Abstract
Owing to the assumed lack of deep-sea macrofossils older than the Late Cretaceous, very little is known about the geological history of deep-sea communities, and most inference-based hypotheses argue for repeated recolonizations of the deep sea from shelf habitats following major palaeoceanographic perturbations. We present a fossil deep-sea assemblage of echinoderms, gastropods, brachiopods and ostracods, from the Early Jurassic of the Glasenbach Gorge, Austria, which includes the oldest known representatives of a number of extant deep-sea groups, and thus implies that in situ diversification, in contrast to immigration from shelf habitats, played a much greater role in shaping modern deep-sea biodiversity than previously thought. A comparison with coeval shelf assemblages reveals that, at least in some of the analysed groups, significantly more extant families/superfamilies have endured in the deep sea since the Early Jurassic than in the shelf seas, which suggests that deep-sea biota are more resilient against extinction than shallow-water ones. In addition, a number of extant deep-sea families/superfamilies found in the Glasenbach assemblage lack post-Jurassic shelf occurrences, implying that if there was a complete extinction of the deep-sea fauna followed by replacement from the shelf, it must have happened before the Late Jurassic.
Keywords: evolution of deep-sea biota, onshore-offshore patterns, in situ diversification, resilience against extinction
1. Introduction
Thanks to intense research efforts during the last few decades, it is now generally acknowledged that the deep sea supports one of the highest levels of biodiversity on Earth, while differing fundamentally from shallow marine and terrestrial ecosystems [1,2]. Yet, the evolutionary processes that have shaped the unique attributes of the deep sea are still controversial. Growing evidence that the deep sea was anything but stable and unchanging through time has challenged the initial concept of the deep sea as a refuge for ancient lineages excluded from shelf habitats. Debates have focused on the impact of variations in temperature, oxygenation and circulation mode on the colonization of deep habitats, and at least for the late Cenozoic, deep-sea sediment cores have provided extensive benthic foraminifer and ostracod microfossil evidence in this respect [3,4]. Biogeographic patterns and molecular clock estimates have yielded dates which predominantly converge to a latest Mesozoic or early Cenozoic origin of the modern deep-sea fauna [5–9]. Testing these hypotheses and exploring older deep-sea biodiversity using direct fossil evidence, however, has been hampered so far by the sparse record of deep-sea faunas older than the Late Cretaceous [6]. The recent discovery of a modern-type echinoderm assemblage from Lower Cretaceous bathyal deposits of the subtropical north Atlantic [10] demonstrated that direct fossil evidence of deep-sea biodiversity exists in sediments beyond Late Cretaceous age and showed that the origin of at least some modern deep-sea groups is to be sought in even older strata.
2. Geological context
We here present fossil remains of a newly discovered diverse deep-marine assemblage retrieved from marls of the Kehlbach and Scheck Members (informally known as ‘Hauptknollenbrekzie’) within the Adnet Formation, exposed in the Glasenbach Gorge, south of Salzburg in the northern Calcareous Alps, Austria. These sediments are interpreted as a giant slump deposit derived from the slope of a submarine high in the former northern Tethys Ocean, and their ammonite fauna indicates an Early Jurassic age (from the late Sinemurian Echioceras raricostatum Zone to the late Pliensbachian Amaltheus margaritatus Zone) [11,12]. Palaeo-depth reconstructions suggest at least 1000 m for the slump components, based on subsidence models of the area, sedimentological comparisons with present-day equivalents and the presence of very similar lithologies directly overlying both oceanic and deeply submerged continental crust in the Jurassic of the north Atlantic [11,13], as well as the absence of light-dependent benthic organisms even on the submarine tops of the source area of the slumping mass [12]. These estimates are supported by our palaeobathymetric analysis using microbioerosion trace fossils in 15 bivalve and brachiopod shell fragments and belemnite rostra. Not a single unequivocal trace of a phototrophic chlorophyte or cyanobacterium, which could indicate a photic environment, was found. Rather, all identified traces are produced by heterotrophic organisms (mostly marine fungi) and are typical constituents of aphotic trace fossil assemblages (figure 1) [14]. Thus, the material studied herein must have been deposited at a palaeo-depth exceeding the lower limit of sunlight influence in the water column, which, in the present case (low palaeo-latitude sea with low water turbidity, deduced from the low sedimentation rate [11]), was between 150 m (deepest regular phototrophic records during settlement experiments [15]) and 370 m (deepest known occurrence of phototrophic microendoliths [16]), and thus well below regular palaeo-shelf depths.
