Abstract
For organisms with complex life cycles, larval environments can modify adult phenotypes. For mosquitoes and other vectors, when physiological impacts of stressors acting on larvae carry over into the adult stage they may interact with infectious dose of a vector-borne pathogen, producing a range of phenotypes for vector potential. Investigation of impacts of a common source of stress, larval crowding and intraspecific competition, on adult vector interactions with pathogens may increase our understanding of the dynamics of pathogen transmission by mosquito vectors. Using Aedes aegypti and the nematode parasite Brugia pahangi, we demonstrate dose dependency of fitness effects of B. pahangi infection on the mosquito, as well as interactions between competitive stress among larvae and infectious dose for resulting adults that affect the physiological and functional ability of mosquitoes to act as vectors. Contrary to results from studies on mosquito–arbovirus interactions, our results suggest that adults from crowded larvae may limit infection better than do adults from uncrowded controls, and that mosquitoes from high-quality larval environments are more physiologically and functionally capable vectors of B. pahangi. Our results provide another example of how the larval environment can have profound effects on vector potential of resulting adults.
Keywords: vector–parasite interaction, mosquito, filarial infection, life history, infectious dose, intraspecific competition
1. Introduction
Parasites by definition exploit and reduce the fitness of their hosts. However, host damage is counterbalanced by the requirement that the host survives and remains healthy enough for the parasite to complete its life cycle [1]. In arthropod vector–parasite associations, parasites are highly dependent on the survival [2] and mobility [3] of the vector. Parasites are ingested during a blood meal, after which some portion of their development, growth or replication takes place within the vector; the vector must then transmit the parasite to a new vertebrate host during a blood meal. Effective transmission thus requires not only that the vector survives the infection, but also that it remains capable of locating and feeding on a vertebrate host. The developmental period of a parasite is often long relative to the vector lifespan [4]; hence, selection is predicted to favour parasites that limit vector mortality and impairment of mobility [2,3]. Despite this prediction, there is often considerable variation within vector populations in physiological and fitness responses to parasitism [4]. This variation probably results from interactions between genetic and environmental factors, particularly those occurring during larval stages [5,6].
For mosquito vectors of pathogens, the role of environmental heterogeneity in altering life history and vector potential has received considerable attention, particularly with respect to biotic and abiotic factors acting on larvae [7–11]. For many mosquitoes, environmental and ecological stressors (e.g. competition, malnourishment and predation threat) are common in larval stages and produce lifelong impacts on physiological and life-history phenotypes of resulting adults [7]. For example, larval crowding is common for mosquitoes and causes competition for limited food which negatively impacts multiple fitness components, prolonging juvenile development and producing smaller adults with decreased longevity and reproductive success [12,13]. These smaller adults tend to take more frequent, smaller blood meals, which has potential to impact both vector and pathogen fitness, as well as population-level vector traits [14]. Larval crowding and food shortage also impact immune function [15,16] and susceptibility to arboviruses in resulting adults, with a greater proportion of stressed mosquitoes becoming competent vectors [10,11]. One hypothesis for the effects of larval stressors on adult physiology is life-history trade-offs, or patterns of energy allocation that favour priority functions (e.g. survival, growth) at the cost of other fitness traits (e.g. defence against parasites) [17]. Larval environments are typically heterogeneous, varying greatly in abiotic and biotic conditions [7]; the result is that trade-off relationships may vary greatly among individuals in a population depending on exposure to stressors across multiple, discrete aquatic habitats [10–13]. This is important for two reasons. First, although impacts of larval stress on adult life history are well studied, we lack a good understanding of the mechanistic basis of those impacts, and of their effects on vector potential. Second, current models used to estimate disease risk typically exclude inter-individual variation in vector traits. For example, vectorial capacity models of population-level pathogen transmission assume that infected and uninfected vectors have equal longevities, birth rates and death rates [18]. These simplifying assumptions are probably unrealistic because infection may impose fitness costs on the vector that impact those precise life-history traits, among others [18]. Models also frequently assume that all infected individuals are equally likely to transmit infection, ignoring individual-level heterogeneity in infectiousness, also known as vector competence [19]. Finally, models often ignore effects of infectious dose and pathogen load on both mosquito and pathogen fitness, despite empirical evidence of such effects [20]. A greater understanding of environmental factors causing variation in vector competence, how parasitism alters vector life-history traits contributing to individual transmission potential, and how environment and parasitism interact is needed. In this paper, we describe experiments investigating the effects of larval rearing environment, parasite dose and their interaction on a model vector–pathogen system involving the mosquito Aedes aegypti and the nematode parasite Brugia pahangi, with the goal of increasing our general understanding of vector–parasite interactions and ultimately, transmission of vector-borne disease.
