Marrying unrelated or distantly related individuals increases the total possible numbers of kin and affines by including a wider net of individuals. An extensive kinship network may be more important in unpredictable environments and for nomadic populations given that it provides a plethora of residential options that may prove vital in times of crises [2,20,21].
Unlike the intensive system examples above, we know of no study that has examined fitness outcomes for kin marriages in forager societies, probably owing to a lack of data. We compiled cross-cultural genealogies to test the optimality proposition that foragers show fitness peaks at low levels of spousal relatedness. Further, we tested whether differences between patterns of fitness by spousal relatedness in foragers and non-foragers could be explained by societal differences in inbreeding, arguably the best measure of intensive kinship [1].
2. Material and methods
Genealogies are from 46 small-scale societies available from online datasets at KinSources (http://kinsources.net) with the addition of Ache foragers from Paraguay [22] (all societies, sample sizes and inbreeding values appear in the electronic supplementary material). The sample is evenly divided into foragers and non-foragers and contains a total of 12 439 marriage records. Primarily pastoral populations, like the Tuareg and Turkish nomads, were included into the non-forager category along with other farming and horticultural societies. Genealogies range in depth from four to 16 generations with an average of 6.5. Calculating spousal relatedness has the problem that different studies have varying degrees of quality in available genealogical information so the depth of the genealogy in number of generations is added to regression models as a quality control variable.
Genealogies allow estimation of the coefficient of co-ancestry between two individuals, or the probability that two alleles at the same locus drawn at random are identical by descent [23]. The coefficient of relatedness (r) between spouses, or spousal relatedness, is twice the coefficient of ancestry, and the inbreeding coefficient of an individual is the coefficient of co-ancestry of the parents. Here, the average coefficient of inbreeding is calculated for each genealogy as the average inbreeding of all individuals. The calculations of coefficients were conducted in Ed Hagen's Descent software (http://itb.biologie.hu-berlin.de/~hagen/Descent/). The following four categories were used to categorize the relatedness between spouses: [0,0.03125), [0.03125,0.0625), [0.0625,0.125) and [0.125,1). These are cut-offs of 1/32, 1/16 and 1/8 levels of relatedness. The latter category represents relatedness greater than or equal to first cousins.
Mixed-effects regression models were estimated using the lme4 package [24] in R. The outcome was the natural log of each couple's number of surviving children (i.e. children who appeared in the genealogies; we first added 1 to this value because the log of 0 is undefined), and the key predictor was spousal relatedness (the lowest bin, [0,0.03125), was designated as the reference group). Models controlled for the number of times each spouse occurred in the marriage records and generational depth of the society's genealogy. To control for generational, family-specific, and society-specific effects, each couple was nested in each spouse's mother and father (individuals with missing codes for a mother and/or father in the genealogy were coded as having a unique mother and/or father), and spouses’ parents were nested in societies.
We first ran the model including only non-foragers, and second including only foragers. To test whether these patterns differed significantly by subsistence type, we estimated a model that included all observations, with the additional predictors subsistence type and its interaction with spousal relatedness. Finally, to test whether differences by subsistence could be attributed to inbreeding levels within societies, we estimated a full model, which added inbreeding and its interaction with spousal relatedness to the previous model.
3. Results
(a). Non-foragers
Sample sizes and parameter estimates for non-foragers appear in the left-hand columns of table 1, and mean fitness by spousal relatedness appears in figure 1. In non-foragers, marriages between individuals with genetic relatedness less than 1/32 were the most common. However, couples from all three other relatedness bins had significantly more surviving children than couples from the lowest relatedness bin.
Table 1.
Parameter estimates for effects of spousal relatedness on fitness by subsistence type. (Husband and wife marriages are the numbers of marriages in which each spouse from the couple was involved. *p < 0.05; **p < 0.001.)
| non-foragers |
foragers |
full model | |||
|---|---|---|---|---|---|
| n | estimate | n | estimate | ||
| relatedness [0, 0.03125) | 6869 | (reference) | 3445 | (reference) | (reference) |
| relatedness [0.03125, 0.0625) | 482 | 0.14** (0.03) | 29 | −0.10 (0.11) | 0.14** (0.03) |
| relatedness [0.0625, 0.125) | 536 | 0.13** (0.03) | 25 | 0.03 (0.12) | 0.13** (0.03) |
| relatedness [0.125, 1) | 1008 | 0.18** (0.03) | 45 | −0.20* (0.09) | 0.18** (0.03) |
| husband marriages | — | −0.01 (0.01) | — | 0.02 (0.02) | 0.00 (0.01) |
| wife marriages | — | −0.17** (0.01) | — | −0.17** (0.02) | −0.17** (0.01) |
| generational depth | — | <0.001 (0.01) | — | 0.04 (0.04) | 0.01 (0.01) |
| subsistence | — | — | — | — | 0.05a(0.07) |
| subsistence × relatedness [0.03125, 0.0625) | — | — | — | — | −0.22 (0.13) |
| subsistence × relatedness [0.0625, 0.125) | — | — | — | — | −0.11 (0.14) |
| subsistence × relatedness [0.125, 1) | — | — | — | — | −0.39** (0.10) |
aMain effect of subsistence, or the difference between non-foragers and foragers at the lowest level of relatedness.
