Abstract
Currently, there is a strong trend towards increasing insecticide-based vector control coverage in malaria endemic countries. The ecological consequence of insecticide applications has been mainly studied regarding the selection of resistance mechanisms; however, little is known about their impact on vector competence in mosquitoes responsible for malaria transmission. As they have limited toxicity to mosquitoes owing to the selection of resistance mechanisms, insecticides may also interact with pathogens developing in mosquitoes. In this study, we explored the impact of insecticide exposure on Plasmodium falciparum development in insecticide-resistant colonies of Anopheles gambiae s.s., homozygous for the ace-1 G119S mutation (Acerkis) or the kdr L1014F mutation (Kdrkis). Exposure to bendiocarb insecticide reduced the prevalence and intensity of P. falciparum oocysts developing in the infected midgut of the Acerkis strain, whereas exposure to dichlorodiphenyltrichloroethane reduced only the prevalence of P. falciparum infection in the Kdrkis strain. Thus, insecticide resistance leads to a selective pressure of insecticides on Plasmodium parasites, providing, to our knowledge, the first evidence of genotype by environment interactions on vector competence in a natural Anopheles–Plasmodium combination. Insecticide applications would affect the transmission of malaria in spite of resistance and would reduce to some degree the impact of insecticide resistance on malaria control interventions.
Keywords: Anopheles gambiae, Plasmodium falciparum, insecticide exposure, insecticide resistance, vector competence, malaria transmission
1. Introduction
Malaria vector control measures have been scaling up in recent years in sub-Saharan Africa with two main interventions: insecticide treated bed-nets (ITN) and indoor residual spraying (IRS), both of which have been shown to be effective for reducing malaria prevalence in Africa [1,2]. However, insecticide resistance has been selected owing to the heavy use of these insecticides for agriculture and public health purposes [3,4] and is developing dramatically throughout Africa in the main malaria vector species, such as Anopheles gambiae [5]. Two major mechanisms of insecticide resistance have been selected in insect vectors: target site mutations and enhanced metabolic detoxification. The molecular basis of target site insensitivity has been characterized in many insect species (reviewed in [3]) and has demonstrated conserved resistant mutations across insect vectors. Non-synonymous mutations in the voltage gated sodium channel (named kdr, for knockdown resistance) [6,7] confer resistance to pyrethroids (PYR) and dichlorodiphenyltrichloroethane (DDT) insecticides, whereas non-synonymous mutations in the ace-1 genes (encoding the acetylcholinesterase) confer cross-resistance to carbamate and organophosphate insecticides (CX and OP, respectively) [8,9]. Metabolic resistance is the result of elevated levels of detoxifying enzymes such as cytochrome P450 monooxygenases, esterases or glutathione-S-transferases [3]. Nowadays, insecticide resistance is widespread and polyfactorial, and allows resistant mosquitoes to survive high doses of insecticides. Nevertheless, insecticide resistance genes may be associated with pleiotropic effects on vector life-history traits (reviewed in [10]), which potentially modify their capacity to transmit pathogens [11–13]. In the main malaria vector A. gambiae, insecticide resistance was recently shown to affect the vector competence for Plasmodium falciparum field isolates in the absence of insecticides: comparing the outcome of infection between mosquito strains sharing a common genetic background except for the resistant alleles (kdr and ace-1R) showed that insecticide resistance mutations increase the prevalence of infection [13].
A growing field of study suggests that environmental factors also influence vector competence and parasite transmission through direct effect on vector immunity or indirect effect on parasite traits (reviewed in [14]). In natural populations, environmental factors are highly variable in time and space and may influence vector–parasite interaction depending also on mosquito and parasite genotypes [15]. Previous studies showed that exposure of Culex pipiens larvae to insect growth regulators affect various traits of the infecting nematode Romanomermis iyengari, such as the body size, the infectivity and the parasite load [16], although no target of this pesticide class is identified. Similarly, it may be hypothesized that insecticides may impede malaria parasite development in the mosquito vector, though no molecular targets for insecticide are known in Plasmodium. Another non-exclusive hypothesis is that environmental factors, such as insecticides, may alter the expression of genetic traits governing vector competence in insecticide-resistant insect [17] and may interfere with the activation of the mosquito immune system and other fitness-related traits, leading to variation of parasite transmission. Consistently, pyrethroid insecticides are associated with immunotoxic effects at least in vertebrates and increased the susceptibility to Plasmodium infection in mice [18].
Increasing coverage of vector control measures will undoubtedly increase the probability of contact of resistant vectors with insecticides at different points of their lifespan. First, when mosquitoes seek to blood feed, they may be in contact with insecticides through ITN. After blood feeding, endophilic-resistant mosquitoes could rest in houses where insecticides might be present through IRS and/or insecticide-treated plastic sheeting. As resistant mosquitoes can ingest higher doses of insecticides owing to prolonged contact with treated materials [19], we hypothesized that the exposure to insecticides would affect vector–parasite interaction in the resistant mosquitoes and have an impact on their vector competence. Moreover, if female mosquitoes feed on gametocyte-infected people, then developing parasites could be in contact with insecticides in the vector at different points of their development.
