Abstract
Inbreeding can cause reductions in fitness, driving the evolution of pre- and postcopulatory inbreeding avoidance mechanisms. There is now considerable evidence for such processes in females, but few studies have focused on males, particularly in the context of postcopulatory inbreeding avoidance. Here, we address this topic by exposing male guppies (Poecilia reticulata) to either full-sibling or unrelated females and determining whether they adjust investment in courtship and ejaculates. Our results revealed that males reduce their courtship but concomitantly exhibit short-term increases in ejaculate quality when paired with siblings. In conjunction with prior work reporting cryptic female preferences for unrelated sperm, our present findings reveal possible sexually antagonistic counter-adaptations that may offset postcopulatory inbreeding avoidance by females.
Keywords: cryptic female choice, sperm competition, inbreeding depression, sexual conflict
1. Introduction
Inbreeding can expose deleterious recessive alleles to selection, thus reducing the fitness of individuals or populations (inbreeding depression [1,2]). Where selection favours inbreeding avoidance, both sexes can adopt mate choice strategies that favour unrelated individuals as mates [3,4]. However, because males and females typically pay different reproductive costs, inbreeding can have asymmetric fitness consequences for both sexes [5–7], thus potentially generating sexually antagonistic responses to inbreeding [8].
In polyandrous species, where females mate with multiple males within a single reproductive episode, females may exploit postcopulatory mechanisms of inbreeding avoidance [e.g. 9] to avoid fertilization by sperm from related males [8,10–12]. In theory, males may also exercise postcopulatory inbreeding avoidance by tailoring the size and/or quality of their ejaculates according to female relatedness [7]. Accordingly, some insect studies have documented male ejaculate tailoring as a response to female relatedness [e.g. 13], although such responses are not universal [14,15].
Here, we examine patterns of male reproductive investment in relation to female relatedness in the guppy (Poecilia reticulata), a polyandrous, livebearing poeciliid fish. Guppies are ideally suited for evaluating pre- and postcopulatory inbreeding avoidance by males. Males exhibit flexible mating strategies, such that individuals readily adjust courtship [16] and adopt alternative mating strategies in response to female cues [17]. Furthermore, there is evidence of ejaculate tailoring by males [18,19], and for their part females exercise cryptic female choice against inbreeding by manipulating sperm velocity to favour unrelated mates [20]. Moreover, inbreeding occurs in natural populations [21], and offspring arising from consanguineous matings exhibit impaired survival [22], reductions in courtship by males [23] and declines in both body size and fertility [24]. Thus, we predicted that males would expend less courtship effort and reduce their expenditure on ejaculates when exposed to related females.
2. Material and methods
Guppies came from an established pedigree (comprising n = 23 families reared through three generations of outbreeding) founded by descendants of wild-caught fish from a feral population in Queensland, Australia. The experimental fish were reared in single-sex tanks until four months old. Two full-sibling males were taken haphazardly from each of the 23 families and assigned to their respective treatment tanks (35 × 19× 22 cm) where they were kept for 3 days to acclimatize. After this period (i.e. day 0), sperm were extracted from anaesthetized males to obtain baseline measures of sperm viability, velocity, length and numbers (see the electronic supplementary material).
After the initial sperm assays (day 0), we placed either a full-sibling or an unrelated stimulus female in each male's tank for 40 days. In both treatments, males had visual and olfactory access to the stimulus female housed within a transparent and perforated plastic drinks bottle (12 cm diameter) in the centre of each tank. Each stimulus female was replaced with a full sibling on days 10, 20 and 30 to prevent familiarity from diminishing male sexual interest [25]. We measured the number of sigmoid displays (an S-shaped posture used during courtship) over a 10 min period, as this behaviour is known to predict male reproductive fitness in guppies [26]. After each trial, the female was anaesthetized and measured for body size (standard length, SL).
We performed sperm assays again to evaluate both short- (5 days) and long-term (40 days) adjustments in ejaculate traits. A short-term period of 5 days was chosen to ensure that males had time to replenish their sperm after the initial baseline sperm assays [27]. The long-term period of 40 days encapsulates the guppy's entire spermatogenetic cycle [28] and therefore accounts for changes in ejaculate traits that depend on sperm maturation. Repeated sperm extractions do not influence male behaviour or survival [19]. The sperm traits considered in this study are associated with male reproductive fitness in poeciliid fishes [29,30].