(a). Study system
Nematode parasites causing lymphatic filariasis of humans (Wuchereria bancrofti, Brugia malayi, Brugia timori) and other mammals (B. pahangi) are vectored exclusively by mosquitoes from multiple genera [21]. Sheathed microfilariae (mf) are ingested during a blood meal from a vertebrate host. Within hours, mf exsheath and perforate the mosquito midgut, escaping into the haemocoel [22]. mf migrate to flight muscle where they undergo two moults, each separated by 2–3 days. The terminal stage in the mosquito (L3) typically occurs 8–9 days post-infection (DPI). L3 worms migrate to the head and proboscis where they may escape during future blood meals, infecting a new host [22]. Worms may remain in the head for the life of the mosquito [23], creating multiple opportunities for transmission. L3 worms are large (approx. 2 mm) relative to the mosquito body (approx. 6–12 mm), hence damage to the mosquito from feeding, movement and induction of immunity is unavoidable [22]. Not surprisingly, infection carries fitness costs on the mosquito, including reduced fecundity, post-infection survival and longevity [24,25]. Fecundity reduction may result from direct competition between vector and parasite for endogenous resources [25], or from diversion of vector resources to immune responses owing to shared components of biochemical pathways (e.g. l-tyrosine, which limits both egg chorion tanning and parasite encapsulation and melanization) [26]. Infection can also induce trade-offs between fecundity and longevity that may affect both vector fitness and the probability of parasite transmission [27]. The extent of trade-offs involving reproduction may also depend on infectious dose. For example, egg production can be inversely proportional to parasite biomass in the A. aegypti/B. pahangi system [24]. For these reasons, investigations of the relationships between parasite dose, survival, reproductive investment and longevity are needed. Infection, and specifically B. pahangi developing in mosquito flight musculature may also reduce flight capability [22,28]. Flight is essential for mosquito fitness, blood meal seeking and parasite transmission [28]; it is thus an important behavioural component of vector potential that should be included in investigations of parasite-induced fitness costs.
Although definitions vary, vector competence is generally described as the ability of a vector to become infectious and subsequently transmit a pathogen [29]. Vector competence is usually inferred by the pathogen reaching its infectious stage in the mosquito tissues associated with transmission (e.g. salivary glands) [29]. This description ignores the possibility that infection may alter mosquito behaviour (e.g. flight capability, host seeking, blood feeding) [28,30] or life history (e.g. post-infection survival) [29] in ways that increase, decrease or eliminate transmission potential [18]. Our experiments focus on effects of the larval environment and infectious dose on physiological and ‘functional’ vector competence. We define functional vector competence as the set of traits contributing to the ability of an individual mosquito to transmit the pathogen effectively given that it has attained physiological competence (e.g. flight capability, host localization, feeding frequency and success). We propose that because vector–pathogen interactions are dynamic and occur in heterogeneous environments, exogenous factors interact to produce a range of phenotypes affecting both aspects of vector competence. In this paper, we first test for dose-dependent impacts of filarial infection on survival and fecundity of mosquitoes from well-fed, homogeneous larval environments. Next, we manipulate the larval environment to test whether larval crowding and infectious dose interact in their effects on likelihood of pathogen transmission by influencing both physiological and functional vector competence. We predict that: (i) for adults reared as larvae in well-fed, homogeneous environments, filarial infection negatively impacts survival and fecundity in a dose-dependent manner; (ii) larval crowding and associated food shortage produces adults that are more susceptible to infection, having higher physiological vector competence; and (iii) larval crowding and infectious dose impinge upon physiology in ways that reduce both fitness and functional vector competence, reducing the number of viable vectors.