Therefore, in this study, we investigated whether exposure to insecticides impact the development of P. falciparum using well-characterized insecticide-resistant strains of A. gambiae s.s. After absorbing insecticides by contact, mosquitoes were blood fed on gametocyte-infected blood, and the impact of insecticides on the prevalence and the intensity of P. falciparum infection were compared with non-exposed mosquitoes.
2. Material and methods
(a). Mosquito strains
Two laboratory reference strains of A. gambiae sensu stricto (called S molecular form before the recent classification of Coetzee et al. [20]) were used in this study. The two strains were resistant to two distinct classes of insecticide: the OP/CX-resistant strain, named Acerkis, and the PYR/DDT-resistant strain, named Kdrkis. Acerkis was obtained by introgression of the resistant ace-1 G119S allele originated from a resistant A. gambiae population collected in Bobo-Dioulasso, Burkina Faso in 2002 into the Kisumu genome [21]. The Kdrkis strain was obtained by introgression of the kdr-west allele (harbouring the L1014F mutation) originating from a pyrethroid-resistant population sampled in Kou Valley, Burkina Faso [6] into the Kisumu genome [13]. Resistant strains were obtained through at least 15 successive backcrosses with the Kisumu strain and selection with propoxur insecticide for the Acerkis strain and permethrin insecticide for Kdrkis, so that the three strains shared a common genetic background at the exception of the locus carrying the insecticide resistance genes [22]. Mosquitoes were kept under standard insectary conditions (27 ± 1°C, 70 ± 8% relative humidity and 12 L : 12 D photoperiod) in the same secure containment facility. Larvae were reared in the same condition at a fixed density in at least five trays (300 first-instar larvae in 700 ml of water per tray) and were fed with TetraminBaby in order to reduce variation in larval growth rate and mosquito size at emergence. After emergence, adults were fed ad libitum on a 5% glucose solution and maintained in 30 × 30 × 30 cm cages.
(b). Insecticide exposure
After emergence of adults from all rearing trays, 2 to 3 days old female mosquitoes of each insecticide-resistant strain were exposed to insecticides using the World Health Organization cylinder test [23]. The OP/CX-resistant strain Acerkis (homozygous for the ace-1 G119S mutation) was exposed for 1 h to 0.1% bendiocarb (CX) and the DDT/pyrethroid strain Kdrkis (homozygous for the kdr L1014F mutation) was exposed for 1 h to 4% DDT. These laboratory strains were used, because no mortality was observed after insecticide exposure even a few days later. A batch of females from each mosquito strain was also exposed to untreated papers to serve as control. After insecticide versus control exposure, female mosquitoes were transferred into insecticide-free cages with access to a 5% glucose solution for 6 h. Then, the glucose solution was removed from the cages, and females were starved for 12 h before blood feeding on P. falciparum gametocyte-infected blood (18 h post-insecticide exposure).
(c). Plasmodium falciparum experimental infection by direct membrane feeding assay
Direct membrane feeding assays were performed as previously described [13]. Briefly, P. falciparum gametocyte carriers were selected by examining thick blood smears from children aged between five and 11 from two villages in southwestern Burkina Faso (Dandé and Soumousso, located 60 km north and 40 km southeast of Bobo-Dioulasso, Burkina Faso, respectively). Children with a gametocyte density of more than 20 per µl of blood were selected, and a venous blood sample (8 ml) was taken. Blood serum was replaced with European naive AB serum to limit the potential effect of natural human transmission blocking immunity [24]. Membrane feeders were filled with 500 µl of reconstituted blood and maintained at 37°C by water jackets. Insecticide-exposed and non-exposed female mosquitoes of the two insecticide-resistant strains were starved for 12 h by removing the glucose solution and they were then allowed to feed simultaneously through a parafilm membrane for up to 30 min on infected blood distributed in two feeders for each condition (strain and insecticide exposure). This procedure was repeated six times, each feeding assay using a different gametocyte-infected blood. Unfed female mosquitoes were discarded and only fully fed mosquitoes of each strain were maintained in large cages (30 × 30 × 30 cm) under standard insectary conditions with a 5% sucrose solution.
(d). Oocyst counting
Blood-fed females were maintained for 7 days in the insectary. Then, midguts were dissected in 0.4% mercurochrome solution, and the infection intensity of each individual female was determined by counting oocysts under a light microscope.
(e). Blood meal size determination
In order to determine whether insecticide exposure would inhibit blood feeding, we compared the blood meal size of females that were exposed or not to insecticide prior to the blood meal. For each mosquito strain and insecticide treatment, 10 individual females from each of the six feeding assays were isolated in Drosophila tubes after blood feeding. Blood meal size was quantified retrospectively by measuring the hematin excreted from individual females as previously described [25]. Hematin excretion was dissolved in 1 ml of a 1% LiCO3 solution, and the absorbance of the resulting mixture was read at 387 nm. Blank was obtained with LiCO3 solution alone, and hematin content was estimated by comparing a standard curve made with porcine serum hematin (Sigma-Aldrich).