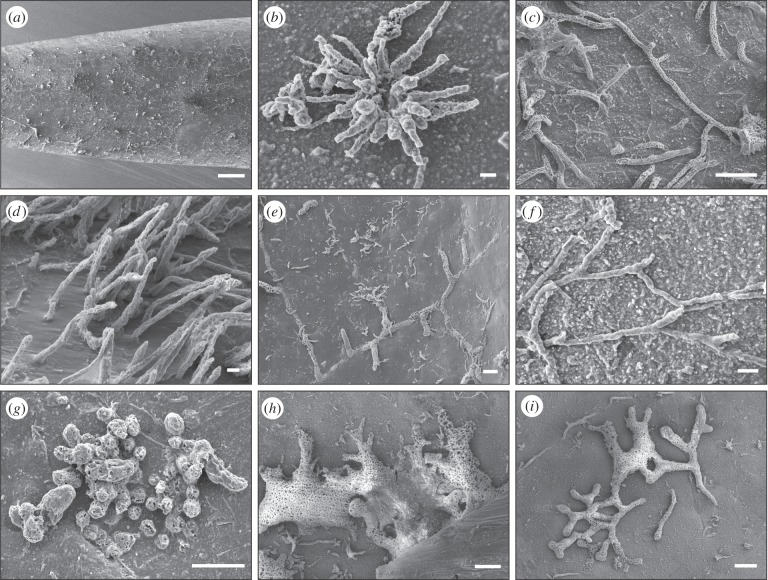
Figure 1.
Scanning electron microscope images of microboring trace fossils (in order of abundance) prepared as epoxy resin casts from mollusc shells from the Sinemurian–Pliensbachian (Early Jurassic) of the Glasenbach section, Austria. (a) Semi-cylindrical resin cast of a belemnite rostrum exhibiting a large number of microborings; (b) the dominant fungal microboring Polyactina araneola, in modern seas produced by the fungus Conchyliastrum; (c) the aphotic index ichnotaxon Orthogonum lineare, producer unknown but most likely a fungus; (d) cluster of Flagrichnus profundus, produced by schizochytrid fungi; (e) Orthogonum giganteum, producer unknown; (f) Saccomorpha isp., presumed fungal trace; (g) Podichnus centrifugalis, attachment scar of a juvenile brachiopod pedicle; (h) Platydendrina convexa, producer unknown; (i) unknown dichotomously branching dendrinid microboring with affinity to the ichnogenus Abeliella. Scale bars equal 1 mm in (a), 10 µm in (b,d,f) and 100 µm in (c,e,g–i).
Further evidence supporting a bathyal palaeo-depth is provided by the ostracod assemblage of the Glasenbach fauna, which includes only forms lacking eyes or other signs of photoreception. In addition, it almost exclusively consists of members of the extant eurybathic Bairdioidea, and thus fundamentally differs from all known coeval ostracod assemblages from shelf depths [17,18] and instead resembles a coeval assemblage found in slope deposits from Turkey [19]. The Glasenbach assemblage thus provides a unique window into Early Jurassic deep-sea biodiversity and predates the late Mesozoic oceanic anoxic events commonly considered to have triggered extinction in the deep sea and subsequent replacement from the shelf.
3. Material and methods
Specimens were collected in the field or picked from washed residues. Figured specimens were deposited at the Natural History Museum in Vienna. Families and, where applicable, superfamilies were chosen as basis for the faunal analyses because they were found to represent a compromise between taxonomic resolution and comparability with modern communities. Identifications of dissociated echinoderm plates followed latest taxonomic recommendations [20–22].