2. Material and methods
(a). Experiment 1: effect of Brugia pahangi infectious dose on survival and fecundity in well-fed mosquitoes
(i). Larval rearing
Rearing protocol was adapted from well-fed groups (i.e. low density) in a previous study [10], with food level and number of larvae determined in preliminary trials to produce a sufficient number of adults in each replicate to accommodate planned assays. Oak leaf infusion was prepared by mixing 115 g dried (50°C, 24 h) live oak (Quercus virginiana) leaves with 3 l of reverse-osmosis-purified (RO) water, and housing the infusion for 10 days at 28 ± 1°C, 60% relative humidity, with 14 L : 10 D photoperiod. Larval rearing habitats (14.2 l clear plastic containers) were prepared by mixing 500 ml sieved (80 μm) oak infusion, 0.3 g yeast : lactalbumin (1 : 1; hereafter ‘artificial larval diet’) and 4 l RO water for a total volume of 4.5 l, then stored in an environmental chamber under conditions described above. Aedes aegypti (Liverpool) eggs were obtained from NIH Filariasis Research Reagent Resource Center (FR3) and were maintained as a colony by blood feeding from anaesthetized (10 : 1 ketamine : xylazine) guinea pigs (Cavia porcellus). Twenty-four hours after container preparation, eggs were hatched in nutrient broth (Difco, 0.4 g l−1 water). Approximately 24 h later, larvae were sieved and 700 first-instars were added to containers, along with 250 ml oak infusion and 0.15 g artificial larval diet. Larval habitats were supplied with ad libitum food for 10 days by adding 250 ml oak infusion every other day, and artificial larval diet (0.15 g) daily, except for 3 days of omission: once on days 2–3 post-larval addition (mean 2.25), once on days 5–6 post-larval addition (mean 5.5) and once on days 8–9 post-larval addition (mean 8.5). Between replicates, oak leaves used for infusion and larval feeding intensity varied, and excess bacteria can be detrimental to larvae. To ensure high survival of larvae and to maximize homogeneity among replicate containers, our methods allowed for omission of food addition over the specified 2 day periods when water clarity was low. Pupae were removed from containers daily and evenly distributed to 500 ml Erlenmeyer flasks representing one of three adult blood feeding treatments: control (uninfected), ‘low’ mf density and ‘high’ mf density. The flasks were covered with ultra-fine insect netting. On the day of adult eclosion, mosquitoes were aspirated into biosafety cages (plastic 4.7 l buckets topped with ultra-fine insect netting) by treatment and given continuous access to 20% sucrose solution. Group cages contained adults emerging over a 3 day period, to reduce differences in mosquito age at the time of blood feeding. Adults were housed in our BioSafety Level 2 containment room at standard insectary conditions (26 ± 1°C, 60% relative humidity, 14 L : 10 D photoperiod with 3 h dawn and dusk phases). Four replicates were carried out in succession (one larval container per replicate) and treated as experimental blocks in the analyses.
(ii). Brugia pahangi infection
Adults 10–12 days post-emergence were offered a blood meal using either B. pahangi-infected gerbils (Meriones unguiculatus; NIH FR3) or uninfected (control) gerbils. Mosquitoes were starved 24–36 h prior to feeding, and each adult cage was fed with only one gerbil. ‘High’ versus ‘low’ mf density were relative terms (i.e. per blood feeding event) based on gerbil blood smear data taken within six months. The objective of this design was to maximize the range of gerbil mf densities within a replicate for use as a continuous variable in analyses. Anaesthetized gerbils (10 : 1 ketamine : xylazine) were placed atop group cages for 30–40 min, and mosquitoes fed through the netting. A regular rotation was employed to provide ample recovery for animals between uses. Gerbil blood smears were obtained via femoral vein puncture (less than or equal to 36 h from feeding) and stained with Giemsa solution. mf were counted using a compound microscope (30×). Visibly engorged mosquitoes were aspirated into new group containers by treatment and age, offered ad libitum 20% sugar solution and housed in our containment room (standard insectary conditions; see above). Males (which do not blood feed) and unfed females were killed and discarded. Four replicates yielded a total of 23 adult cages of blood-fed mosquitoes: seven ‘control’ cages (one to two per replicate); eight ‘low mf’ cages (two per replicate) and eight ‘high mf’ cages (two per replicate).