We used mixed-effects models to analyse variation in courtship and ejaculate traits. All models included relatedness treatment as a fixed factor and family identity as a random effect. Sigmoid data exhibited a negative binomial error distribution and were analysed using a generalized linear mixed-effects model [31] within the glmmADMB [32] package of R [33]. Female standard length was included as a covariate, as male guppies exhibit preferences for larger females [34,35]. Interactions between covariates and relatedness treatment were not significant. The analysis of ejaculate traits was performed using the lme4 [36] package of R and included the additional fixed effects of time (two levels) and time-by-treatment interactions. Male ID was also included as a random effect to account for repeated sampling of individuals across time periods. Five males had no sperm and one was found to have no intact sperm bundles, so these six individuals were excluded from the analyses (n = 3 from each treatment).
3. Results
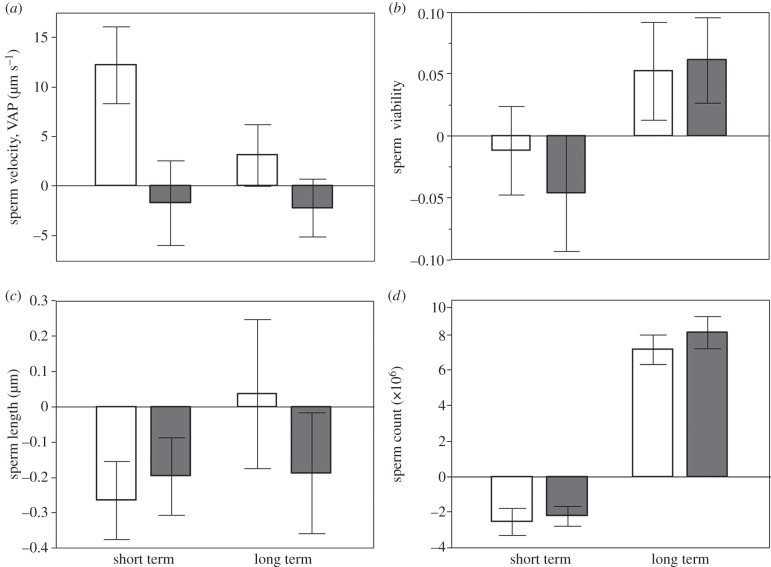
Males directed significantly more courtship towards unrelated females (table 1 and figure 1). However, males housed with related females produced sperm with higher average path velocity (VAP) than those housed with unrelated females (table 2). This effect was predominantly attributable to short-term adjustments in VAP (figure 2a), although the treatment-by-time interaction was marginally non-significant (table 2). There was no significant effect of treatment on the remaining ejaculate traits (table 2 and figure 2b–d).
Table 1.
Effect of male–female relatedness on male sigmoid displays. Results include parameter estimates (±s.e.), test statistics (z), and significance (p). Sample size n = 41.
| source | estimates (±s.e.) | z-value | p-value |
|---|---|---|---|
| relatedness treatment | 1.86 (±0.82) | 2.27 | 0.02 |
| female standard length (mm) | −0.31 (±0.33) | −0.94 | 0.35 |
Figure 1.
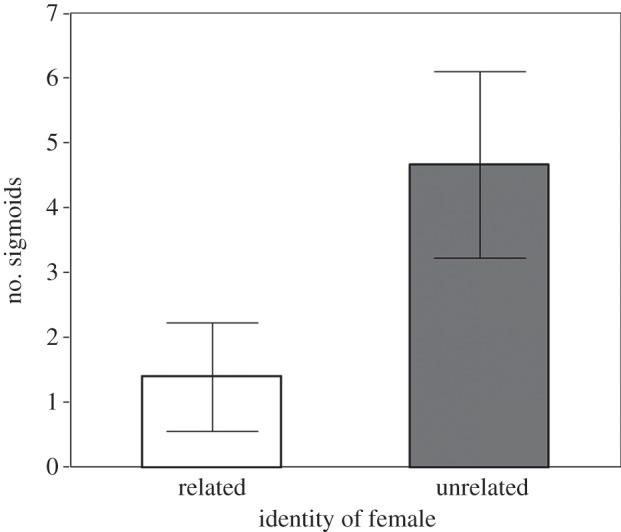
The mean (±s.e.) number of courtship displays (sigmoids) by male guppies. White bar indicates related treatment; grey bar indicates unrelated treatment.
Table 2.