To assess the origin, resilience and bathymetric range shifts of the deep-sea fauna, we classified the families/superfamilies of the Glasenbach assemblage as well as coeval shelf (less than 200 m palaeo-depth) faunas [20,21,23–36] as extinct, or extant and, according to their present-day bathymetric distribution, typically deep, typically shallow, or without depth preference (eurybathic) (figure 3; electronic supplementary material, table 1). Our bathymetric classification was based on the averaged mean depth distribution of the extant species of the families/superfamilies (calculated by averaging the upper and lower distribution boundaries for each species and by computing the arithmetic mean of the obtained values for each family/superfamily): typically deep—averaged mean depth greater than 500 m; typically shallow—averaged mean depth shallower than 200 m; eurybathic—averaged mean depth between 200 and 500 m. The upper boundary of the deep sea is commonly set between 200 and 500 m, in line with the fading of seasonal variations in physical parameters (e.g. temperature) and of the influence of sunlight [2,6,37–39]. We adopted a conservative approach using the 500 m boundary to minimize the impact of potential outliers from shelf depths. Microbioerosion trace fossils were analysed by applying the vacuum cast-embedding method and scanning electron microscopic imaging to visualize the delicate microborings in decalcified polymer-resin casts [14]. Statistic tests were performed using the software package PAST v. 2.13 [40].
Figure 3.
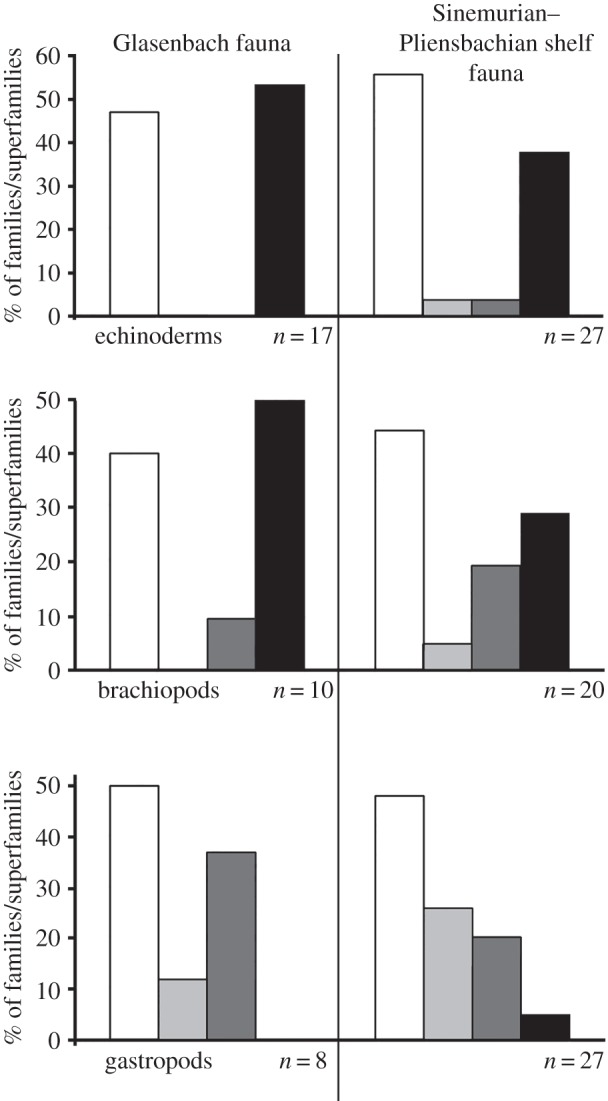
Percentages of extinct, modern shallow, modern eurybathic and modern deep families/superfamilies of echinoderms, brachiopods and gastropods in the Sinemurian–Pliensbachian Glasenbach fauna and coeval shallow-water (less than 200 m) communities. White, extinct; light grey, extant typically shallow; dark grey, extant eurybathic; black, extant typically deep.