(iii). Survivorship and fecundity measurements
Post-infection mortality was recorded from group cages at 0.5, 1, 2, 4, 6, 12, 24 and 48 h. Mosquitoes surviving to 48 h were transferred into individual screened polyethylene cups (height = 11.43 cm, diameter = 11.91 cm) with an oviposition cup (black polystyrene beaker lined with seed germination paper, holding approx. 40 ml of RO water) and offered constant 20% sugar solution. Mortality was recorded daily until 45 DPI, when remaining individuals were killed. On the day of death for each individual, the number of eggs in the oviposition cup was recorded, and the abdomen was dissected to count the number of retained mature eggs, which is common in investigations of mosquito fecundity (e.g. [31,32]) and avoids underestimates of reproductive investment arising from early death [32]. This is particularly important for A. aegypti because females tend to spread their eggs among multiple containers over several days [33]. Wings were removed and wing lengths measured using ImageJ software and a dissecting microscope at 40×. Wing length is commonly used as a measure of size, and as an indicator of the impact of the larval rearing environment on adult quality (e.g. [12]).
(b). Experiment 2: interactive effects of larval crowding and infectious dose on physiological and functional vector competence
(i). Larval rearing
Rearing methods were adapted from [10,11] with food level and numbers of larvae determined in preliminary trials to produce a sufficient number of surviving adult females for planned assays. Oak infusion was prepared as in experiment 1 and incubated for 7 days. Two larval habitat containers were then prepared by mixing 500 ml oak infusion, 0.2 g artificial larval diet and 4 l RO water. Both containers aged 3 days, after which A. aegypti (Liverpool) first-instar larvae were randomly assigned to one of the two larval treatments, ‘uncrowded’ (250 larvae) or ‘crowded’ (500 larvae). Two hundred and fifty millilitres of oak infusion and 0.15 g of artificial larval diet were added to each container with larvae. Artificial larval diet (0.15 g) was added to each container on the fifth day after larval addition, and every subsequent third day until completion of larval development. Pupae were removed from containers daily and transferred to 500 ml Erlenmeyer flasks by larval density treatment. Adults were transferred on the day of eclosion into cages grouped by 3 day cohorts (as above) and offered a constant supply of 20% sucrose solution. Seven replicates (one ‘crowded’ and one ‘uncrowded’ cohort of larvae per replicate) were reared in this way, in succession. Replicates were treated as experimental blocks in the analyses.
(ii). Brugia pahangi infection
Adults (4–6 days old) were starved for 24–36 h and then offered a blood meal using B. pahangi-infected gerbils (as above). Blood samples were collected at the time of feeding, and mf counted (see above). Visibly engorged mosquitoes were aspirated into new biosafety containers based on replicate, emergence date, blood feed date and gerbil, offered constant 20% sugar solution and housed in containment (standard insectary conditions; see above). Males and unfed females were killed and discarded. Seven replicates produced 37 adult blood-fed cages, 22 from ‘crowded’ larvae and 15 from ‘uncrowded’ larvae.
(iii). Survival, parasite load, parasite success and flight capability
Post-infection mortality was recorded at 0.5, 1, 2, 4, 8, 12 and 24 h, then daily until all females either died naturally or were killed for dissection. Subsets (1–12) of mosquitoes from each cage were randomly chosen for dissection at 8, 9, 10, 11 and 12 DPI. The number dissected each day was determined on day 8, by dividing the number of surviving females by the number of days in the dissection period (5). We developed a simple behavioural assay for mosquito mobility. Prior to dissection, each individual was aspirated into an empty biosafety cage topped with netting. After a 3 min acclimation period, the mosquito was touched on a rear leg with a metal spatula three times, at 30 s intervals. Flight scores were assigned as: 1 = no flight; 2 = mobile, but no sustained flight (less than 3 s); or 3 = sustained flight (more than 3 s). Mean scores for the three trials were analysed. Mosquitoes were then killed by aspiration into pure ethanol (more than or equal to 20 s) and placed on glass slide. Using micro-dissection tools, the head, thorax and abdomen were separated, and tissues were teased apart in a drop of sterile phosphate buffered saline. Worms were counted using a compound microscope at 30×. Wing lengths were obtained as described above.