Effect of male–female relatedness on ejaculate traits in guppies. Test parameters (χ2) and significance levels (p) are from linear mixed-effects models. Sample size n = 40. Sperm viability, proportion live sperm.
| source | χ2 | p-value |
|---|---|---|
| VAP (µm s−1) | ||
| relatedness treatment | 4.48 | 0.03 |
| time | 4.51 | 0.03 |
| treatment × time | 3.67 | 0.06 |
| sperm viability | ||
| relatedness treatment | 0.17 | 0.68 |
| time | 10.74 | 0.001 |
| treatment × time | 0.70 | 0.40 |
| sperm length (µm) | ||
| relatedness treatment | 0.11 | 0.73 |
| time | 1.83 | 0.17 |
| treatment × time | 1.64 | 0.20 |
| sperm count (×106) | ||
| relatedness treatment | 0.66 | 0.42 |
| time | 340.17 | <0.001 |
| treatment × time | 0.36 | 0.55 |
Figure 2.
Change in mean (±s.e.) sperm velocity (a), viability (proportion live sperm) (b), length (c) and number (d) over short term (difference from baseline to day 5) and long term (difference from baseline to day 40). White bars indicate related treatment; grey bars indicate unrelated treatment.
4. Discussion
We show that male guppies are capable of kin recognition and adjust both their courtship and sperm quality accordingly. Males decreased their courtship but produced ejaculates comprising faster swimming sperm when exposed to siblings. Although this finding contrasts with most other studies, it is not without precedent. In red junglefowl (Gallus gallus), males initiate copulations more readily with unrelated females, but when copulations do occur they inseminate more sperm with sisters [8]. Interestingly, as with guppies, female junglefowl exercise cryptic preferences for unrelated male sperm [8], and thus males of both species may adopt sperm investment strategies that counter cryptic female choice against inbreeding.
Our results may reflect sex-specific responses to inbreeding, whereby males tolerate higher levels of inbreeding than females [5–7]. However, while this latter possibility is consistent with our finding for sperm velocity (relatively higher with siblings), it is at odds with our finding that males reduce (precopulatory) mating effect when paired with sisters.
An alternative explanation for our findings is that when females mate with siblings they are more likely to seek additional mates (e.g. where sperm competition/cryptic female choice biases paternity towards less related males [e.g. 37]). Accordingly, the degree of male–female relatedness may be associated with the level of sperm competition, which in turn can favour a reduction in ejaculate investment under high-‘intensity’ sperm competition scenarios [38]. Thus, our findings for sperm velocity could be interpreted as a response to the heightened intensity of sperm competition when males encounter sibling females.
Finally, although the mechanism underlying kin recognition is unknown in guppies (and in our case may involve familiarity cues developed during pregnancy), other fish species depend on odours linked to genes of the major histocompatibility complex (MHC) to identify kin [e.g. 39,40]. Female guppies have been shown to preferentially associate with particular males based on olfactory cues alone [41], and future work could profitably focus on the MHC as a basis for these odour-based mating preferences.
Acknowledgements
We thank Cameron Duggin for technical assistance and two anonymous reviewers for comments.
References
- 1.Keller LF, Waller DM. 2002. Inbreeding effects in wild populations. Trends Ecol. Evol. 17, 230–241. ( 10.1016/S0169-5347(02)02489-8) [DOI] [Google Scholar]
- 2.Pusey A, Wolf M. 1996. Inbreeding avoidance in animals. Trends Ecol. Evol. 11, 201–206. ( 10.1016/0169-5347(96)10028-8) [DOI] [PubMed] [Google Scholar]
- 3.Hoffman JI, Forcada J, Trathan PN, Amos W. 2007. Female fur seals show active choice for males that are heterozygous and unrelated. Nature 445, 912–914. ( 10.1038/nature05558) [DOI] [PubMed] [Google Scholar]