4. Results and discussion
Among the several thousand available specimens, we distinguished at least 68 species belonging to four different phyla, i.e. echinoderms (ophiuroids, asteroids, echinoids and crinoids), molluscs (gastropods), brachiopods and crustaceans (ostracods) (figure 2 and electronic supplementary material).
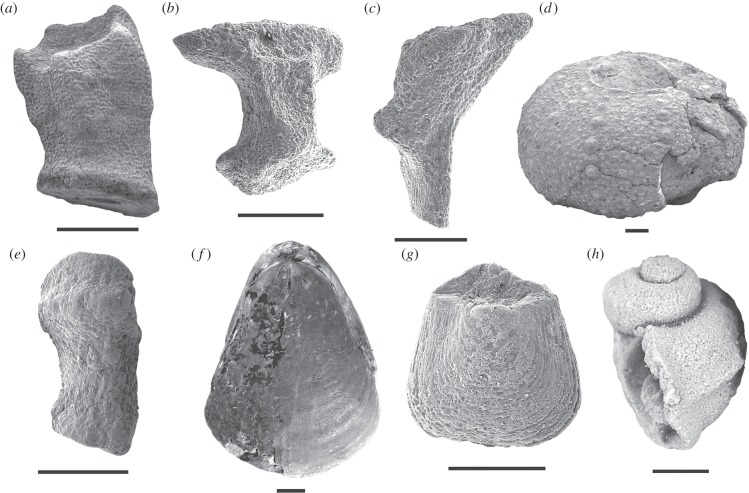
Figure 2.
Representatives of modern deep-sea groups from the Sinemurian–Pliensbachian (Early Jurassic) of the Glasenbach section, Austria. (a) Eudesicrinus cuneatus (Eudesicrinidae, Crinoidea), cup in lateral view; (b) Benthopectinidae (Asteroidea) new genus and species, ambulacral in actinal view; (c) Pterasteridae (Asteroidea) genus and species indeterminate, ambulacral in actinal view; (d) Aspidodiadematidae (Echinoidea) new genus and species, test in latero-oral view; (e) Ophiomycetidae (Ophiuroidea) new genus and species, lateral arm plate in external view; (f) Securithyris adnethensis (Dyscolioidea, Rhynchonellata), in dorsal view; (g) Zellania sp. (Gwynioidea, Rhynchonellata), in dorsal view; (h) Pleurotomarioidea (Gastropoda) genus and species indeterminate. Scale bars equal 500 µm (a–d, f–g), 200 µm (e) and 1000 µm (h).
This assemblage includes the extant, typically deep ophiomycetid ophiuroids, basal pterasterid asteroids and gwynioid brachiopods. These fossils predate the hitherto oldest record of these groups from Middle Jurassic shallow-water deposits by more than 25 Myr [20,21,23,24]. In addition, four extant deep-sea groups present in the Glasenbach assemblage, namely benthopectinid asteroids, eudesicrinid crinoids and aspidodiadematid as well as extinct stem-group irregular echinoids, either coincide with, or slightly predate, the oldest known occurrences at shelf depths [20,21,25], depending on whether the Glasenbach assemblage is considered as late Pliensbachian or late Sinemurian in age. A deep-sea origin or at least an immediate expansion to the deep sea following a shallow origin must be assumed for these groups, suggesting that in situ origination of family/superfamily-level diversity in the deep sea, as opposed to colonization by taxa from the shelf, played a greater role in shaping modern deep-sea biodiversity than previously thought. This highlights the potential of deep-sea environments to produce and export higher taxonomic diversity [41,42], and suggests that at least some fossil shelf occurrences of modern deep-sea groups may be temporary expansions into shallow water rather than indicators of an onshore origin.