(c). Statistical analysis
Survival analysis for both experiments used proportional hazards models (PROC PHREG, SAS v. 9.3), with gerbil mf density as a continuous variable in both cases. For ease of presentation, we show predicted survival curves for particular mf densities (3000, 2000, 1000, 300 and 30 per 20 μl blood, and 0 if applicable). Mosquitoes for which death could be interpreted as a laboratory artefact (e.g. stuck to tape; injured during aspiration) were censored observations. Conclusions of our analyses were not sensitive to our definition of censoring, hence we used this broad definition.
For experiment 1, we also analysed effects of the continuous variable ‘gerbil mf density’ on mosquito egg production (retained + laid) using generalized linear models (PROC GENMOD, SAS v. 9.3) with negative binomial error distribution and log link function. We omitted any females dying before 72 h, as these females would have died before producing eggs.
For experiment 2, effects of larval crowding and gerbil mf density class on parasite load (=total worms in the mosquito) and proportion of successful worms (=reaching the head) were analysed via mixed effects general linear models (PROC MIXED, SAS 9.3). mf density was classified as low (less than 100 mf per 20 µl blood), intermediate (more than or equal to 100, less than 1000 mf per 20 µl blood) or high (more than or equal to 1000 mf per 20 µl blood). We treated mf density as a class variable for this analysis because exploratory analysis indicated that the dependent variables (parasite load, proportion successful worms and flight ability) were all nonlinearly related to mf density (figure 4, and tables 2 and 3), and we elected against fitting arbitrary polynomials to the data. We chose these density classes to have order-of-magnitude steps over the range of mf densities observed. Flight ability was analysed via analysis of covariance (PROC MIXED, SAS v. 9.3) testing for effects of larval crowding, DPI, mf density class (as above) and all interactions, with parasite load as a covariate. For all analyses significant effects were further examined by pairwise comparisons of least-squares means using the Tukey–Kramer method with experimentwise α = 0.05.
Figure 4.
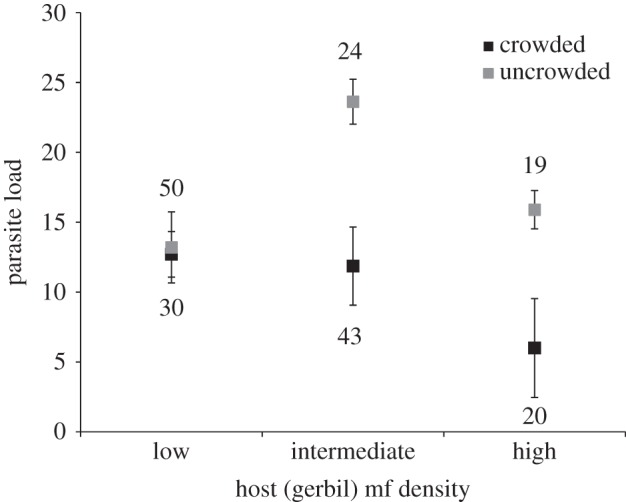
Least-squares means (±s.e.) for vector total parasite load for combinations of larval crowding and gerbil mf density level (both class variables) in dissected mosquitoes. Numbers beside each point indicate number of mosquitoes for that least-squares mean. Statistical model shown in table 1.
Table 2.
ANOVA for the proportion of total worms in the head (worm success) of dissected mosquitoes (n = 186). (Significant effects are shown in italics. mf density classes defined in table 1.)
| effect | num d.f. | den d.f. | F-value | Pr > F |
|---|---|---|---|---|
| larval crowding | 1 | 126 | 7.85 | 0.0059 |
| mf density | 2 | 126 | 4.79 | 0.0099 |
| days post-infection (DPI) | 3 | 126 | 22.39 | <0.0001 |
| larval crowding × mf density | 2 | 126 | 0.72 | 0.4894 |
| larval crowding × DPI | 3 | 126 | 1.03 | 0.3835 |
| DPI × mf density | 6 | 126 | 1.02 | 0.4174 |
| larval crowding × mf density × DPI | 6 | 126 | 0.82 | 0.556 |
Table 3.