(f). Statistical analysis
To analyse the effect of insecticide exposure on parasite infection, the data consisted of two response variables: the status of infection, named infection: infected (1) or not (0) for each individual, and the number of parasites present in infected individuals, named intensity expressed by the number of oocysts per infected midgut. In the analyses, we used five explanatory variables: genotype (a two-level categorical variable: ace-1R, the OP/CX-resistant allele and kdr, the PYR/DDT-resistant allele); insecticide exposure (a two-level categorical variable: ‘exposed’ to insecticide, or ‘control’); donor (a categorical variable, each gametocyte carrier representing a level); gametocyte density of the blood donor (an ordinal variable ordered from the lowest to the highest, denoted ‘gam. density’); and feeder (a categorical variable, each feeder representing a distinct level). The values of the gametocyte density are clustered between 104 and 160 gametocytes µl−1 of blood with two extreme values of 48 and 280 gametocytes µl−1 of blood. Therefore, the gametocyte density was used as an ordinal variable rather than as a numerical one to avoid any bias in the analysis. The genotype variable was used instead of strain because they share a common genetic background and differ only by the presence/absence of the locus involved in insecticide resistance. We performed two types of analyses: one on the whole dataset and including the genotype variable with all interactions; a second analysis for each mosquito strain separately. The maximal models included the variables genotype (only to analyse all strains together), gam. density and exposure and their interactions as fixed effects. The donor and feeder variables were used as random variable to account for the nested data structure, i.e. the correlation between individuals from the same experimental infection (or blood donor) or from the same feeding batch. The prevalence at the oocyst stage was analysed on 1154 female mosquitoes among six feeding assays. For the analysis of the infection intensity, only individuals that developed at least one oocyst were included (n = 726 among six feeding assays).
Statistical analyses were performed with statistical analysis software (SAS Institute Inc., Cary, NC). For each analysis, the random structure was selected and compared with a generalized linear model with no random effect based on the lowest Akaike information criterion (AIC). The prevalence at oocyst stage was analysed using the GLIMMIX procedure (SAS) with a binomial error structure, and the oocyst intensity was analysed using the GLIMMIX procedure implemented with a specified variance function to account for overdispersion [26,27]. For all analyses, the random structure with the donor alone gives the lowest AIC. This procedure performs a type III hypothesis for the fixed effect variables and computes the F-statistic based on Satterthwaite's approximation. The mean prevalence and intensity of infection among all feeding assays were computed and compared between mosquito strains taking into account for multiple testing using the Bonferroni procedure. Hematin content was analysed on 233 blood-fed females of both strains that were exposed or not to insecticides and compared using a Bonferroni-adjusted pairwise t-test.
3. Results
We first investigated whether exposure to insecticides has affected blood feeding. To do this, we measured and compared hematin content of blood-fed females that were exposed to insecticides or to a non-impregnated paper (n = 233). No differences were found between females that were exposed or not to insecticides in both strains (Bonferroni-adjusted pairwise t-test, p = 0.99 and p = 1 for Acerkis and Kdrkis, respectively).
We then analysed the effect of the genotype, insecticide exposure, gametocyte density and all interactions on the prevalence of infection on 1154 blood-fed females of both strains from six feeding assays (table 1) either together or separately. When analysing both strains together, insecticide exposure and gametocyte density had a significant effect on the prevalence of infection (F1,1141 = 53.51, p < 0.001 and F5,1141 = 39.21, p < 0.001, respectively; table 1a). The genotype variable is also significant (F1,1141 = 6.76, p = 0.0094) showing that the prevalence in Kdrkis (73.37% ± 2.99) was greater than in Acerkis (67.27% ± 3.22), which is consistent with previous observations [13]. The significant genotype by gametocyte density interaction (F5,1141 = 4.77, p = 0.0003) indicated that the effect of the gametocyte density on the prevalence of infection is different between strains. When analysing the prevalence of infection on Acerkis individuals only (n = 612), the minimal model showed that the insecticide exposure and gametocyte density have a significant influence (F1,605 = 33.90, p < 0.001 and F1,605 = 25.73, p < 0.001, respectively; table 1b). When considering Kdrkis individuals only (n = 542), similar results was obtained: insecticide exposure and gametocyte density influencing significantly the prevalence of infection (F1,535 = 20.16, p < 0.001 and F5,535 = 16.72, p < 0.001, respectively; table 1b). Overall, the prevalence of infection was lower in mosquitoes that were exposed to insecticide compared with the non-exposed counterparts: bendiocarb caused a 26% prevalence reduction in Acerkis (52.99% ± 4.25 in insecticide-exposed compared with 79.08% ± 3.01 in control, Bonferroni-adjusted p < 0.001) and DDT caused a 19% prevalence reduction in Kdrkis (62.40% ± 4.13 in insecticide exposed compared with 81.85% ± 2.93 in control, Bonferroni-adjusted p < 0.001; figure 1).
