Abstract
Microbial biofilms induce larval settlement for some invertebrates, including corals; however, the chemical cues involved have rarely been identified. Here, we demonstrate the role of microbial biofilms in inducing larval settlement with the Caribbean coral Porites astreoides and report the first instance of a chemical cue isolated from a marine biofilm bacterium that induces complete settlement (attachment and metamorphosis) of Caribbean coral larvae. Larvae settled in response to natural biofilms, and the response was eliminated when biofilms were treated with antibiotics. A similar settlement response was elicited by monospecific biofilms of a single bacterial strain, Pseudoalteromonas sp. PS5, isolated from the surface biofilm of a crustose coralline alga. The activity of Pseudoalteromonas sp. PS5 was attributed to the production of a single compound, tetrabromopyrrole (TBP), which has been shown previously to induce metamorphosis without attachment in Pacific acroporid corals. In addition to inducing settlement of brooded larvae (P. astreoides), TBP also induced larval settlement for two broadcast-spawning species, Orbicella (formerly Montastraea) franksi and Acropora palmata, indicating that this compound may have widespread importance among Caribbean coral species.
Keywords: biofilm, chemical ecology, coral recruitment, larval settlement, Pseudoalteromonas
1. Introduction
Coral cover on Caribbean reefs has declined in recent decades, with many reefs shifting from coral-dominated ecosystems to those dominated by macroalgae [1,2]. Recovery of coral populations on degraded reefs is dependent on recruitment of new individuals [1], and genetic diversity maintained through the input of sexually produced propagules may be important for stress tolerance and future reproductive success within coral populations [3]. The recruitment process in corals encompasses three main life-history stages: (i) development of competent larvae either within the coral colony (brooders) or within the water column (broadcast spawners), (ii) settlement (attachment followed by metamorphosis) of larvae onto appropriate substrata, and (iii) post-settlement survival of juvenile corals [4]. Survival of juvenile coral recruits may be dependent on the type of substratum chosen by larvae during settlement [5,6], suggesting that the appropriate choice of settlement substratum is critical for recruitment success.
Similar to other invertebrates, coral larvae are able to discriminate among settlement locations by responding to a variety of physical and chemical factors (see reviews [7–9]). The exact nature of the cues that induce coral larval settlement is still not completely understood. Some crustose coralline algae (CCA) provide positive settlement cues for many coral species; and in some cases, the settlement response can be elicited by chemical extracts of the CCA, indicating that larvae are responding to chemical cues [5,6,10,11]. However, not all CCA species are equal in their ability to induce settlement, and larvae of some coral species exhibit preferences for particular CCA species [5,6]. Other coral species do not settle on CCA at all, but instead settle preferentially on naturally biofilmed, non-CCA carbonate surfaces [12].
Biofilm bacteria living on surfaces of preferred settlement substrata may be responsible for producing positive settlement cues for some corals. Webster et al. [13] found that Acropora microphthalma larvae settle in response to microbial biofilms, regardless of the presence of CCA, and that alterations in the bacterial community within the biofilm coinciding with biofilm age affect the amount of settlement. Several bacterial strains isolated from a variety of marine biofilms induce settlement to varying degrees in the Pacific coral Pocillopora damicornis [14]. A bacterial strain (Pseudoalteromonas sp. A3) isolated from the surface of the CCA Hydrolithon onkodes induces settlement in two Pacific coral species, Acropora millepora and A. willisae, when in the presence of carbonate structure [15]. The activity of this bacterium has been attributed to the compound tetrabromopyrrole (TBP); however, purified TBP induces spontaneous metamorphosis without attachment [16].
While evidence is accumulating that biofilm bacteria provide positive settlement cues for some corals, studies on the role of bacteria in coral recruitment have been limited to species found in the Pacific, and the mechanisms involved are still unknown. The aim of this study was to examine the role of biofilm bacteria in the settlement of Caribbean corals and to determine whether biofilm bacteria induce larval settlement through the production of chemical cues.
2. Material and methods
(a). Effects of biofilms on larval settlement
(i). Larval collection and handling
For experiments testing the response of Porites astreoides larvae to live biofilms, adult coral heads were collected from Wonderland Reef in the lower Florida Keys (24°33.62′ N, 81°30.08′ W) on 27 May 2011 and transported in coolers to Mote's Tropical Research Lab (TRL) in Summerland Key, Florida, USA where they were maintained in flowing seawater raceways. Larvae were collected on the nights of 28–31 May 2011, following the methods outlined by Kuffner et al. [17] (electronic supplementary material).
(ii). Natural bacterial assemblages
Natural biofilms were allowed to form for 15 or 21 days on limestone settlement tiles (5 × 5 × 1.2 cm) that were prepared from slabs of Keystone Coral Flagging (Select Stone Co, Ft. Pierce, FL). Tiles were placed on white fibreglass rods spaced approximately 5.5 cm apart using spacers made from 1.5 cm diameter Tygon tubing to ensure biofilm formation on all sides. The resulting tile racks were attached directly to the reef near Looe Key, Florida (24°33.214′ N, 81°22.749′ W) on 6 and 12 May 2011 to prepare tiles with 21- and 15-day-old biofilms. All tiles were collected from the reef on 27 May 2011 and transferred to flow-through raceways at TRL, where they remained until use. Naturally biofilmed tiles were brushed gently with a toothbrush to remove macrofouling organisms prior to use in experiments.
To determine whether biofilm age affected larval settlement, tiles were placed into autoclave-sterilized glass jam jars with lids (200 ml; Weck Jars, Crystal Lake, IL) filled with 90 ml of filter-sterilized seawater (FSW). Each jar contained one of two treatments: a 15-day-biofilmed tile or a 21-day-biofilmed tile (n = 8). Controls were prepared with only FSW, no tile (n = 8), to demonstrate that larvae will not settle in the absence of a positive settlement cue. Forty 1-day-old larvae (released 28 May, used 29 May) were added to each jar in 10 ml FSW. Number of larvae settled (attached and metamorphosed), number metamorphosed but not attached, and total number surviving were counted and recorded after 24 and 48 h to allow for adequate larval response. Because larval response to all treatments in this experiment was greater after 48 h, those data are reported. Larval response was adequate after 24 h in subsequent experiments.