Figure 1.
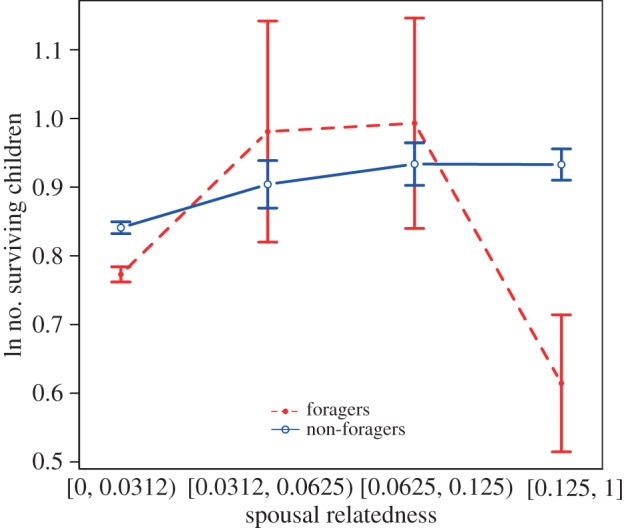
Mean fitness by spousal relatedness and subsistence type. Error bars are standard errors of the mean. (Online version in colour.)
(b). Foragers
Sample sizes and parameter estimates for foragers appear in the third and fourth columns of table 1. As seen for non-foragers, forager marriages between individuals with genetic relatedness less than 1/32 were also the most common. However, this tendency was much more pronounced in foragers: the ratio of marriages in the lowest relatedness bin to marriages in the highest relatedness bin was 7 : 1 in non-foragers, compared with 77 : 1 in foragers. The pattern of fitness across relatedness bins also differed for foragers. Specifically, couples in the highest relatedness bin had significantly fewer surviving children than couples in the lowest relatedness bin. Neither intermediate fitness relatedness bins differed significantly from the lowest relatedness bin.
(c). Contrasting non-foragers to foragers
Results from the model that tested for differences between subsistence regimes appear in the last column of table 1. The main effect of subsistence was not significant in this model, indicating that foragers and non-foragers in the lowest relatedness bin did not differ in their fitness. However, the interaction between subsistence level and the highest relatedness bin was statistically significant, indicating that fitness is higher for non-foragers at higher levels of spousal relatedness.
(d). The role of inbreeding
When society-level inbreeding and its interaction with spousal relatedness were added to the previous model, none of the interactions between spousal relatedness and subsistence level remained statistically significant (table 2), indicating that differences in the relationship between spousal relatedness and fitness were fully explained by societal differences in inbreeding. All of the interactions between relatedness and society-level inbreeding were positive and statistically significant (table 2), indicating that higher levels of societal inbreeding were associated with a more positive relationship between spousal relatedness and fitness, even after controlling for subsistence type. The relationship between spousal relatedness and fitness for each quartile of society-level inbreeding are shown in figure 2. The relationship between spousal relatedness and fitness is positive for the highest quartile of inbreeding values, and negative for the lowest quartile of inbreeding values.
Table 2.
Parameter estimates for effects of spousal relatedness on fitness by subsistence type and inbreeding. (Husband and wife marriages are the numbers of marriages in which each spouse from the couple was involved. **p < 0.001.)
| estimate | |
|---|---|
| relatedness [0, 0.03125) | (reference) |
| relatedness [0.03125, 0.0625) | 0.00 (0.04) |
| relatedness [0.0625, 0.125) | 0.00 (0.05) |
| relatedness [0.125, 1) | −0.04 (0.05) |
| husband marriages | 0.00 (0.01) |
| wife marriages | −0.18** (0.01) |
| generational depth | 0.01 (0.01) |
| subsistence | 0.03 (0.08) |
| subsistence × relatedness [0.03125, 0.0625) | −0.10 (0.13) |
| subsistence × relatedness [0.0625, 0.125) | −0.01 (0.14) |
| subsistence × relatedness [0.125, 1) | −0.18 (0.11) |
| inbreeding × relatedness [0.03125, 0.0625) | 8.07** (1.76) |
| inbreeding × relatedness [0.0625, 0.125) | 6.14** (1.28) |
| inbreeding × relatedness [0.125, 1) | 7.39** (1.07) |
Figure 2.