ANOVA for flight scores. (Higher scores indicate greater flight ability (see text for mean flight scores). Significant effects are shown in italics. mf density classes defined in table 1.)
| effect | num d.f. | den d.f. | F-value | Pr > F |
|---|---|---|---|---|
| larval crowding | 1 | 147 | 0.57 | 0.4512 |
| mf density | 2 | 147 | 21.53 | <0.0001 |
| days post-infection (DPI) | 4 | 147 | 3.06 | 0.0185 |
| total parasite load | 1 | 147 | 4.53 | 0.035 |
| larval crowding × mf density | 2 | 147 | 2.12 | 0.1236 |
| larval crowding × DPI | 4 | 147 | 1.12 | 0.347 |
| DPI × mf density | 8 | 147 | 1.79 | 0.0837 |
| larval crowding × mf density × DPI | 6 | 147 | 1.95 | 0.0767 |
3. Results
(a). Experiment 1: dose dependency of fitness responses to infection
Mean ± s.e. wing length of females across all replicates was 2.76 ± 0.02 mm (n = 132). There were 15 females (4.7%) still living when the post-infection mortality study ended (45 DPI). We found a significant negative effect of gerbil mf density on hazard of death (n = 320, χ21 = 17.95, p < 0.0001; figure 1), with a hazard ratio = 1.144 per 500 mf. There was a marginally significant effect of adult emergence date group (χ21 = 3.84, p = 0.05) indicating that faster developing mosquitoes had a greater hazard of death. There was a negative effect of egg production on hazard of death (χ21 = 79.23, p < 0.0001, hazard ratio = 0.98); a parameter estimate less than 0 and hazard ratio less than 1.0 indicate that laying more eggs is associated with decreased hazard of death.
Figure 1.
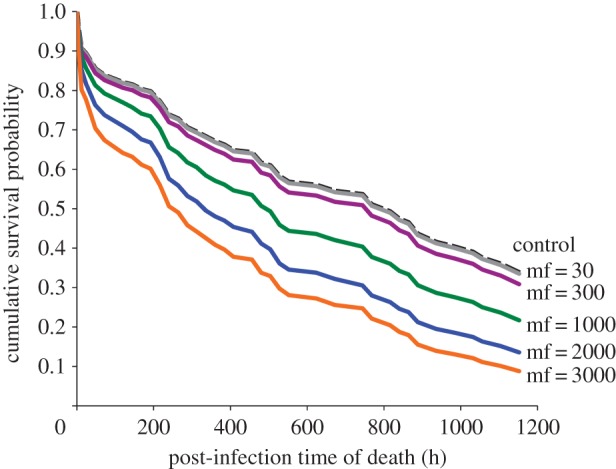
Predicted survival versus time for ad libitum fed mosquitoes at six gerbil microfilarial (mf) densities (per 20 μl blood). Predicted values from PROC PHREG, SAS 9.3; final model included gerbil mf density and number of eggs produced (retained + laid) as continuous variables, and adult emergence date and replicate as class variables. See text for statistical results. (Online version in colour.)
Generalized linear model analysis indicated a significant negative effect of gerbil mf density on egg production (n = 179, Wald χ21 = 6.06, p = 0.0138; figure 2). We found no significant effect of wing length on the number of eggs produced in a subset of females (n = 81) for which wings could be measured (Wald χ21 = 0.86, p = 0.3536), and no interaction of wing length with mf density (Wald χ21 = 3.44, p = 0.0638). Thus, wing length was removed from the final analyses. We further tested a model of a quadratic relationship between eggs and mf density, the quadratic term was not significant (p > 0.10) and Akaike information criterion with correction for sample size for this model was greater than that for the linear model.
Figure 2.
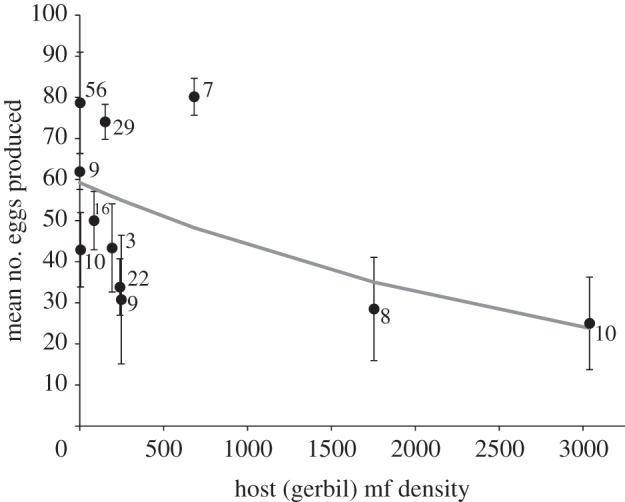
Observed means and predicted number of eggs laid + retained for mosquitoes at various gerbil mf densities. Predicted relationship results from a generalized linear model using gerbil mf density as the sole predictor (PROC GENMOD, SAS 9.3) with negative binomial error distribution and log link function. Numbers adjacent to each point indicate the number of adult females used for mean fecundity for that gerbil mf density. See Material and methods for the structure of the model.