Naturally biofilmed tiles (21-day-old) were treated with antibiotics to determine the impacts of reduced bacterial load on the settlement behaviour of P. astreoides larvae. Ten tiles containing a 21-day-old biofilm were placed in 100 ml of seawater-antibiotic solution (1000/250/250 mg l−1 penicillin/streptomycin/gentamycin) for 12 h. Afterwards, antibiotic-treated tiles and naturally biofilmed tiles (n = 10) were rinsed by dipping twice in FSW and placed in 90 ml of FSW in 200 ml glass jam jars. Controls were prepared with only FSW, no tile, to demonstrate that larvae will not settle in the absence of a positive settlement cue. Fifty 4-day-old larvae (released 30 May, used 3 June) were added to each jar in 10 ml of FSW. Number of larvae settled (attached and metamorphosed), number metamorphosed but not attached and total number surviving were counted and recorded after 24 h.
(iii). Monospecific bacterial biofilms
Four species of CCA (Paragoniolithon solubile, Hydrolithon boergesenii, Lithoporella atlantica and Titanoderma prototypum) were collected from the fringing reef near Looe Key on 5 May 2011, placed in individual sterile bags (WhirlPak) and transported to TRL. Each piece of CCA was identified and rinsed under a stream of 15 ml FSW. The surface (approx. 1–5 cm2) was swabbed with a sterile cotton swab. Swabs were placed in 1 ml FSW in sterile microcentrifuge tubes and vortexed at high speed for 1 min. A 1 : 10 dilution was prepared in FSW, and 100 μl aliquots of both the full concentration and diluted bacterial solutions were plated using the spread plate method on marine broth agar (MBA), seawater agar and seawater agarose. Media recipes are given in the electronic supplementary material. Colonies were picked from these plates, and individual bacterial strains were isolated using the streak plate method on MBA. Glycerol stocks were prepared and stored at −80°C.
Monospecific biofilms were prepared on ceramic frag discs (Bulk Reef Supply, Minneapolis, MN) by placing one autoclaved disc into each of five wells in a sterile, polystyrene six-well plate and adding 5 ml sterile marine broth (MB) to each well. Five replicate wells were inoculated with 100 µl of liquid bacterial culture for each of 16 bacterial strains (electronic supplementary material, table S1). Five wells were left uninoculated to act as a negative MB control. After 45 h, discs were transferred to approximately 5 ml FSW in clean, sterile six-well plates and soaked for 1 h. Discs were then transferred to sterile Petri dishes containing 40 ml FSW. Twenty 1-day-old larvae (released 29 May, used 30 May) in 10 ml FSW were added to each dish. Autoclave-sterilized un-biofilmed discs that were not soaked in marine broth (non-marine broth, NMB) and no disc (FSW) controls were also prepared. Number of larvae settled (attached and metamorphosed), number metamorphosed but not attached and total number surviving were counted after 24 h.
(iv). Identification of bacterial isolates
16S rRNA gene sequencing was used to identify each isolate. The 16S rRNA gene fragment was amplified directly from colonies using a Phire Animal Tissue Direct PCR Kit (Finnzymes/Thermo Fisher Scientific, Lafayette, CO). Universal bacterial 16S rRNA primers (27F, AGAGTTTGATCMTGGCTCAG; 1492R, TACGGYTACCTTGTTACGACTT) were added to each reaction at a final concentration of 0.5 µM following the manufacturer's protocol. Unpurified PCR reactions were submitted to Beckman Coulter Genomics (Danvers, MA) for single-pass sequencing. Resulting sequences were aligned to each other, based on 97% sequence similarity, and sequences were compared to NCBI/GenBank (http://blast.ncbi.nlm.nih.gov) and the Ribosomal Database Project (http://rdp.cme.msu.edu) databases to determine phylogenetic affiliation.
The settlement inducing strain PS-MBA-5 was subjected to further sequencing to obtain better phylogenetic placement within the Pseudoalteromonads. DNA was isolated from PS-MBA-5 cells using the Wizard DNA Purification kit (Promega Biosciences, San Luis Obispo, CA) and the 16S rRNA gene was amplified using 16S rRNA primers (8F, AGAGTTTGATCCTGGCTCAG; 1492R, ACGGCTACCTTGTTACGACTT) (Integrated DNA Technologies, Coralville, IA). PCR products were cleaned using the QIAquick PCR Purification Kit (Qiagen, Hilden, Germany) and submitted to the Smithsonian Institution Laboratories of Analytical Biology (Suitland, MD) for sequencing. Sequencing was performed with 8F and 1492R, as well as an internal primer (533–551, E. coli K12-MG1655; GGTAATACGGAGGGTGCGA), to increase coverage of the entire sequence. Based on comparison with NCBI/GenBank, PS-MBA-5 was identified as belonging to the genus Pseudoalteromonas and given the strain name Pseudoalteromonas sp. PS5. All sequences were submitted to GenBank under accession nos. KF733508–KF733524. See the electronic supplementary material for detailed PCR reaction profiles.
(b). Effects of Pseudoalteromonas sp. PS5 extracts on larval settlement
Effects of Pseudoalteromonas sp. PS5 crude organic extracts on larval settlement were tested with the brooding coral P. astreoides and the active compound from the crude extract was elucidated via bioassay-guided fractionation. The active compound was then tested in isolation over a range of concentrations with P. astreoides, Acropora palmata and Orbicella franski.
(i). Extract preparation and fractionation
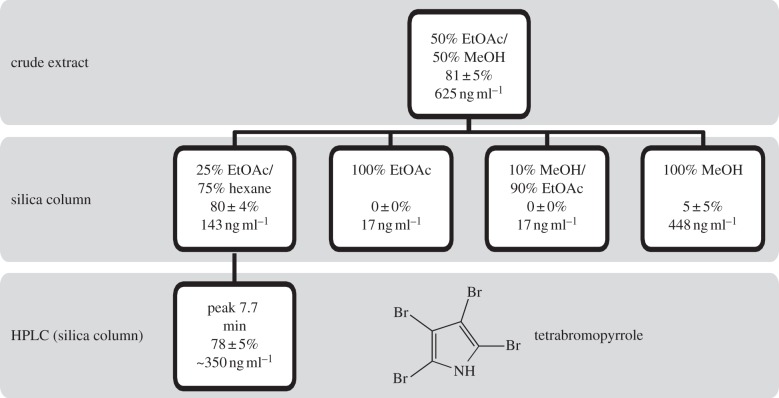
One hundred microlitres of 72 h Pseudoalteromonas sp. PS5 liquid culture was spread onto each of 20 MBA plates and grown for 48 h to create thick bacterial lawns. Bacteria were scraped from the surface of the agar using a sterile razor blade and lyophilized. Dried bacteria were extracted overnight in an excess of methanol/ethyl acetate (1 : 1). Solvents were removed by SpeedVac and extracts stored at −20°C. The crude extract was separated into four fractions by applying the extract to a silica column and eluting sequentially with 75 : 25 hexane/ethyl acetate, ethyl acetate, 90 : 10 ethyl acetate/methanol and methanol. The four fractions were tested in larval settlement assays and the active column fraction (75 : 25 hexane/ethyl acetate) was further separated using high-pressure liquid chromatography (HPLC). See the electronic supplementary material for detailed HPLC protocol. HPLC peaks were tested in larval settlement assays, and a single active compound was identified as TBP. TBP was repurified before every set of bioassays to ensure that the compound had not degraded. TBP remained stable for a month or more, as long as it was stored frozen.