Both the Glasenbach assemblage and the coeval shelf faunas contain a similar percentage of extinct taxa (figure 3). This intuitively suggests similar resilience against extinction in both shallow and deep-water habitats, but it does not take differential bathymetric range shifts between the two environments into account. To test for such diverging evolutionary dynamics, we compared the number of extant families/superfamilies in the Glasenbach assemblage and in coeval shelf faunas that retained their Early Jurassic depth preference until today versus those that did not. In the case of the Glasenbach assemblage these are the typically deep and eurybathic groups and in the case of the shelf fauna, the typically shallow and eurybathic groups. Among the echinoderms, significantly more deep-sea inhabitants retained their Early Jurassic depth preference to the present day than did coeval shelf inhabitants (Fisher's exact test, p = 0.0002); among gastropods, these proportions are indistinguishable. Although more deep-sea brachiopods retained their Early Jurassic depth preference than those in shallow water, this difference is not statistically significant (Fisher's exact test, p = 0.1), possibly because many extant brachiopods are most common at depths between 100 and 300 m [23] and are thus classified as eurybathic rather than typically deep according to the criteria applied here. Thus, depth-related turnover, reflecting how many extant groups have undergone shifts in their preferred depth distribution since the Early Jurassic, was higher at shallow depths than in the deep sea among echinoderms and probably in brachiopods, but not among gastropods. These results call for caution when using a single taxonomic group as a model to explore evolutionary patterns controlling deep-sea biodiversity.
The Glasenbach assemblage lacks extant shallow-water families/superfamilies, except for a single gastropod superfamily (Neritoidea). Thus, it seems that once families/superfamilies had colonized the deep sea, they either remained there or became extinct. This implies first that deep-sea environments provide higher evolutionary stability than shallow-water environments, and second that bathymetric shifts, in contrast to bathymetric extensions, from the deep sea to shallow waters are unlikely. Many extant deep-sea families/superfamilies present in the Glasenbach assemblage occur also in Early Jurassic shallow-water settings. Their restriction to the deep sea today thus results from a restriction of their once broad bathymetric range. This loss of shallow-water representatives among the once eurybathic groups seems to be a continuous process through geological time [43], as suggested by the last shelf occurrences of some of the Glasenbach families/superfamilies: e.g. Eudesicrinidae in the Late Jurassic [21], Zeillerioidea in the Early Cretaceous [23], and Pleurotomarioidea in the Cenozoic [44].
Several present-day deep-sea families, including Benthopectinidae, Aspidodiadematidae, Eudesicrinidae and Ophiomycetidae, lack a post-Jurassic fossil record at shelf depths but were already present in the Early Jurassic Glasenbach deep-sea assemblage. Had there been a complete extinction of the deep-sea fauna followed by recolonization from shelf seas since the Early Jurassic [1,5], it must have happened before the Late Jurassic. This further highlights the resilience of the deep sea against major palaeoceanographic perturbations and sheds further doubt on the global extent and/or devastating nature of the so-called anoxic events during the late Mesozoic [10,45–47].
5. Conclusion
In summary, our results imply that two macroevolutionary processes have concurrently contributed to deep-sea biodiversity since the Early Jurassic: the potential to generate higher-level taxonomic diversity and resilience against major palaeoceanographic perturbations. We show for the first time, to our knowledge, that higher level taxa can originate in the deep sea, expand to shelf depths and survive in the deep sea even after having gone extinct in the shallow seas again. We speculate that the resilience of the deep sea results from the sheer size of the environment combined with the great dispersal potential of the deep-sea benthos, increasing the chance for taxa to survive in deep-sea refuges and to subsequently re-expand. The potential to generate diversity might result from the many unique attributes of the deep-sea environment [2], which require unique adaptations that can only evolve in situ. Whatever the causes, our results shed new light on the role of the deep sea as a macroevolutionary source and reservoir of biodiversity, as well as on its potential to contribute to shallow-water diversity. The latter point in particular calls for a careful reappraisal of the impact of deep-sea trawling and mining activities on marine biodiversity.
Acknowledgements
We thank H. Hess for assistance with the identification of crinoid material, and G. Moosleitner for providing part of the fossil material.
Funding statement
Part of this study benefited from the support of EU-funded Synthesys grant nos. SE-TAF-2674 and SE-TAF-1297 to B.T., and GB-TAF-2781 to A.D. The study of brachiopods was supported also by the Hungarian Scientific Research Fund (OTKA K77451). Further financial support was provided by the Deutsche Forschungsgemeinschaft through grants Ki802/6-1 and Ki802/8-1 to S.K.