(b). Experiment 2: interactive effects of larval crowding and infectious dose on adult fitness and vector traits
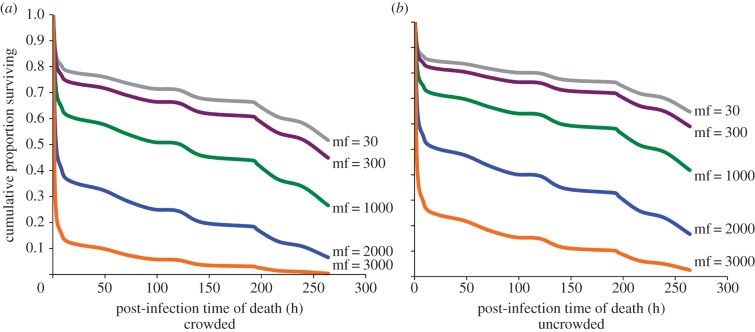
Mean ± s.e. wing lengths of females from crowded versus uncrowded larval densities were 2.70 ± 0.03 mm (n = 59) and 3.00 ± 0.03 mm (n = 52), respectively, and the groups differed significantly (t109 = 7.86, p < 0.0001). These sizes are consistent with previous larval competition studies for A. aegypti reared at high and low densities [10]. Survival analysis yielded a significant effect of gerbil mf density on survival (n = 210, Wald χ21 = 90.99, p < 0.0001). Hazard of death increased with mf density (hazard ratio = 1.54 per 500 mf). There was a significant negative effect of larval crowding on survival (Wald χ21 = 12.97, p = 0.0003, hazard ratio for low density relative to high density of 0.615; figure 3). There was no interaction between gerbil mf density and larval crowding (Wald χ21 = 2.3089, p = 0.1286); hence, the interaction term was omitted from the final model. Survival varied significantly among replicates (Wald χ25 = 47.6442, p < 0.0001), probably a result of combined effects of random variation among individual mosquitoes and among experimental gerbils.
Figure 3.
Panels (a,b) predicted survival versus time for adults from crowded (a) versus uncrowded (b) larval conditions at gerbil microfilarial (mf) density = 30, 300, 1000, 2000 and 3000 mf per 20 µl blood. Predicted values from PROC PHREG, SAS 9.3; final model included rearing density, gerbil mf density as a continuous variable, and replicate. See text for statistical results. See Material and methods for the experimental design. (Online version in colour.)
Mixed model ANOVA with replicate as a random variable yielded significant effects of gerbil mf density, DPI, larval crowding and interactions of DPI × gerbil mf density and larval crowding × mf density on vector parasite load (table 1). Among least-squares means for the DPI × gerbil mf density interaction, only mosquitoes assayed at 10 DPI and fed on gerbils with low mf density, differed significantly from every other group in parasite load. The significant interaction of larval crowding and gerbil mf density arose because mosquitoes from uncrowded larval conditions had significantly greater total parasite load when exposed to ‘intermediate’ and ‘high’ gerbil mf densities; however, when exposed to ‘low’ gerbil mf densities, the groups do not differ (figure 4). There were significant effects of larval crowding, DPI and gerbil mf density, but not of any interactions, on parasite success (i.e. proportion in the head or proboscis relative to the body total) (table 2). Worms were significantly more successful (least-squares mean [±s.e. bounds] N) in adults from uncrowded conditions (39.5% [34.5%, 44.5%] 93) than from crowded conditions (21.7% [17.4%, 26.4%] 93). Success was also significantly higher in adults fed on low mf density gerbils (42.7% [36.8%, 248.8%] 80) than in adults fed on gerbils with intermediate mf density (21.6% [17.5%, 26.1%] 67). Success when fed on high mf density gerbils (27.2% [17.4%, 26.4%] 93) did not differ from that of the other two mf density groups. As expected based on known developmental patterns, worm success increased with DPI (data not shown).