(ii). Larval collection and handling
For experiments testing effects of Pseudoalteromonas sp. PS5 extracts on larval settlement, larvae were collected from adult P. astreoides colonies as described above during the new moon of May 2012 (crude extracts) and May 2013 (isolated TBP).
Acropora palmata gametes were collected approximately 50 m south of Carrie Bow Cay, Belize (16°48.18′ N, 88°04.93′ W) on the night of 28 July 2013 and larvae reared following the methods of Ritson-Williams et al. [6] (electronic supplementary material). Fertilized eggs developed into competent larvae within 5 days and were used in experiments on 2 August 2013.
Orbicella franksi colonies were collected on 23–25 August 2013 from Raph's Wall near Carrie Bow Cay, Belize (16°46.775′ N, 88°04.513′ W) and transported directly to Carrie Bow Cay. Corals were kept under the dock during the day and brought into the laboratory 30–60 min prior to sunset to monitor for spawning. In the laboratory, they were kept in buckets in the dark. Gamete bundles were collected using plastic transfer pipettes. Gamete bundles were allowed to break apart naturally, and sperm and eggs were separated by filtering gametes through 100 µm mesh. Eggs and sperm from different colonies were mixed and allowed to sit together for approximately 1 h to enable fertilization. Fertilized eggs were rinsed and transferred to 3 l mixing bowls in FSW. Water was kept stagnant and changed every 8–12 h for 4 days until larvae were competent.
(iii). Settlement assays
Settlement assays were performed in sterile, polystyrene six-well plates with 10 ml FSW. Extracts or pure TBP were dissolved in dimethyl sulfoxide (DMSO) and 10 µl was added to each assay well. In addition to the treatments being tested, each experiment included a solvent control that was prepared by adding 10 µl DMSO, an FSW control with nothing added and a positive control containing the CCA Hydrolithon boergesenii, which is known to induce settlement in a variety of coral species [6]. Immediately after treatments were added, 10 larvae were counted into each well in a minimal amount of seawater. Ages of the larvae post-release (P. astreoides) and post-fertilization (A. palmata and O. franksi) used in experiments are given in the electronic supplementary material, table S2.
Five replicate wells were used per treatment except for the test of crude extracts with P. astreoides, in which 10 replicate wells were used per treatment. Treatments were randomly assigned to wells within six-well plates. The number of larvae settled, the number unattached and metamorphosed, and the number of total surviving larvae were recorded after 6 and 24 h. For A. palmata larvae, six-well plates containing settled larvae were left under flowing seawater in the flow-through seawater system at Carrie Bow Cay and the number of juvenile corals surviving was counted after four weeks.
(iv). Structural elucidation of tetrabromopyrrole
NMR data were collected on an ECA-600 spectrometer (JEOL, Tokyo, Japan) operating at 600.17 MHz for 1H and 150.9 MHz for 13C. 1H NMR chemical shift was referenced to residual CHCl3 observed at δ 7.24 and 13C NMR chemical shifts were referenced to CDCl3 observed at δ 77.0. HRMS data were obtained using an Agilent Technologies (Santa Clara, CA) 6210 LC-TOF mass spectrometer equipped with an APCI/ESI multimode ion source detector at the Mass Spectrometer Facility at the University of California, Riverside, California.
(c). Statistical analyses
All experiments were analysed for both settlement (attachment and metamorphosis) and total metamorphosis (attached + unattached). Proportion data were arcsine square root transformed and analysed with either a t-test or one-way ANOVA using SigmaPlot. When data did not meet the assumption of normality they were analysed with a non-parametric equivalent (Mann–Whitney rank sum test or Kruskal–Wallis one-way ANOVA on ranks). Comparisons were made between treatments and controls following ANOVA analyses using Dunnett's test unless otherwise noted. Treatments were considered significantly different from controls when p < 0.05.
3. Results
Larvae responded to assay conditions in one of three ways. They attached to substrata and subsequently underwent metamorphosis (settlement), failed to attach but still underwent metamorphosis, or remained swimming larvae. The percentages of larvae that underwent complete settlement as well as those that underwent metamorphosis without first attaching are analysed as separate values here. Total percentage metamorphosis includes larvae that settled and those that metamorphosed without attaching. Limestone tiles that were allowed to develop a natural biofilm in the field for 15 days induced 10.3 ± 2.4% (mean ± s.e.) settlement and 2.5 ± 1.1% metamorphosed in the water without attaching. Older biofilms on tiles that were conditioned for 21 days on the reef induced over twice as much settlement (25.3 ± 3.9%) compared with the 15-day-old biofilms, with a similar number of larvae metamorphosing without attachment (3.4 ± 0.8%). Both settlement and total metamorphosis (attached + unattached) were significantly higher on older biofilms (figure 1; p = 0.012 and 0.003, respectively). Tiles that contained 21-day-old biofilms that were treated with antibiotics induced significantly less settlement (2.0 ± 1.5%) than tiles on which the biofilms remained untreated (18.0 ± 3.9%; figure 2; p < 0.001).
Figure 1.
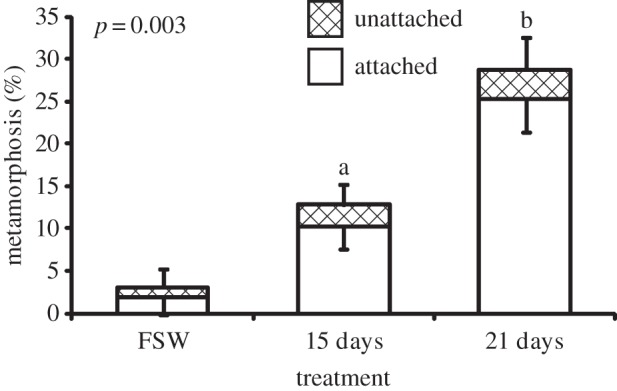
Response of P. astreoides larvae to 15- and 21-day-old biofilms on limestone tiles. FSW control contained only FSW and no tile, and was not included in statistical analyses. Bars represent mean percentage metamorphosis after 48 h including attached (white) and unattached (hatched) larvae (n = 8, 40 larvae per replicate). Error bars represent ±s.e. for total metamorphosis (upper) and attached (lower). Letters above bars represent significant differences (p = 0.012) in settlement (attached) between treatments according to a t-test; p-values given for total metamorphosis.