Table 1.
Statistical analyses of the prevalence of oocysts. Significance of variables obtained after selection of the minimal mixed effect model is presented for the analysis of the whole dataset (a) or for the analyses of each strain separately (b). (geno. genotype; gam. density, gametocyte density; dfn and dfs are the degree of freedom of the numerator and the denominator, respectively.)
| source | dfn | dfs | F | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| (a) | ||||||||
| genotype | 1 | 1141 | 6.76 | 0.0094 | ||||
| insecticide exposure | 1 | 1141 | 53.51 | p < 0.001 | ||||
| gametocyte density | 5 | 1141 | 39.21 | p < 0.001 | ||||
| geno. × gam. density | 5 | 1141 | 4.77 | 0.0003 | ||||
| Acerkis |
Kdrkis |
|||||||
| source | dfn | dfs | F | p-value | dfn | dfs | F | p-value |
| (b) | ||||||||
| insecticide exposure | 1 | 605 | 33.90 | p < 0.001 | 1 | 535 | 20.16 | p < 0.001 |
| gametocyte density | 5 | 605 | 25.73 | p < 0.001 | 5 | 535 | 16.72 | p < 0.001 |
Figure 1.
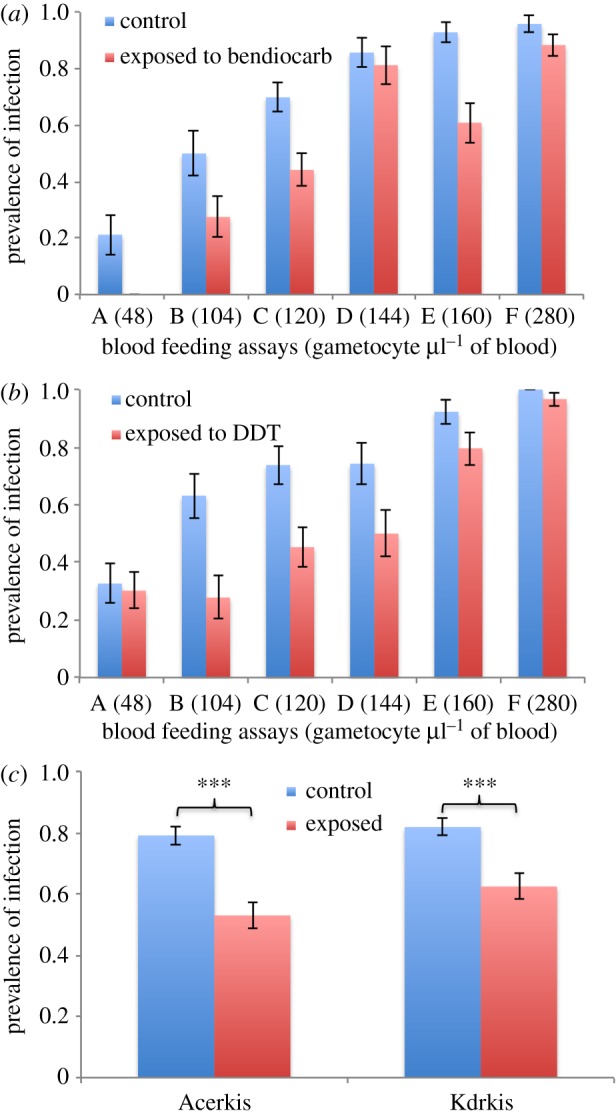
Prevalence of oocyst infection in insecticide exposed and unexposed An. gambiae strains. (a,b) Histograms presenting the prevalence of oocyst-infected females for each Acerkis and Kdrkis strain, respectively, and for each feeding assay. The gametocyte density for each blood donor (per µl of blood) is indicated in brackets. (c) presents the mean prevalence for each insecticide-resistant strain among all six feeding assays. Prevalence of infection in insecticide exposed and control mosquitoes are indicated in red (right hand) columns and blue (left hand) columns, respectively. Bars above and below the means represent the standard errors of the mean. Tests of significance were corrected for multiple testing using the Bonferroni procedure. Asterisks indicate the significance level: *p < 0.05; **p < 0.01; ***p < 0.001. (Online version in colour.)