References
- 1.Rex MA, Etter RJ. 2010. Deep-sea biodiversity: pattern and scale. Cambridge, MA: Harvard University Press. [Google Scholar]
- 2.Ramirez-Llodra E, et al. 2010. Deep, diverse and definitely different: unique attributes of the world's largest ecosystem. Biogeosciences 7, 2851–2899. ( 10.5194/bg-7-2851-2010) [DOI] [Google Scholar]
- 3.Yasuhara M, Hunt G, Cronin T, Okahashi H. 2009. Temporal latitudinal-gradient dynamics and tropical instability of deep-sea species diversity. Proc. Natl Acad. Sci. USA 106, 21 717–21 720. ( 10.1073/pnas.0910935106) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kawagata S, Hayward BW, Grenfell HR, Sabaa A. 2005. Mid-Pleistocene extinction of deep-sea foraminifera in the North Atlantic Gateway (ODP sites 980 and 982). Palaeogeogr. Palaeoclim. Palaeoecol. 221, 267–291. ( 10.1016/j.palaeo.2005.03.001) [DOI] [Google Scholar]
- 5.Jacobs DK, Lindberg DR. 1998. Oxygen and evolutionary patterns in the sea: onshore/offshore trends and recent recruitment of deep-sea faunas. Proc. Natl Acad. Sci. USA 95, 9396–9401. ( 10.1073/pnas.95.16.9396) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Smith AB, Stockley B. 2005. The geological history of deep-sea colonization by echinoids: roles of surface productivity and deep-water ventilation. Proc. R. Soc. B 272, 865–869. ( 10.1098/rspb.2004.2996) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Strugnell JM, Rogers AD, Prodöhl PA, Collins M, Allcock AL. 2008. The thermohaline expressway: the Southern Ocean as a centre of origin for deep-sea octopuses. Cladistics 24, 853–860. ( 10.1111/j.1096-0031.2008.00234.x) [DOI] [PubMed] [Google Scholar]
- 8.Williams ST, et al. 2013. Cenozoic climate change and diversification on the continental shelf and slope: evolution of gastropod diversity in the family Solariellidae (Trochoidea). Ecol. Evol. 3, 887–917. ( 10.1002/ece3.513) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Vrijenhoek RC. 2013. On the instability and evolutionary age of deep-sea chemosynthetic communities. Deep-Sea Res. II 92, 189–200. ( 10.1016/j.dsr2.2012.12.004) [DOI] [Google Scholar]
- 10.Thuy B, Gale AS, Kroh A, Kucera M, Numberger-Thuy LD, Reich M, Stöhr S. 2012. Ancient origin of the modern deep-sea fauna. PLoS ONE 7, e46913 ( 10.1371/journal.pone.0046913) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bernoulli D, Jenkyns HC. 1970. A Jurassic basin: the Glasenbach Gorge, Salzburg, Austria. Verh. Geol. B.-A. 1970, 504–531. [Google Scholar]
- 12.Böhm F. 2003. Lithostratigraphy of the Adnet Group (Early to Middle Jurassic, Salzburg, Austria). In Stratigraphia Austriaca (ed. Piller WE.), pp. 231–268. Österr. Akad. Wiss., Schriftenr. Erdwiss. Komm. 16. [Google Scholar]
- 13.Bernoulli D, Jenkyns HC. 2009. Ancient oceans and continental margins of the Alpine-Mediterranean Tethys: deciphering clues from Mesozoic pelagic sediments. Sedimentology 56, 149–190. ( 10.1111/j.1365-3091.2008.01017.x) [DOI] [Google Scholar]
- 14.Wisshak M. 2012. Microbioerosion. In Trace fossils as indicators of sedimentary environments. Developments in Sedimentology, issue 64 (eds Knaust D, Bromley R.), pp. 213–243. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 15.Vogel K, Gektidis M, Golubic S, Kiene WE, Radtke G. 2000. Experimental studies on microbial bioerosion at Lee Stocking Island, Bahamas and One Tree Island, Great Barrier Reef, Australia: implications for paleoecological reconstructions. Lethaia 33, 190–204. ( 10.1080/00241160025100053) [DOI] [Google Scholar]