Figure 2.
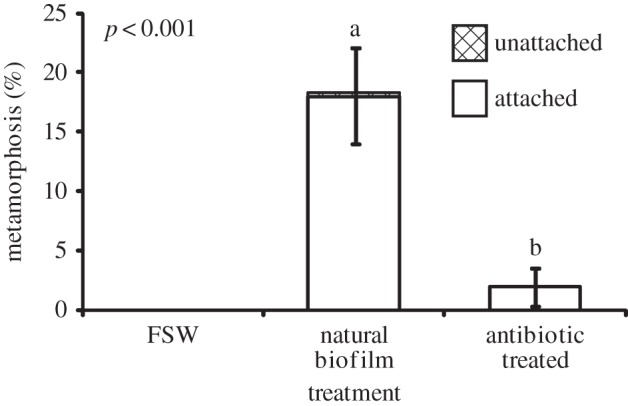
Response of P. astreoides larvae to natural 21-day-old biofilms and 21-day-old biofilms treated with antibiotics. FSW control contained only FSW and no tile, and was not included in statistical analyses. Bars represent mean percentage metamorphosis after 24 h including attached (white) and unattached (hatched) larvae (n = 8, 50 larvae per replicate). Error bars represent ±s.e. for total metamorphosis (upper) and attached (lower). Letters above bars represent significant differences (p < 0.001) in settlement (attached) between treatments according to a t-test; p-values given for total metamorphosis.
Out of 16 bacterial strains isolated from the surfaces of CCA, only one induced settlement of P. astreoides larvae (electronic supplementary material, table S1). Isolate PS-MBA-5 induced 31.0 ± 7.0% settlement and 52.0 ± 17.6% total metamorphosis, which was significantly higher than MB controls (2.0 ± 1.2%, p = 0.002 and 4.0 ± 1.9%, p = 0.022, respectively; figure 3). No other tested bacterial strain induced more than 1.0% settlement or total metamorphosis (data not shown). Isolate PS-MBA-5 was identified as belonging to the genus Pseudoalteromonas and was designated as Pseudoalteromonas sp. PS5 (figure 4). A BLAST search found 146 strains with more than 99% sequence similarity to Pseudoalteromonas sp. PS5. Of those that indicated isolation source (114), the majority were isolated from seawater (46%). Nine per cent were isolated from corals, 4% from CCA, 6% from other macroalgae and 6% from biofilms on abiotic surfaces (electronic supplementary material, table S3).
Figure 3.
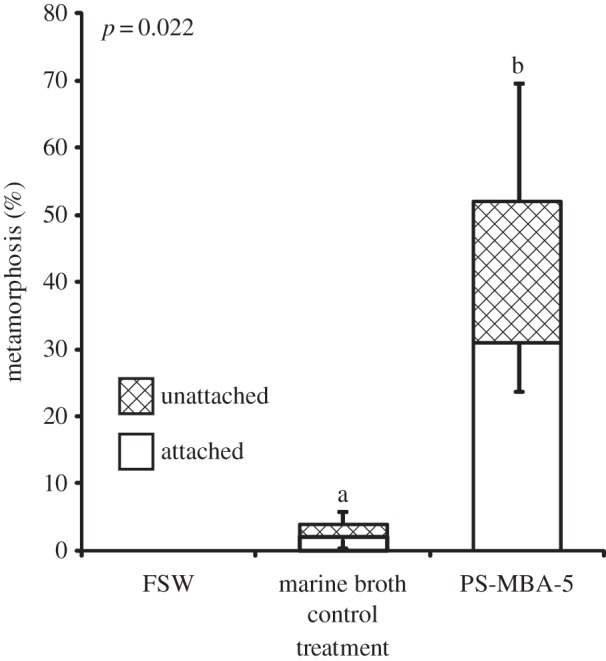
Response of P. astreoides larvae to monospecific biofilms of bacterial strain PS-MBA-5 (Pseudoalteromonas sp. PS5) compared with sterile MB media controls. FSW control contained only FSW and no tile, and was not included in statistical analyses. Bars represent mean percentage metamorphosis after 24 h including attached (white) and unattached (hatched) larvae (n = 5, 20 larvae per replicate). Error bars represent ±s.e. for total metamorphosis (upper) and attached (lower). Letters above bars represent significant differences (p < 0.002) in settlement (attached) between treatments according to a t-test; p-values given for total metamorphosis.
Figure 4.
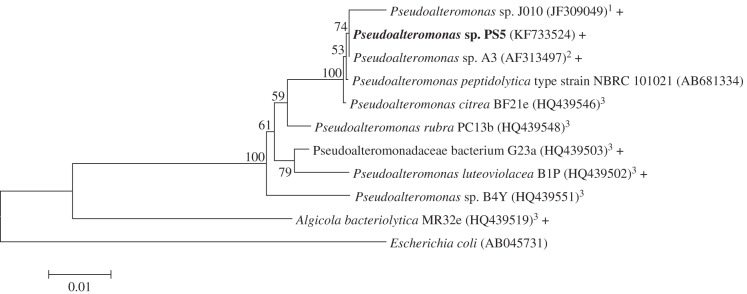
Phylogenetic relationship of Pseudoalteromonas strains that have been tested for their ability to induce coral larval settlement. Strains that have been shown to induce coral larval settlement are indicated by a plus symbol (+); Pseudoalteromonas sp. PS5 from this study is shown in bold. Tree is based on 1360 bp of the 16S rRNA gene using Minimum Evolution method (Jukes–Cantor model). Numbers beside nodes indicate the level of bootstrap support as a percentage out of 1000 replicates. Evolutionary analyses were conducted in MEGA5. Superscript numbers denote as follows: 1Tebben et al. [16], 2Negri et al. [15], 3Tran & Hadfield [14].
Crude organic extracts (625 ng ml−1) from cultures of Pseudoalteromonas sp. PS5 induced significantly higher settlement (69.0 ± 6.9%) in P. astreoides larvae compared with solvent controls (3.0 ± 3.0%) within 6 h (figure 5; p < 0.001). An additional 11.0 ± 4.1% of larvae metamorphosed unattached in response to Pseudoalteromonas sp. PS5 extracts. After 24 h, the number of settled larvae increased to 81.0 ± 4.6% in crude extract treatments, and the number of unattached metamorphosed larvae increased to 18.0 ± 4.7%. In the solvent control, there were no metamorphosed larvae after 24 h (figure 5; p < 0.05). At both time periods, Pseudoalteromonas sp. PS5 extracts elicited as much settlement as positive CCA controls (figure 5).