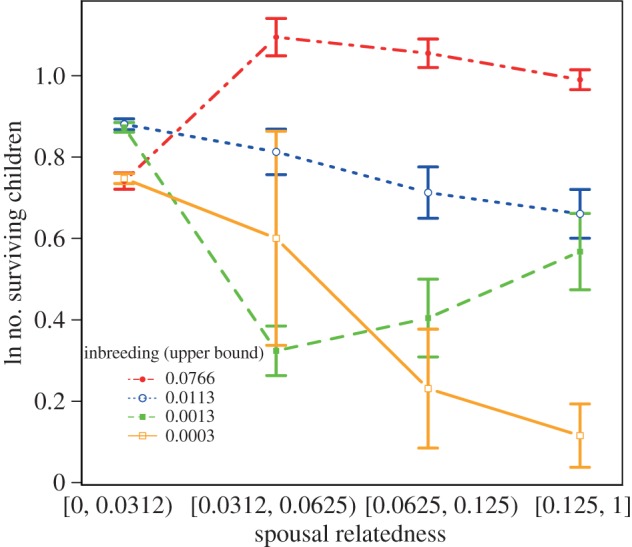
Mean fitness by spousal relatedness and society-level inbreeding quartile. Error bars are standard errors of the mean. (Online version in colour.)
4. Discussion
We find that fitness outcomes increase with spousal relatedness for non-foragers but decrease for foragers. The moderating effect of subsistence could be fully explained by differences in society-level inbreeding values. Specifically, the relationship between spousal relatedness and fitness was positive for societies with the highest levels of inbreeding (societies with intensive kinship systems) and negative for societies with the lowest levels of inbreeding (extensive kinship systems).
Given the positive relationship between spousal relatedness and fitness among non-foragers, one might wonder why marriage between first cousins is not the modal type of marriage in more societies. One possibility is inbreeding depression in future generations: this study did not measure the relationship between spousal relatedness and number of grandchildren, but a previous study found that children of couples related at the level of second cousins or higher have lower levels of survival and reproduction [9]. Additionally, factors other than family ties also influence individuals’ and their parents’ decisions about marriage [22,25]. Regardless, spousal relatedness is clearly influenced by social norms. The observed 7 : 1 ratio of couples in the lowest relatedness bin to couples in the highest relatedness bin in non-foragers is probably much lower than the ratio of unrelated potential spouses to marriage-age-appropriate, opposite sex cousins.
An alternative explanation that could account for at least some of the cross-societal differences in the relationship between spousal relatedness and fitness is that individuals with good marriage prospects may have higher reproductive potential. For example, potential spouses with the largest numbers of resources may be favoured for cousin marriages in societies with intensive kinship systems. Endogamy might be strategic only for individuals of the highest social status, for whose descendants endogamy might be most likely to maximize wealth and genes associated with social success [26]. Thus, some of the differences in fitness by spousal relatedness may not be attributable to spousal relatedness per se, but to individual differences that affect both fitness and spousal relatedness, like wealth.
Finally, although the sample used in this study was the largest and most diverse of any sample used to address the relationship between spousal relatedness and fitness, it was certainly not exhaustive. We encourage other researchers to test whether our findings hold in other traditional societies as well.
Acknowledgements
We thank Craig Palmer and Ray Hames for comments on a previous draft.
Funding statement
Financial support was provided by a National Geographic Society Research and Exploration grant (no. 9165-12).
References
- 1.Walker RS, Bailey DH. 2014. Marrying kin in small-scale societies. Am. J. Hum. Biol. 26, 384–388. ( 10.1002/ajhb.22527) [DOI] [PubMed] [Google Scholar]
- 2.Bugos PE. 1985. An evolutionary ecological analysis of the social organization of the Ayoreo of the northern Gran Chaco. PhD dissertation, Northwestern University, Evanston, IL, USA. [Google Scholar]
- 3.Flinn MV, Low BS. 1986. Resource distribution, social competition, and mating patterns in human societies. In Ecological aspects of social evolution (eds Rubenstein D, Wrangham R.), pp. 217–243. Princeton, NJ: Princeton University Press. [Google Scholar]
- 4.Apostolou M. 2007. Sexual selection under parental choice: the role of parents in the evolution of human mating. Evol. Hum. Behav. 28, 403–409. ( 10.1016/j.evolhumbehav.2007.05.007) [DOI] [Google Scholar]
- 5.Walker RS, Hill K. In press. Causes, consequences, and kin bias of human group fissions. Hum. Nat. [DOI] [PubMed] [Google Scholar]
- 6.Patai R. 1965. The structure of endogamous unilineal descent groups. Southwestern J. Anthropol. 21, 325–350. [Google Scholar]
- 7.Walker RS. In press. Amazonian horticulturalists live in larger, more related groups than hunter-gatherers. Evol. Hum. Behav.