- 16.Lukas KJ. 1978. Depth distribution and form among common microboring algae from the Florida continental shelf. Geol. Soc. Am., Abstr. with Progr. 10, 448. [Google Scholar]
- 17.Boomer I, Ainsworth NR. 2009. Lower Jurassic (Hettangian-Toarcian). In Ostracods in British stratigraphy (eds Whittaker JE, Hart MB.), pp. 175–197. London, UK: The Micropalaeontological Society. [Google Scholar]
- 18.Lord AR. 1988. Ostracoda of the Early Jurassic Tethyan Ocean. In Evolutionary biology of Ostracoda (eds Hanai T, Ikeya N, Ishizaki K.), pp. 855–868. Tokyo, Japan: Kodansha. [Google Scholar]
- 19.Lord AR, Lambourne DC. 1991. Lower Jurassic ostracods from the western Pontides, Turkey. Geol. Rom 27, 381–387. [Google Scholar]
- 20.Gale AS. 2011. The phylogeny of post-Palaeozoic Asteroidea (Neoasteroidea, Echinodermata). Spec. Pap. Palaeontol. 85, 5–112. [Google Scholar]
- 21.Hess H. 2011. Treatise on invertebrate paleontology. Part T, revised, Echinodermata 2, volume 3, Crinoidea Articulata. Lawrence, KS, USA: KU Paleontological Institute, The University of Kansas. [Google Scholar]
- 22.Thuy B, Stöhr S. 2011. Lateral arm plate morphology in brittle stars (Echinodermata: Ophiuroidea): new perspectives for ophiuroid micropalaeontology and classification. Zootaxa 3013, 1–47. [Google Scholar]
- 23.Williams A, et al. 1997–2007. Treatise on invertebrate paleontology. Part H, revised, Brachiopoda, volumes 1–6. Lawrence, KS, USA: KU Paleontological Institute, The University of Kansas. [Google Scholar]
- 24.Thuy B, Meyer CA. 2012. The pitfalls of extrapolating modern depth ranges to fossil assemblages: new insights from Middle Jurassic brittle stars (Echinodermata: Ophiuroidea) from Switzerland. Swiss J. Palaeontol. 132, 5–21. ( 10.1007/s13358-012-0048-5) [DOI] [Google Scholar]
- 25.Kroh A, Smith AB. 2010. The phylogeny and classification of post-Palaeozoic echinoids. J. Syst. Palaeontol. 8, 147–212. ( 10.1080/14772011003603556) [DOI] [Google Scholar]
- 26.Conti MA, Szabó J. 1989. A revision of the Jurassic gastropod fauna from Cape San Vigilio (S-Alps, Italy), published by M. Vacek (1886). Fragmenta Mineral. Palaeontol. 14, 29–40. [Google Scholar]
- 27.Gatto R, Monari S. 2010. Pliensbachian gastropods form Venetian Southern Alps (Italy) and their palaeobiogeographical significance. Palaeontology 53, 771–802. ( 10.1111/j.1475-4983.2010.00961.x) [DOI] [Google Scholar]
- 28.Gründel J. 1999. Gastropoden aus dem höheren Lias von Grimmen, Vorpommern (Deutschland). Archiv. Geschiebekde. 2, 629–672. [Google Scholar]
- 29.Gründel J. 2001. Gastropoden aus dem Jura der südamerikanischen Anden. Freiberger Forschungsh. C 492, 43–84. [Google Scholar]
- 30.Gründel J. 2003. Gastropoden aus dem Unteren Lias (Ober-Hettangium bis Unter Sinemurium) Südwestdeutschlands. Stuttgart Beitr. Naturkde. B 340, 1–55. [Google Scholar]
- 31.Gründel J. 2007. Jurassische Gastropoden aus der Betakalkbank (oberes Sinemurium, obere Obtusum-Zone) Südwestdeutschlands. Stuttgart. Beitr. Naturk. B 370, 1–29. [Google Scholar]