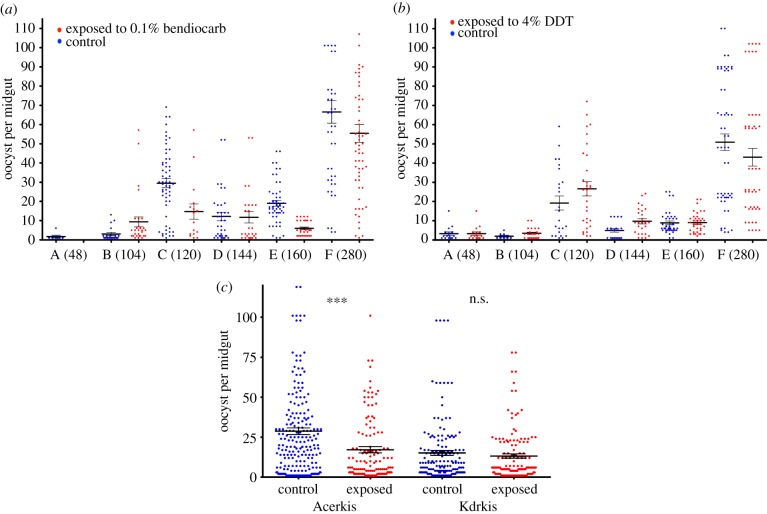
Among the 1154 females of both strains dissected for oocyst detection, 726 individuals carried at least one oocyst (nAcerkis = 378 and nKdrkis = 348) and were included in the analysis for infection intensity (table 2). The minimal model obtained on the whole dataset (table 2a) revealed that the effect of insecticide exposure had a significant effect (F1,712 = 6.3, p = 0.0123) as well as the gametocyte density (F5,712 = 99.87, p < 0.001) and the genotype (F1,712 = 5.21, p = 0.0228). The significant interaction of insecticide exposure by gametocyte density (F5,712 = 3.52, p = 0.00389) indicated that the effect of insecticides depends on the gametocyte density. In addition, the significant genotype by insecticide exposure interaction (F1,712 = 4.88, p = 0.0274) indicated that the effect of insecticide exposure on infection intensity is different in the two insecticide-resistant strains. When analysing the intensity of infection on Acerkis individuals only, insecticide exposure, gametocyte density and the interaction of both had a significant effect on the intensity of infection (F1,367 = 10.03, p = 0.0017, F5,367 = 54.69, p < 0.001 and F4,367 = 3.83, p = 0.0046, respectively; table 2b). By contrast, analysing Kdrkis individuals separately revealed a significant effect of gametocyte density only (F5,342 = 48.66, p < 0.001; table 2b). Comparing the computed means revealed that insecticide exposure decreased significantly the number of oocysts in the Acerkis strain (mean of 15.82 ± 2.38 oocysts per midgut in mosquitoes exposed to bendiocarb compared with 23.94 ± 3.49 in control, Bonferroni-adjusted p < 0.001), but not in Kdrkis (18.95 ± 2.82 oocysts per midgut in mosquitoes exposed to DDT compared with 17.09 ± 2.58 in control, Bonferroni-adjusted p = 0.20; figure 2).
Table 2.
Statistical analyses of the intensity of oocyst infection. Significance of variables obtained after selection of the minimal mixed effect model is presented for the whole dataset (a) or for each strain separately (b). (expo., insecticide exposure; gam. density, gametocyte density; dfn and dfs are the degree of freedom of the numerator and the denominator, respectively.)
| source | dfn | dfs | F | p-value | ||||
|---|---|---|---|---|---|---|---|---|
| (a) | ||||||||
| genotype | 1 | 712 | 5.21 | 0.0228 | ||||
| insecticide exposure | 1 | 712 | 6.3 | 0.0123 | ||||
| gametocyte density | 5 | 712 | 99.87 | p < 0.001 | ||||
| expo. × genotype | 1 | 712 | 4.88 | 0.0274 | ||||
| expo. × gam. density | 5 | 712 | 3.52 | 0.0038 | ||||
| Acerkis |
Kdrkis |
|||||||
| source | dfn | dfs | F | p-value | dfn | dfs | F | p-value |
| (b) | ||||||||
| insecticide exposure | 1 | 367 | 10.03 | 0.0017 | — | — | — | — |
| gametocyte density | 5 | 367 | 54.69 | p < 0.001 | 5 | 342 | 48.66 | p < 0.001 |
| expo. × gam. density | 4 | 367 | 3.83 | 0.0046 | — | — | — | — |
Figure 2.
Mean number of oocysts per midgut in insecticide exposed and control An. gambiae strains. Number of oocysts per female midgut is presented as a scatter dot plot for each mosquito strain and for each feeding assay ((a) Acerkis; (b) Kdrkis); and for each mosquito strain among all six feeding assays (c). The gametocyte density for each blood donor (per µl of blood) is indicated in parentheses. Blue (left hand black) dots represent the infection intensity for individual mosquitoes that were not exposed to insecticides and red (right hand grey) dots for mosquitoes that were exposed for 1 h to insecticides. Bars above and below the means represent the standard errors of the mean. Tests of significance were corrected for multiple testing using the Bonferroni procedure. Asterisks indicate the significance level: *p < 0.05; **p < 0.01; ***p < 0.001. (Online version in colour.)