- 4.Lemaître J-F, Ramm SA, Hurst JL, Stockley P. 2012. Inbreeding avoidance behaviour of male bank voles in relation to social status. Anim. Behav. 83, 453–457. ( 10.1016/j.anbehav.2011.11.017) [DOI] [Google Scholar]
- 5.Kokko H, Ots I. 2006. When not to avoid inbreeding. Evolution 60, 467–475. ( 10.1111/j.0014-3820.2006.tb01128.x) [DOI] [PubMed] [Google Scholar]
- 6.Parker GA. 1979. Sexual selection and sexual conflict. In Sexual selection and reproductive competition in insects (eds Blum MS, Blum NA.), pp. 123–166. New York, NY: Academic Press. [Google Scholar]
- 7.Parker GA. 2006. Sexual conflict over mating and fertilization: an overview. Phil. Trans. R. Soc. B 361, 235–259. ( 10.1098/rstb.2005.1785) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Pizzari T, Lovlie H, Cornwallis CK. 2004. Sex-specific, counteracting responses to inbreeding in a bird. Proc. R. Soc. Lond. B 271, 2115–2121. ( 10.1098/rspb.2004.2843) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bretman A, Wedell N, Tregenza T. 2004. Molecular evidence of post-copulatory inbreeding avoidance in the field cricket Gryllus bimaculatus. Proc. R. Soc. Lond. B 271, 159–164. ( 10.1098/rspb.2003.2563) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Firman RC, Simmons LW. 2008. Polyandry facilitates postcopulatory inbreeding avoidance in house mice. Evolution 62, 603–611. ( 10.1111/j.1558-5646.2007.00307.x) [DOI] [PubMed] [Google Scholar]
- 11.Hosken DJ, Blanckenhorn WU. 1999. Female multiple mating, inbreeding avoidance, and fitness: it is not only the magnitude of costs and benefits that counts. Behav. Ecol. 10, 462–464. ( 10.1093/beheco/10.4.462) [DOI] [Google Scholar]
- 12.Zeh JA, Zeh DW. 1997. The evolution of polyandry II: post-copulatory defenses against genetic incompatibility. Proc. R. Soc. Lond. B 264, 69–75. ( 10.1098/rspb.1997.0010) [DOI] [Google Scholar]
- 13.Lewis Z, Wedell N. 2009. Male moths reduce sperm investment in relatives. Anim. Behav. 77, 1547–1550. ( 10.1016/j.anbehav.2009.03.013) [DOI] [Google Scholar]
- 14.Simmons LW, Thomas ML. 2008. No postcopulatory response to inbreeding by male crickets. Biol. Lett. 4, 183–185. ( 10.1098/rsbl.2007.0578) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stockley P. 1999. Sperm selection and genetic incompatibility: does relatedness of mates affect male success in sperm competition? Proc. R. Soc. Lond. B 266, 1663–1669. ( 10.1098/rspb.1999.0829) [DOI] [Google Scholar]
- 16.Houde AE. 1997. Sex, color, and mate choice in guppies. Princeton, NJ: Princeton University Press. [Google Scholar]
- 17.Evans JP, Kelley JL, Ramnarine IW, Pilastro A. 2002. Female behaviour mediates male courtship under predation risk in the guppy. Behav. Ecol. Sociobiol. 52, 496–502. ( 10.1007/s00265-002-0535-6) [DOI] [Google Scholar]
- 18.Bozynski CC, Liley NR. 2003. The effect of female presence on spermiation, and of male sexual activity on ‘ready’ sperm in the male guppy. Anim. Behav. 65, 53–58. ( 10.1006/anbe.2002.2024) [DOI] [Google Scholar]
- 19.Gasparini C, Peretti AV, Pilastro A. 2009. Female presence influences sperm velocity in the guppy. Biol. Lett. 5, 792–794. ( 10.1098/rsbl.2009.0413) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gasparini C, Pilastro A. 2011. Cryptic female preference for genetically unrelated males is mediated by ovarian fluid in the guppy. Proc. R. Soc. B 278, 2495–2501. ( 10.1098/rspb.2010.2369) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Johnson AM, Chappell G, Price AC, Rodd FH, Olendorf R, Hughes KA. 2010. Inbreeding depression and inbreeding avoidance in a natural population of guppies (Poecilia reticulata). Ethology 116, 448–457. ( 10.1111/j.1439-0310.2010.01763.x) [DOI] [Google Scholar]
- 22.Nakadate M, Shikano T, Taniguchi N. 2003. Inbreeding depression and heterosis in various quantitative traits of the guppy, Poecilia reticulata. Aquaculture 220, 219–226. ( 10.1016/S0044-8486(02)00432-5) [DOI] [Google Scholar]