- 32.Hess H. 2006. Crinoids (Echinodermata) from the Lower Jurassic (Upper Pliensbachian) of Arzo, southern Switzerland. Schweizer Pal. Abh. 126, 1–143. [Google Scholar]
- 33.Schubert S, Gründel J, Nützel A. 2008. Early Jurassic (Upper Pliensbachian) gastropods from the Herforder Liasmulde (Bielefeld, Northwest Germany). Paläontol. Z. 82, 7–30. ( 10.1007/BF02988430) [DOI] [Google Scholar]
- 34.Thuy B, Gale AS, Reich M. 2011. A new echinoderm Lagerstätte from the Pliensbachian (Early Jurassic) of the French Ardennes. Swiss J. Pal. 130, 173–185. ( 10.1007/s13358-010-0015-y) [DOI] [Google Scholar]
- 35.Szabó J. 2002. A new euomphalomorph gastropod genus in the faunula, listed by Vadász (1915) from Alsórákos (Persani Mts, Romania, ?Early Jurassic). Fragmenta Palaeontol. Hungarica 20, 61–67. [Google Scholar]
- 36.Thuy B. 2013. Temporary expansion to shelf depths rather than an onshore-offshore trend: the shallow-water rise and demise of the modern deep-sea brittle star family Ophiacanthidae (Echinodermata: Ophiuroidea). Eur. J. Taxon. 48, 1–242. ( 10.5852/ejt.2013.48) [DOI] [Google Scholar]
- 37.Gage JD, Tyler PA. 1991. Deep-sea biology: a natural history of organisms at the deep-sea floor. Cambridge, UK: Cambridge University Press. [Google Scholar]
- 38.UNESCO. 2009. Global open oceans and deep seabed (GOODS) – biogeographic classification. Paris, France: UNESCO-IOC. [Google Scholar]
- 39.Glover AG, Higgs N, Horton T. 2014. World register of deep-sea species. (http://www.marinespecies.org/deepsea accessed on 25 February 2014). [Google Scholar]
- 40.Hammer Ø, Harper DAT, Ryan PD. 2001. PAST: palaeontological statistics software package for education and data analysis. Palaeontol. Electr. 4, 1–9. [Google Scholar]
- 41.Lindner A, Cairns SD, Cunningham CW. 2008. From offshore to onshore: multiple origins of shallow-water corals from deep-sea ancestors. PLoS One 3, e2429 ( 10.1371/journal.pone.0002429) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pante E, France SC, Couloux A, Cruaud C, McFadden CS, Samadi S, Watling L. 2012. Deep-sea origin and in-situ diversification of chrysogorgiid octocorals. PLoS ONE 7, e38357 ( 10.1371/journal.pone.0038357) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jablonski D. 2005. Evolutionary innovations in the fossil record: the intersection of ecology, development, and macroevolution. J. Exp. Zool. 304B, 504–519. ( 10.1002/jez.b.21075) [DOI] [PubMed] [Google Scholar]
- 44.Hickman CS. 1976. Pleurotomaria (Archaeogastropoda) in the Eocene of the Northeastern Pacific: a review of Cenozoic biogeography and ecology of the genus. J. Paleontol. 50, 1090–1102. [Google Scholar]
- 45.Little CTS, Vrijenhoek RC. 2003. Are hydrothermal vent animals living fossils? Trends Ecol. Evol. 18, 582–588. ( 10.1016/j.tree.2003.08.009) [DOI] [Google Scholar]
- 46.Kiel S, Little CTS. 2006. Cold seep mollusks are older than the general marine mollusk fauna. Science 313, 1429–1431. ( 10.1126/science.1126286) [DOI] [PubMed] [Google Scholar]
- 47.Kiel S, Wiese F, Titus AL. 2012. Shallow-water methane-seep faunas in the Cenomanian Western Interior Seaway: no evidence for onshore-offshore adaptations to deep-sea vents. Geology 40, 839–842. ( 10.1130/G33300.1) [DOI] [Google Scholar]