Figure 5.
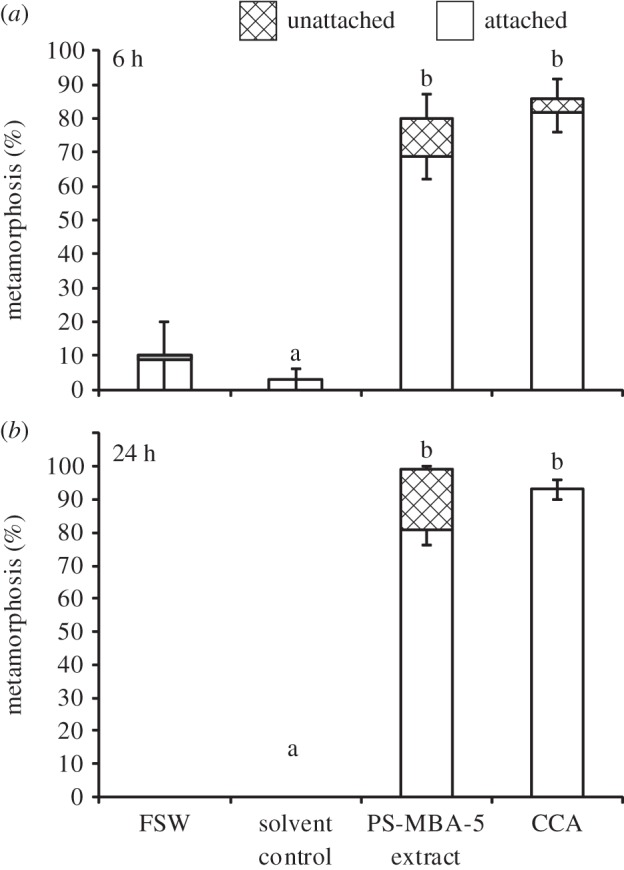
Response of P. astreoides larvae to crude extracts (625 ng ml−1) from bacterial strain PS-MBA-5 (Pseudoalteromonas sp. PS5) compared to solvent (DMSO) and Hydrolithon boergesenii (CCA) controls. FSW controls were not included in statistical analyses. Bars represent mean percentage metamorphosis after (a) 6 h and (b) 24 h including attached (white) and unattached (hatched) larvae (n = 10, 10 larvae per replicate). Error bars represent ±s.e. for total metamorphosis (upper) and attached (lower). There was a significant effect of treatment on settlement and total metamorphosis after both 6 and 24 h according to a one-way ANOVA or ANOVA on ranks (p < 0.001 for each). Letters above bars represent significant differences (p < 0.05) in settlement (attached) among treatments according to a Tukey test.
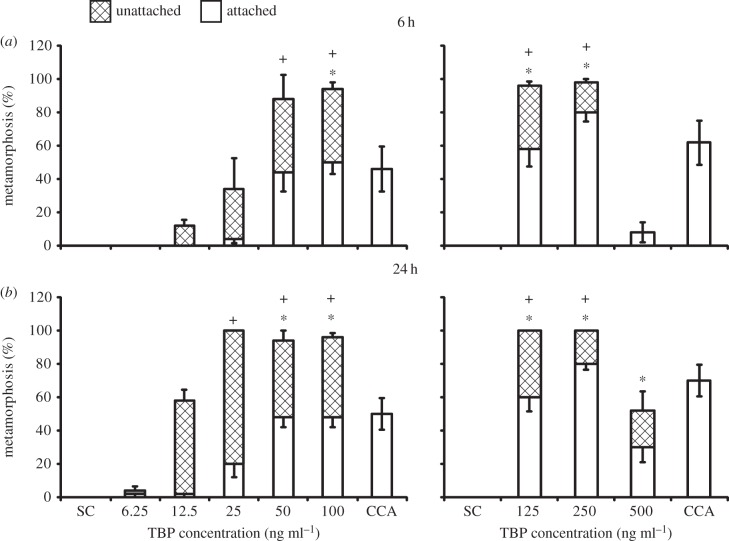
The active compound from the crude extract was isolated using bioassay-guided fractionation. Of four fractions separated using silica column chromatography, only the most non-polar induced a significant amount of settlement compared with solvent controls (figure 6). That fraction was subjected to HPLC separation and only one peak induced settlement (figure 6; p = 0.002). The active peak was identified as TBP based on high-resolution mass spectrometry ([M–H]− observed m/z = 381.6735, calculated for C479Br281Br2 N m/z = 381.6729) and 1H (δ 8.42) and 13C NMR (δ 102.4 and δ 99.8) [18]. The activity of TBP was concentration dependent. The amount of settlement increased with increasing TBP concentration and was significantly different from solvent controls in treatments subjected to 100 ng ml−1 or higher after 6 h and in those subjected to a minimum of 50 ng ml−1 after 24 h (figure 7; p < 0.05). Total metamorphosis, including those larvae that metamorphosed without attaching, was significantly increased at concentrations as low as 50 ng ml−1 after 6 h and 25 ng ml−1 after 24 h (figure 7). Activity of TBP peaked at a concentration of 250 ng ml−1, inducing 80.0 ± 5.5% settlement and 98.0 ± 2.0% total metamorphosis after only 6 h (figure 7). After 24 h, total metamorphosis had increased to 100% (figure 7). At higher concentrations (500 ng ml−1), the amount of settlement and total metamorphosis decreased. After 6 h in the 500 ng ml−1 treatment, there was an average of 8.0 ± 5.8% settled larvae, and no larvae unattached and metamorphosed (figure 7). This was not significantly different from the solvent control. After 24 h, the number of settled larvae had increased to 30.0 ± 8.9%, making it significantly different from the solvent control, which had no settlement (figure 7; p < 0.05).
Figure 6.
Bioassay-guided isolation of the settlement inducing compound TBP. Solvents used are shown in boxes with the percentage settlement (mean ± s.e.) of P. astreoides and extract concentrations below.
Figure 7.