- 23.Mariette M, Kelley JL, Brooks R, Evans JP. 2006. The effects of inbreeding on male courtship behaviour and coloration in guppies. Ethology 112, 807–814. ( 10.1111/j.1439-0310.2006.01236.x) [DOI] [Google Scholar]
- 24.van Oosterhout C, Smith AM, Hanfling B, Ramnarine IW, Mohammed RS, Cable J. 2007. The guppy as a conservation model: implications of parasitism and inbreeding for reintroduction success. Conserv. Biol. 21, 1573–1583. [DOI] [PubMed] [Google Scholar]
- 25.Kelley JL, Graves JA, Magurran AE. 1999. Familiarity breeds contempt in guppies. Nature 401, 661–662. ( 10.1038/44314) [DOI] [PubMed] [Google Scholar]
- 26.Evans JP, Magurran AE. 2001. Patterns of sperm precedence and predictors of paternity in the Trinidadian guppy. Proc. R. Soc. Lond. B 268, 719–724. ( 10.1098/rspb.2000.1577) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuckuck C, Greven H. 1997. Notes on the mechanically stimulated discharge of spermiozeugmata in the guppy, Poecilia reticulata: a quantitative approach. Z. Fischkunde 4, 73–88. [Google Scholar]
- 28.Billard R, Puissant C. 1969. La spermatogenèse de Poecilia reticulata II: la production spermatogénétique. Ann. Biol. Anim. Bioch. Biophys. 9, 307–313. ( 10.1051/rnd:19690301) [DOI] [Google Scholar]
- 29.Boschetto C, Gasparini C, Pilastro A. 2011. Sperm number and velocity affect sperm competition success in the guppy (Poecilia reticulata). Behav. Ecol. Sociobiol. 65, 813–821. ( 10.1007/s00265-010-1085-y) [DOI] [Google Scholar]
- 30.Smith CC. 2012. Opposing effects of sperm viability and velocity on the outcome of sperm competition. Behav. Ecol. 23, 820–826. ( 10.1093/beheco/ars036) [DOI] [Google Scholar]
- 31.Faraway JJ. 2005. Extending the linear model with R: generalized linear, mixed effects and nonparametric regression models. CRC Texts in Statistical Science London, UK: Chapman & Hall. [Google Scholar]
- 32.Fournier DA, Skaug HJ, Ancheta J, Ianelli J, Magnusson A, Maunder M, Nielsen A, Sibert J. 2012. AD Model Builder: using automatic differentiation for statistical inference of highly parameterized complex nonlinear models. Optim. Methods Softw. 27, 233–249. ( 10.1080/10556788.2011.597854) [DOI] [Google Scholar]
- 33.R Development Core Team. 2013. A language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing; (http://www.R-project.org/). [Google Scholar]
- 34.Dosen LD, Montgomerie R. 2004. Female size influences mate preferences of male guppies. Ethology 110, 245–255. ( 10.1111/j.1439-0310.2004.00965.x) [DOI] [Google Scholar]
- 35.Herdman EJE, Kelly CD, Godin JGJ. 2004. Male mate choice in the guppy (Poecilia reticulata): do males prefer larger females as mates? Ethology 110, 97–111. ( 10.1111/j.1439-0310.2003.00960.x) [DOI] [Google Scholar]
- 36.Bates D, Maechler M, Bolker BM, Walker S. 2014. Linear mixed-effects models using Eigen and S4. (http://cran.r-project.org/web/packages/lme4/index.html) [Google Scholar]
- 37.Harano T, Katsuki M. 2012. Female seed beetles, Callosobruchus chinensis, remate more readily after mating with relatives. Anim. Behav. 83, 1007–1010. ( 10.1016/j.anbehav.2012.01.022) [DOI] [Google Scholar]
- 38.Parker GA, Ball MA, Stockley P, Gage MJG. 1996. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. Lond. B 263, 1291–1297. ( 10.1098/rspb.1996.0189) [DOI] [Google Scholar]
- 39.Olsén KH, Grahn M, Lohm J, Langefors Å. 1998. MHC and kin discrimination in juvenile Arctic charr, Salvelinus alpinus (L.). Anim. Behav. 56, 319–327. ( 10.1006/anbe.1998.0837) [DOI] [PubMed] [Google Scholar]
- 40.Reusch TBH, Haberli MA, Aeschlimann PB, Milinski M. 2001. Female sticklebacks count alleles in a strategy of sexual selection explaining MHC polymorphism. Nature 414, 300–302. ( 10.1038/35104547) [DOI] [PubMed] [Google Scholar]
- 41.Shohet AJ, Watt PJ. 2004. Female association preferences based on olfactory cues in the guppy, Poecilia reticulata. Behav. Ecol. Sociobiol. 55, 363–369. ( 10.1007/s00265-003-0722-0) [DOI] [Google Scholar]