- 8.Chagnon N. 1980. Kin selection theory, kinship, marriage and fitness among the Yanomamö Indians. In Sociobiology: beyond nature/nurture? (eds Alexander R, Tinkle D.), pp. 545–571. New York, NY: Westview. [Google Scholar]
- 9.Helgason A, Palsson S, Gudbjartsson DF, Kristjansson T, Stefansson K. 2008. An association between the kinship and fertility of human couples. Science 319, 813–816. ( 10.1126/science.1150232) [DOI] [PubMed] [Google Scholar]
- 10.Bittles A, Grant J, Sullivan S, Hussain R. 2002. Does inbreeding lead to decreased human fertility? Ann. Hum. Biol. 29, 111–130. ( 10.1080/03014460110075657) [DOI] [PubMed] [Google Scholar]
- 11.Bittles A, Black M. 2010. Consanguinity, human evolution, and complex diseases. Proc. Natl Acad. Sci. 107(Suppl. 1), 1779–1786. ( 10.1073/pnas.0906079106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chapais B. 2008. Primeval kinship: how pair bonding gave birth to human society. Cambridge, MA: Harvard University Press. [Google Scholar]
- 13.Lévi-Strauss C. 1969. Elementary structures of kinship. Boston, MA: Beacon Press. [Google Scholar]
- 14.Borgerhoff Mulder M, et al. 2009. Intergenerational wealth transmission and the dynamics of inequality in small-scale societies. Science 326, 682–688. ( 10.1126/science.1178336) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Van Den Berghe PL, Mesher GM. 1980. Royal incest and inclusive fitness. Am. Ethnol. 7, 300–317. ( 10.1525/ae.1980.7.2.02a00050) [DOI] [Google Scholar]
- 16.Wrangham RW, Wilson ML, Muller MN. 2006. Comparative rates of violence in chimpanzees and humans. Primates 47, 14–26. ( 10.1007/s10329-005-0140-1) [DOI] [PubMed] [Google Scholar]
- 17.Hill KR, et al. 2011. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science 331, 1286–1289. ( 10.1126/science.1199071) [DOI] [PubMed] [Google Scholar]
- 18.Binford LR. 1980. Willow smoke and dogs’ tails: hunter-gatherer settlement systems and archaeological site formation. Am. Antiquity 45, 4–20. ( 10.2307/279653) [DOI] [Google Scholar]
- 19.Kelly RL. 1983. Hunter–gatherer mobility strategies. J. Anthropol. Res. 39, 277–306. [Google Scholar]
- 20.Yellen J, Harpending H. 1972. Hunter–gatherer populations and archaeological inference. World Archaeol. 4, 244–253. ( 10.1080/00438243.1972.9979535) [DOI] [PubMed] [Google Scholar]
- 21.MacDonald DH, Hewlett BS. 1999. Reproductive interests and forager mobility. Curr. Anthropol. 40, 501–524. ( 10.1086/200047) [DOI] [Google Scholar]
- 22.Hill K, Hurtado AM. 1996. Ache life history: the ecology and demography of a foraging people. New York, NY: Aldine de Gruyter. [Google Scholar]
- 23.Wright S. 1922. Coefficients of inbreeding and relationship. Am. Nat. 56, 330–338. ( 10.1086/279872) [DOI] [Google Scholar]
- 24.Bates D, Maechler M, Bolker B. 2011. lme4: linear mixed-effects models using S4 classes. R package v. 0.999375–42. See http://cran.r-project.org/web/packages/lme4/index.html.
- 25.Apostolou M. 2010. Parental choice: what parents want in a son-in-law and a daughter-in-law across 67 pre-industrial societies. Br. J. Psychol. 101, 695–704. ( 10.1348/000712609X480634) [DOI] [PubMed] [Google Scholar]
- 26.Clark G. 2014. The son also rises: surnames and the history of social mobility. Princeton, NJ: Princeton University Press. [Google Scholar]