Response of P. astreoides larvae to different concentrations of TBP after (a) 6 h and (b) 24 h. Hydrolithon boergesenii (CCA) controls were not included in statistical analyses. Bars represent mean percentage metamorphosis after 6 and 24 h including attached (white) and unattached (hatched) larvae (n = 5, 10 larvae per replicate). Error bars represent ±s.e. for total metamorphosis (upper) and attached (lower). There was a significant effect of treatment on settlement and total metamorphosis after both 6 and 24 h according to a one-way ANOVA or ANOVA on ranks (p < 0.001 for each). Symbols above bars represent significant differences (p < 0.05) in settlement (*) and total metamorphosis (+) from the solvent control (SC) according to Dunnett's test.
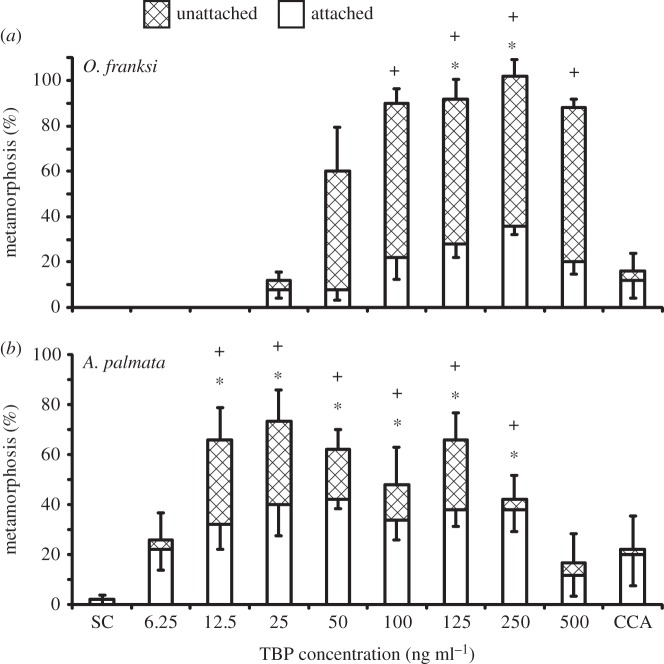
For both spawning species (A. palmata and O. franksi), there was little settlement in any of the treatments after 6 h (data not shown). However, both species showed increases in settlement and total metamorphosis in response to TBP after 24 h. The amount of total metamorphosis for O. franksi was significantly greater than solvent controls at concentrations of 100 ng ml−1 (90.0 ± 6.3%, p < 0.05) and higher (figure 8). Settlement was significantly induced at 125 ng ml−1 (28.0 ± 5.8%, p < 0.05) and 250 ng ml−1 (36.0 ± 4.0%, p < 0.05; figure 8). Total metamorphosis and settlement were significantly induced by TBP in A. palmata at concentrations between 12.5 and 250 ng ml−1 with settlement peaking at 42.0 ± 3.7% in treatments containing 50 ng ml−1 (p < 0.05; figure 8). Acropora palmata juveniles were counted again four weeks later and there was at least 50.0% survival in all TBP treatments except for the 500 ng ml−1 treatment, which had 33.3 ± 1.9% survival (electronic supplementary material, figure S1). There was no significant difference in survival among larvae treated with TBP and FSW controls (p = 0.569).
Figure 8.
Response of (a) O. franksi and (b) A. palmata larvae to different concentrations of TBP after 24 h. Hydrolithon boergesenii (CCA) controls were not included in statistical analyses. Bars represent mean percentage metamorphosis after 24 h including attached (white) and unattached (hatched) larvae (n = 5 except for 25 and 500 ng ml−1, n = 6; 10 larvae per replicate). Error bars represent ±s.e. for total metamorphosis (upper) and attached (lower). There was a significant effect of treatment on settlement and total metamorphosis according to a one-way ANOVA or ANOVA on ranks (p < 0.001 for each). Symbols above bars represent significant differences (p < 0.05) in settlement (*) and total metamorphosis (+) from the solvent control (SC) according to Dunnett's test.
4. Discussion
Microbial biofilms have been identified as positive settlement cues for many invertebrate larvae (reviewed in [7,19,20]), including several coral species [12–15]. For at least some corals, changes in the composition of the bacterial communities within biofilms coincide with differences in settlement rates, often resulting in higher settlement rates on more mature biofilms [13,21]. Similarly, we found that larvae from the Caribbean coral P. astreoides settled on naturally biofilmed tiles, and that tiles conditioned for 21 days induced more than twice as much settlement compared with tiles that were conditioned for 15 days (figure 1). Although we did not characterize the composition of the bacterial community within these biofilms, the successional colonization of surfaces in the marine environment has been demonstrated repeatedly and it can be assumed that biofilms of different ages probably represent different assemblages of organisms [7,22,23]. It is also possible that tiles conditioned for 21 days contained larger amounts of CCA compared with those conditioned for 15 days; however, we did not quantify the amount of CCA on tiles, because it appeared to be minimal. Larval settlement was nearly eliminated when 21-day-old biofilms were treated with a strong antibiotic cocktail, further supporting the hypothesis that bacteria are necessary for inducing the larval response to the biofilm (figure 2). The lack of settlement on tiles treated with antibiotics suggests that larvae respond to cues produced by active living bacteria and not simply to physical components of the biofilm matrix, which would remain intact under antibiotic treatment.
It has been suggested that larvae have evolved to respond to mature biofilms, because biofilms represent a surface that has been consistently submerged for long periods of time and is therefore an appropriate substratum for settlement [19]. Our results suggest that the basis for this larval behaviour may be the presence of specific bacterial strains, or combinations of strains, that are directly beneficial to larval health and post-settlement survival. Porites astreoides larvae selectively settled in response to monospecific biofilms of the strain Pseudoalteromonas sp. PS5 (figure 3). Larvae did not settle in response to any of the other 15 bacterial strains tested, demonstrating that they are capable of distinguishing among different strains. Bacterial strains in the genus Pseudoalteromonas have previously been identified as inducers of coral larval settlement and have also been recognized for their importance in the settlement of other invertebrate larvae [14–16,19,24]. The 16S rRNA gene sequence of Pseudoalteromonas sp. PS5 is highly similar (99%) to the 16S sequences of two strains (Pseudoalteromonas sp. A3 and Pseudoalteromonas sp. J010) isolated from the CCA Hydrolithon onkodes and Neogoniolithon fosliei (respectively) that have been reported to induce metamorphosis in Pacific corals A. millepora and A. willisae (figure 4) [15,16]. Although these strains induced metamorphosis, very few A. millepora and A. willisae larvae attached prior to metamorphosis in the absence of carbonate structure. By contrast, in our experiments with the Caribbean coral P. astreoides, 31.0 ± 7.0% of larvae underwent complete attachment and metamorphosis in response to monospecific biofilms of Pseudoalteromonas sp. PS5 (figure 3).