4. Discussion
We investigated the influence of mosquito exposure to insecticides on P. falciparum infection. To do this, we exposed two insecticide-resistant strains of A. gambiae s.s. (sharing a common genetic background) to distinct insecticides prior to providing them with a blood meal containing infective gametocytes. In both strains, insecticide exposure significantly reduced the prevalence of P. falciparum oocysts. The carbamate insecticide bendiocarb also reduced significantly the number of oocysts among infected Acerkis individuals. Prevalence and intensity of P. falciparum infection in mosquito vectors were previously shown to be differentially affected depending on the initial infection intensity [28]. When intensity decreased, prevalence is expected to decrease (which we observed with bendiocarb treatment) or not to change. However, in Kdrkis individuals exposed to DDT, prevalence decreased, whereas infection intensity was not affected. This suggests that the effect of insecticide exposure on parasite development depends on the mosquito individual as some cleared the infection and others were not affected. We previously showed that the kdr allele affects differentially the prevalence and intensity of infection, which is consistent with two distinct mechanisms regulating these two modalities as previously suggested [29]. All together, these results showed a negative impact of mosquito exposure to insecticides on parasite development in its vector. One assumption is that insecticides may act directly on P. falciparum resulting in parasite toxicity mediated through an unknown target. Another assumption is that insecticide exposure could have an indirect effect through induced modifications of mosquito physiology. Indeed, insecticides modulate the expression of several genes, particularly those related to xenobiotic detoxification and mitochondrial redox metabolism [30], and increased cytochromes P450 expression which are known to amplify the oxidative stress [31]. This may interfere with parasite development as oxidative stress induced by blood meal, and Plasmodium ingestion was shown to play critical role in controlling the infection [32,33]. Although our experimental design did not allow testing for oxidative stress induced by insecticide exposure; we may conjecture that insecticides prevent Plasmodium development through higher reactive oxygen species level and increased cytochrome P450 expression. The presence of xenobiotics may probably affect various steps of Plasmodium sporogony (i.e. oocsyt and sporozoites development) directly or indirectly and would deserve further attention for giving a full picture of the insecticide impact on Anopheles–Plasmodium interactions.
We recently showed that target-site mutations responsible for insecticide-resistant strains increase the probability of P. falciparum infection following ingestion of blood containing gametocytes in the absence of insecticide [13] suggesting an increased parasite transmission in resistant populations. By contrast, our present results suggest that a prolonged exposure to insecticides on resistant mosquitoes may protect mosquitoes from infection when they take an infectious blood meal in the following hours. Therefore, these results evidence a mosquito genotype by environment interaction on vector competence. Indeed, while insecticide-resistant mutations increase the vector competence of A. gambiae for P. falciparum, insecticide exposure has the opposite effect on mosquitoes carrying those mutations. This is, to our knowledge, the first evidence of a genotype by environment interaction on vector competence in a natural Anopheles–Plasmodium combination [14]. Therefore, insecticides may have a more complex impact on malaria epidemiology than merely to reduce malaria transmission through reduction of vector density. Thus, both insecticide applications and insecticide resistance will shape mosquito–parasite coevolutionary dynamics.
In conclusion, we provide evidence that insecticides impede parasite development in insecticide-resistant colonies of A. gambiae. Widespread concomitant insecticide resistance mechanisms and increasing insecticide application may thus influence malaria transmission and epidemiology. This highlights the need to better understand the relationships between insecticide resistance and vector competence in varying insecticide environments for predicting long-term consequences of vector control measures on the dynamic of malaria transmission. Further studies using natural vector–parasite combinations in their ecological context should help implementation and development of sustainable control strategies.
Acknowledgements
We thank all children and their parents for participating in this study as well as to the local authorities for their support. We are very grateful to the IRSS staff in Burkina Faso for technical assistance. We thank the Laboratoire Mixte International LAMIVECT, Bobo-Dioulasso, Burkina Faso for technical support.
Ethical approval was obtained from the Centre Muraz Institutional Ethics Committee under the ethical clearance number 003-2009/cE-cM. All human volunteers were enrolled after written informed consent from the participant and/or their legal guardians.
Funding statement
This study was supported by the European Community's Seventh Framework Programme (FP7/2007–2013) under grant agreements no. 242095 and no. 223736. H.A. was supported by a postdoctoral fellowship from IRD.