Not all Pseudoalteromonas strains are capable of inducing settlement. Of the 16 strains that we tested, three were Pseudoalteromonas spp., but only one induced settlement (electronic supplementary material, table S1). This is consistent with patterns seen by Tran & Hadfield [14], who found that Pseudoalteromonas strains with highly similar 16S rRNA sequences did not always elicit the same settlement responses (figure 4). Closely related Pseudoalteromonas strains have been shown to produce different suites of secondary metabolites, and this may explain why some strains induce settlement while others do not [25]. These results highlight that the presence of shared particular functions and/or functional genes is not implied by sequence similarity in phylogenetic marker genes, such as 16S rRNA.
Crude organic extracts of Pseudoalteromonas sp. PS5 elicited the same settlement response as monospecific biofilms, demonstrating that this bacterium induces larval settlement through the production of active organic compounds. Bioassay-guided fractionation of the crude extract led to the isolation of the compound TBP. This compound induced P. astreoides settlement within 6 h of exposure and was most active in concentrations of 50–250 ng ml−1 (figure 7). TBP also induced settlement of two spawning corals A. palmata and Orbicella franksi after 24 h of exposure and within similar concentration ranges (figure 8). Four weeks after settlement, A. palmata larvae that were exposed to TBP appeared healthy, having similar levels of survival compared with controls, and exhibiting normal development, including colonial polyp development, zooxanthellae uptake and polyp extension. It should be noted that TBP is unstable in isolation [16,18], and therefore the actual active concentration may be slightly lower than that reported here. However, we isolated fresh compound before each set of assays and found consistent activity within the time periods that we used the compound.
TBP has been shown to induce metamorphosis in the Pacific coral A. millepora [16]. However, A. millepora larvae failed to attach when exposed to TBP and therefore did not complete the settlement process in the presence of this compound. The difference in the response of A. millepora and the three Caribbean species (P. astreoides, A. palmata and O. franksi) to this compound may be related to the concentrations of the compound tested. The range of concentrations at which TBP induced full settlement in P. astreoides and O. franksi was just under one order of magnitude between the lowest and the highest active concentrations. At lower concentrations, larvae responded by undergoing metamorphosis without attaching, similar to the results shown for A. millepora. The lack of attachment in A. millepora could also be a result of inherent differences among the coral species; however, the Caribbean species tested represent individuals from both brooding and spawning corals, and from three different genera. The universal induction of larval settlement among the Caribbean coral species tested suggests that the activity of this compound may be widespread. Further testing of this compound with other coral species across multiple geographical areas and over a range of concentrations is necessary to determine the diversity of coral species that can recognize this settlement cue.
Pseudoalteromonas spp. are well known for the production of bioactive metabolites, including antibacterial and antifouling compounds [24,26]. Coral larvae may have evolved to detect specific bacteria such as Pseudoalteromonas sp. PS5 because they eliminate potential pathogens and/or reduce the settlement of competitors (i.e. macroalgae). Strains of Pseudoalteromonas are commonly found associated with corals at all life-history stages, and have demonstrated antibiotic activity against the coral pathogens Serratia marcescens, Vibrio shiloi, V. corallilyticus and Thalassomonas loyana [27–30]. In addition to signalling the presence of potentially beneficial Pseudoalteromonas strains, the compound TBP exhibits strong antibiotic activity against both human pathogens and marine bacteria, and may alter the bacterial flora within marine biofilms, making them hospitable to corals during their vulnerable early life-history stages [18].
This study adds further evidence that bacteria produce cues for coral larval settlement. The ability of corals to discriminate among different bacterial strains probably provides a mechanism by which corals select suitable settlement substrata. The independent isolation of closely related bacterial strains from the Pacific and the Caribbean that produce the same chemical compound and impact coral larval behaviour indicates that these bacteria may play an important role in coral recruitment worldwide. A better understanding of the distribution of these bacteria and/or the compound TBP in natural environments is necessary to determine how influential these bacteria are on coral recruitment processes on a global scale.
Acknowledgements
We thank R. Ritson-Williams for assistance with coral and CCA collection and identification, and S. Box for advice on statistical analyses. We also thank S. Gunasekera for structure elucidation of TBP. N. Fogarty, M. Jones, Z. Foltz, K. Olsen, A. Wood, M. Henley, H. Noren and C. Ross assisted with larval collection and maintenance. J. Houk, H. Granger, L. Spiers and E. Wile assisted with experiments.
Belize Fisheries Department provided permits to conduct research at Carrie Bow Cay, Belize. In the Florida Keys, coral larvae and CCA were collected under permit nos. FKNMS-2010-023, FKNMS-2010-080 and FKNMS-2013-021.
Data accessibility
DNA sequences are available at GenBank (accession nos. KF733508–KF733524).
Funding statement
Funding was provided by Mote Protect Our Reef Grant program (POR-2010-29, POR-2011-21 and POR-2012-3), the Dart Foundation and Smithsonian Competitive Grants Program for Science. This is contribution no. 950 of the Smithsonian Marine Station at Fort Pierce and no. 958 of the CCRE program.