References
- 1.WHO. 2012. World Malaria report, pp. 23–30. Geneva, Switzerland: World Health Organization; See http://www.who.int/iris/bitstream/10665/78945/1/9789241564533_eng.pdf. [Google Scholar]
- 2.Kelly-Hope L, Ranson H, Hemingway J. 2008. Lessons from the past: managing insecticide resistance in malaria control and eradication programmes. Lancet Infect. Dis. 8, 387–389. ( 10.1016/S1473-3099(08)70045-8) [DOI] [PubMed] [Google Scholar]
- 3.Labbé P, Alout H, Djogbénou L, Weill M, Pasteur N. 2011. Evolution of resistance to insecticide in disease vectors. In Genetics and evolution of infectious diseases (ed. Tibayrenc M.), pp. 363–409. Amsterdam, The Netherlands: Elsevier. [Google Scholar]
- 4.Corbel V, N'Guessan R. 2013. Distribution, mechanisms, impact and management of insecticide resistance in malaria vectors: a pragmatic review. In Anopheles mosquitoes - new insights into malaria vectors (ed. Manguin S.), pp. 579–633. InTech; See: http://www.intechopen.com/books/anopheles-mosquitos-newinsights-into-malaria-vectors/distribution-mechanisms-impact-and-management-of-insecticide-resistance-in-malaria-vectors-a-pragmat. [Google Scholar]
- 5.Ranson H, N'Guessan R, Lines J, Moiroux N, Nkuni Z, Corbel V. 2011. Pyrethroid resistance in African anopheline mosquitoes: what are the implications for malaria control? Trends Parasitol. 27, 91–98. ( 10.1016/j.pt.2010.08.004) [DOI] [PubMed] [Google Scholar]
- 6.Martinez-Torres D, Chandre F, Williamson MS, Darriet F, Berge JB, Devonshire AL, Guillet P, Pasteur N, Pauron D. 1998. Molecular characterization of pyrethroid knockdown resistance (kdr) in the major malaria vector Anopheles gambiae s.s. Insect Mol. Biol. 7, 179–184. ( 10.1046/j.1365-2583.1998.72062.x) [DOI] [PubMed] [Google Scholar]
- 7.Ranson H, Jensen B, Vulule JM, Wang X, Hemingway J, Collins FH. 2000. Identification of a point mutation in the voltage-gated sodium channel gene of Kenyan Anopheles gambiae associated with resistance to DDT and pyrethroids. Insect Mol. Biol. 9, 491–497. ( 10.1046/j.1365-2583.2000.00209.x) [DOI] [PubMed] [Google Scholar]
- 8.Weill M, et al. 2003. Insecticide resistance in mosquito vectors. Nature 7, 7–8. [DOI] [PubMed] [Google Scholar]
- 9.Alout H, Berthomieu A, Hadjivassilis A, Weill M. 2007. A new amino-acid substitution in acetylcholinesterase 1 confers insecticide resistance to Culex pipiens mosquitoes from Cyprus. Insect Biochem. Mol. Biol. 37, 41–47. ( 10.1016/j.ibmb.2006.10.001) [DOI] [PubMed] [Google Scholar]
- 10.Raymond M, Berticat C, Weill M, Pasteur N, Chevillon C. 2001. Insecticide resistance in the mosquito Culex pipiens: what have we learned about adaptation? Genetica 112–113, 287–96. [PubMed] [Google Scholar]
- 11.McCarroll L, Hemingway J. 2002. Can insecticide resistance status affect parasite transmission in mosquitoes? Insect Biochem. Mol. Biol. 32, 1345–1351. ( 10.1016/S0965-1748(02)00097-8) [DOI] [PubMed] [Google Scholar]
- 12.Vezilier J, Nicot A, Gandon S, Rivero A. 2010. Insecticide resistance and malaria transmission: infection rate and oocyst burden in Culex pipiens mosquitoes infected with Plasmodium relictum. Malaria J. 9, 379 ( 10.1186/1475-2875-9-379) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Alout H, Ndam NT, Sandeu MM, Djégbe I, Chandre F, Dabiré RK, Djogbénou LS, Corbel V, Cohuet A. 2013. Insecticide resistance alleles affect vector competence of Anopheles gambiae s.s. for Plasmodium falciparum field isolates. PLoS ONE 8, e63849 ( 10.1371/journal.pone.0063849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lefèvre T, Vantaux A, Dabiré KR, Mouline K, Cohuet A. 2013. Non-genetic determinants of mosquito competence for malaria parasites. PLoS Pathog. 9, e1003365 ( 10.1371/journal.ppat.1003365) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lambrechts L, Halbert J, Durand P, Gouagna LC, Koella JC. 2005. Host genotype by parasite genotype interactions underlying the resistance of anopheline mosquitoes to Plasmodium falciparum. Insect Mol. Biol. 4, 3 ( 10.1186/1475-2875-4-3) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Suman DS, Brey CW, Wang Y, Sanad M, Shamseldean MSM, Gaugler R. 2013. Effects of insect growth regulators on the mosquito–parasitic nematode Romanomermis iyengari. Parasitol. Res. 112, 817–824. ( 10.1007/s00436-012-3201-6) [DOI] [PubMed] [Google Scholar]
- 17.Lambrechts L, Chavatte J-M, Snounou G, Koella JC. 2006. Environmental influence on the genetic basis of mosquito resistance to malaria parasites. Proc. R. Soc. B 273, 1501–1506. ( 10.1098/rspb.2006.3483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Suwanchaichinda C, Khamkong P, Worasuttayangkurn L, Satayavivad J. 2005. Deltamethrin exposure affects host resistance to Plasmodium infection in mice. Environ. Toxicol. Pharmacol. 20, 77–82. ( 10.1016/j.etap.2004.10.004) [DOI] [PubMed] [Google Scholar]
- 19.Chandre F, Darriet F, Duchon S, Finot L, Manguin S, Carnevale P, Guillet P. 2000. Modifications of pyrethroid effects associated with kdr mutation in Anopheles gambiae. Med. Vet. Entomol. 14, 81–88. ( 10.1046/j.1365-2915.2000.00212.x) [DOI] [PubMed] [Google Scholar]
- 20.Coetzee M, Hunt RH, Wilkerson R, Della Torre A, Coulibaly MB, Besansky NJ. 2013. Anopheles coluzzii and Anopheles amharicus, new members of the Anopheles gambiae complex. Zootaxa 3619, 1–29. ( 10.11646/zootaxa.3619.3.2) [DOI] [PubMed] [Google Scholar]
- 21.Djogbénou L, Weill M, Hougard JM, Raymond M, Akogbéto M, Chandré F. 2007. Characterization of insensitive acetylcholinesterase (ace-1R) in Anopheles gambiae (Diptera: Culicidae): resistance levels and dominance. J. Med. Entomol. 44, 805–810. ( 10.1603/0022-2585(2007)44[805:COIAAI]2.0.CO;2) [DOI] [PubMed] [Google Scholar]
- 22.Berticat C, Boquien G, Raymond M, Chevillon C. 2002. Insecticide resistance genes induce a mating competition cost in Culex pipiens mosquitoes. Genet. Res. 79, 41–47. ( 10.1017/S001667230100547X) [DOI] [PubMed] [Google Scholar]
- 23.WHO. 1998. Test procedures for insecticides resistance monitoring in malaria vectors, bio-efficacy and persistence of insecticides treated surfaces, pp. 14–20. Geneva, Switzerland: World Health Organization; See http://www.who.int/malaria/publications/atoz/who_cds_cpc_mal_98_12/en/index.html. [Google Scholar]
- 24.Gouagna LC, Bonnet S, Gounoue R, Verhave JP, Eling W, Sauerwein R, Boudin C. 2004. Stage-specific effects of host plasma factors on the early sporogony of autologous Plasmodium falciparum isolates within Anopheles gambiae. Trop. Med. Int. Health 9, 937–48. ( 10.1111/j.1365-3156.2004.01300.x) [DOI] [PubMed] [Google Scholar]
- 25.Briegel H. 1980. Determination of uric-acid and hematin in a single sample of excreta from blood-fed insects. Experientia 36, 1428 ( 10.1007/BF01960142) [DOI] [Google Scholar]
- 26.Sinden RE, et al. 2007. Progression of Plasmodium berghei through Anopheles stephensi is density-dependent. PLoS Pathog. 3, e195 ( 10.1371/journal.ppat.0030195) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Vaughan JA. 2007. Population dynamics of Plasmodium sporogony. Trends Parasitol. 23, 63–70. ( 10.1016/j.pt.2006.12.009) [DOI] [PubMed] [Google Scholar]
- 28.Harris C, Morlais I, Churcher TS, Awono-Ambene P, Gouagna LC, Dabiré RK, Fontenille D, Cohuet A. 2012. Plasmodium falciparum produce lower infection intensities in local versus foreign Anopheles gambiae populations. PLoS ONE 7, e30849 ( 10.1371/journal.pone.0030849) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harris C, Lambrechts L, Rousset F, Abate L, Nsango SE, Fontenille D, Morlais I, Cohuet A. 2010. Polymorphisms in Anopheles gambiae immune genes associated with natural resistance to Plasmodium falciparum. PLoS Pathog. 6, e1001112 ( 10.1371/journal.ppat.1001112) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vontas J, Blass C, Koutsos AC, David J-P, Kafatos FC, Louis C, Hemingway J, Christophides GK, Ranson H. 2005. Gene expression in insecticide resistant and susceptible Anopheles gambiae strains constitutively or after insecticide exposure. Insect Mol. Biol. 14, 509–521. ( 10.1111/j.1365-2583.2005.00582.x) [DOI] [PubMed] [Google Scholar]
- 31.Morgan ET. 1997. Regulation of cytochromes P450 during inflammation and infection. Drugs Metab. Rev. 29, 1129–1188. ( 10.3109/03602539709002246) [DOI] [PubMed] [Google Scholar]
- 32.Kumar S, Christophides GK, Cantera R, Charles B, Han YS, Meister S, Dimopoulos G, Kafatos FC, Barillas-Mury C. 2003. The role of reactive oxygen species on Plasmodium melanotic encapsulation in Anopheles gambiae. Proc. Natl. Acad. Sci. USA 100, 14 139–14 144. ( 10.1073/pnas.2036262100) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Molina-Cruz A, DeJong RJ, Charles B, Gupta L, Kumar S, Jaramillo-Gutierrez G, Barillas-Mury C. 2008. Reactive oxygen species modulate Anopheles gambiae immunity against bacteria and Plasmodium. J. Biol. Chem. 283, 3217–3223. ( 10.1074/jbc.M705873200) [DOI] [PubMed] [Google Scholar]