References
- 1.Mumby PJ, Steneck RS. 2008. Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol. Evol. 23, 555–563. ( 10.1016/j.tree.2008.06.011) [DOI] [PubMed] [Google Scholar]
- 2.Gardner TA, Cote IM, Gill JA, Grant A, Watkinson AR. 2003. Long-term region-wide declines in Caribbean corals. Science 301, 958–960. ( 10.1126/science.1086050) [DOI] [PubMed] [Google Scholar]
- 3.Baums IB, Devlin-Durante MK, Polato NR, Xu D, Giri S, Altman NS, Ruiz D, Parkinson JE, Boulay JN. 2013. Genotypic variation influences reproductive success and thermal stress tolerance in the reef building coral, Acropora palmata. Coral Reefs 32, 703–717. ( 10.1007/s00338-013-1012-6) [DOI] [Google Scholar]
- 4.Ritson-Williams R, Arnold SN, Fogarty N, Steneck RS, Vermeij MJ, Paul VJ. 2009. New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smith. Contrib. Mar. Sci. 38, 437–457. ( 10.5479/si.01960768.38.437) [DOI] [Google Scholar]
- 5.Harrington L, Fabricius K, De'ath G, Negri A. 2004. Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 85, 3428–3437. ( 10.1890/04-0298) [DOI] [Google Scholar]
- 6.Ritson-Williams R, Paul VJ, Arnold SN, Steneck RS. 2010. Larval settlement preferences and post-settlement survival of the threatened Caribbean corals Acropora palmata and A. cervicornis. Coral Reefs 29, 71–81. ( 10.1007/s00338-009-0555-z) [DOI] [Google Scholar]
- 7.Hadfield MG, Paul VJ. 2001. Natural chemical cues for settlement and metamorphosis of marine-invertebrate larvae. In Marine chemical ecology (eds McClintock J, Baker B.), pp. 431–462. Boca Raton, FL: CRC Press. [Google Scholar]
- 8.Rodriguez SR, Ojeda FP, Inestrosa NC. 1993. Settlement of benthic marine-invertebrates. Mar. Ecol. Progr. Ser. 97, 193–207. ( 10.3354/Meps097193) [DOI] [Google Scholar]
- 9.Gleason DF, Hofmann DK. 2011. Coral larvae: From gametes to recruits. J. Exp. Mar. Biol. Ecol. 408, 42–57. ( 10.1016/j.jembe.2011.07.025) [DOI] [Google Scholar]
- 10.Morse DE, Hooker N, Morse ANC, Jensen RA. 1988. Control of larval metamorphosis and recruitment in sympatric agariciid corals. J. Exp. Mar. Biol. Ecol. 116, 193–217. ( 10.1016/0022-0981(88)90027-5) [DOI] [Google Scholar]
- 11.Heyward AJ, Negri AP. 1999. Natural inducers for coral larval metamorphosis. Coral Reefs 18, 273–279. ( 10.1007/s003380050193) [DOI] [Google Scholar]
- 12.Golbuu Y, Richmond RH. 2007. Substratum preferences in planula larvae of two species of scleractinian corals, Goniastrea retiformis and Stylaraea punctata. Mar. Biol. 152, 639–644. ( 10.1007/s00227-007-0717-x) [DOI] [Google Scholar]
- 13.Webster NS, Smith LD, Heyward AJ, Watts JEM, Webb RI, Blackall LL, Negri AP. 2004. Metamorphosis of a scleractinian coral in response to microbial biofilms. Appl. Environ. Microbiol. 70, 1213–1221. ( 10.1128/Aem.70.2.1213-1221.2004) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tran C, Hadfield MG. 2011. Larvae of Pocillopora damicornis (Anthozoa) settle and metamorphose in response to surface-biofilm bacteria. Mar. Ecol. Progr. Series 433, 85–96. ( 10.3354/Meps09192) [DOI] [Google Scholar]
- 15.Negri AP, Webster NS, Hill RT, Heyward AJ. 2001. Metamorphosis of broadcast spawning corals in response to bacteria isolated from crustose algae. Mar. Ecol. Progr. Ser. 223, 121–131. ( 10.3354/meps223121) [DOI] [Google Scholar]
- 16.Tebben J, Tapiolas DM, Motti CA, Abrego D, Negri AP, Blackall LL, Steinberg PD, Harder T. 2011. Induction of larval metamorphosis of the coral Acropora millepora by tetrabromopyrrole isolated from a Pseudoalteromonas bacterium. PLoS ONE 6, e19082 ( 10.1371/journal.pone.0019082) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kuffner I, Walters L, Becerro MA, Paul VJ, Ritson-Williams R, Beach K. 2006. Inhibition of coral recruitment by macrolagae and cyanobacteria. Mar. Ecol. Progr. Ser. 323, 107–117. ( 10.3354/meps323107) [DOI] [Google Scholar]
- 18.Andersen RJ, Wolfe MS, Faulkner DJ. 1974. Autotoxic antibiotic production by a marine chromobacterium. Mar. Biol. 27, 281–285. ( 10.1007/Bf00394363) [DOI] [Google Scholar]
- 19.Hadfield MG. 2011. Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Annu. Rev. Mar. Sci. 3, 453–470. ( 10.1146/annurev-marine-120709-142753) [DOI] [PubMed] [Google Scholar]
- 20.Pawlik JR. 1992. Chemical ecology of the settlement of benthic marine invertebrates. Oceanogr. Mar. Biol. Annu. Rev. 30, 273–335. [Google Scholar]
- 21.Webster NS, Soo R, Cobb R, Negri AP. 2011. Elevated seawater temperature causes a microbial shift on crustose coralline algae with implications for the recruitment of coral larvae. Isme J. 5, 759–770. ( 10.1038/ismej.2010.152) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wahl M. 1989. Marine Epibiosis .1. Fouling and antifouling—some basic aspects. Mar. Ecol. Progr. Ser. 58, 175–189. ( 10.3354/Meps058175) [DOI] [Google Scholar]
- 23.Whalan S, Webster NS. 2014. Sponge larval settlement cues: the role of microbial biofilms in a warming ocean. Sci. Rep. 4, 4072 ( 10.1038/srep04072) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bowman JP. 2007. Bioactive compound synthetic capacity and ecological significance of marine bacterial genus Pseudoalteromonas. Mar. Drugs 5, 220–241. ( 10.3390/Md504220) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Vynne NG, Mansson M, Gram L. 2012. Gene sequence based clustering assists in dereplication of Pseudoalteromonas luteoviolacea strains with identical inhibitory activity and antibiotic production. Mar. Drugs 10, 1729–1740. ( 10.3390/Md10081729) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holmstrom C, Egan S, Franks A, McCloy S, Kjelleberg S. 2002. Antifouling activities expressed by marine surface associated Pseudoalteromonas species. Fems Microbiol. Ecol. 41, 47–58. ( 10.1111/J.1574-6941.2002.Tb00965.X) [DOI] [PubMed] [Google Scholar]
- 27.Sharp KH, Distel D, Paul VJ. 2012. Diversity and dynamics of bacterial communities in early life stages of the Caribbean coral Porites astreoides. Isme J. 6, 790–801. ( 10.1038/ismej.2011.144) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kvennefors ECE, Sampayo E, Kerr C, Vieira G, Roff G, Barnes AC. 2012. Regulation of bacterial communities through antimicrobial activity by the coral holobiont. Microb. Ecol. 63, 605–618. ( 10.1007/s00248-011-9946-0) [DOI] [PubMed] [Google Scholar]
- 29.Ritchie KB. 2006. Regulation of microbial populations by coral surface mucus and mucus-associated bacteria. Mar. Ecol. Progr. Ser. 322, 1–14. ( 10.3354/Meps322001) [DOI] [Google Scholar]
- 30.Nissimov J, Rosenberg E, Munn CB. 2009. Antimicrobial properties of resident coral mucus bacteria of Oculina patagonica. Fems Microbiol. Lett. 292, 210–215. ( 10.1111/j.1574-6968.2009.01490.x) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
DNA sequences are available at GenBank (accession nos. KF733508–KF733524).