Table 1.
ANOVA for effects of larval rearing conditions, number of DPI and mf density on total parasite load in dissected mosquitoes (n = 186). (mf density is a class variable categorizing gerbil microfilaremia per 20 µl blood (low (less than 100), intermediate (more than or equal to 100, less than 1000) and high (more than or equal to 1000)). Effects significant at p < 0.05 are shown in italics.)
| effect | num d.f. | den d.f. | F-value | Pr > F |
|---|---|---|---|---|
| larval crowding | 1 | 156 | 11.02 | 0.0011 |
| mf density | 2 | 156 | 3.06 | 0.0498 |
| days post-infection (DPI) | 3 | 156 | 2.47 | 0.0638 |
| larval crowding × mf density | 2 | 156 | 6.42 | 0.0021 |
| larval crowding × DPI | 3 | 156 | 1.9 | 0.1312 |
| DPI × mf density | 6 | 156 | 2.38 | 0.0313 |
| larval crowding × mf density × DPI | 6 | 156 | 1.98 | 0.0715 |
Flight score was significantly affected by mf density and DPI (table 3), but there was no effect of larval crowding or of any interactions (table 3). Flight scores (least-squares mean ± s.e., N) were significantly greater at low gerbil mf densities (2.5 ± 0.2, 80) than at intermediate (1.7 ± 0.1, 63) and high (1.8 ± 0.2, 39) gerbil mf densities, and high and intermediate mf density groups did not differ. Flight scores were significantly lower on days 11 + 12 (1.2 ± 0.2, 47) than on day 10 (2.3 ± 0.2, 37). The covariate of parasite load had a significant negative effect on flight score (slope ± s.e. = −0.109 ± 0.0055).
4. Discussion
Mosquitoes can differ greatly in responses to parasitism, including post-infection survival, sustained parasite load and likelihood of progressing to vector competent status [11]. Only a portion of pathogen-exposed mosquitoes will become competent vectors of disease [10,18]. We postulated that some of this variation exists because environmental factors (i.e. stressors) acting on larval stages impinge on adult physiology, and that interactions between resulting adult body condition and ingested parasites contribute to differences among individuals in both physiological and functional vector competence. For A. aegypti reared in well-fed, homogeneous conditions, post-infectious blood meal survival is significantly and negatively associated with the microfilarial density of the vertebrate blood host (i.e. our proxy for infectious dose). We detected a marginal effect of larval developmental pace on post-infection survival, suggesting that earlier emerging adults are less likely to survive to vector competent stages. This is surprising because for well-fed larvae, adult eclosion occurred quickly and more or less synchronously (all adults emerged over less than or equal to 6 days). In poor larval conditions, a greater degree of developmental asynchrony is expected [34], and differences between early versus late groups may be more pronounced. Our results suggest that differences in larval developmental pace in poor conditions might increase natural variability in adult post-filarial infection survival.
Filarial infection can disrupt the predicted positive relationship between size and fecundity [31], thus our observed lack of correlation between these traits is not unexpected. Gerbil mf density significantly and negatively impacted mosquito egg production, a result consistent with other studies [24,26]. One possible mechanism of this trade-off is l-tyrosine limitation in the melanin formation pathway, which links reproduction and immunity [26]. Production of melanin is critical for egg chorion tanning, cuticular sclerotization, wound healing and, most importantly, immune defences critical for combating filarial worms [35]. l-tyrosine supply within an individual is finite, thus fecundity reduction may result from increased allocation of this resource into immune defence [26]. Surprisingly, we observed a significant positive relationship between longevity and egg production. This apparent relationship could be spurious, as adults that live longer for any reason have increased opportunity for oviposition. A proper test for costs of reproduction to longevity will require direct manipulation of investment into reproduction [32].
Larval crowding significantly increased post-infection mortality across all gerbil mf density levels. Adults from uncrowded larvae had greater total parasite load at intermediate and high mf levels; however, at low mf levels no measurable differences were observed. Thus, infectious dose seems to interact with larval rearing conditions to compound fitness effects in a dose-dependent manner. Mosquitoes fed on the highest gerbil mf density levels experienced severe mortality, perhaps because those mf levels were uncommonly high and A. aegypti (Liverpool) are highly susceptible to infection; this combination of factors could have enhanced pathology. However, our low and intermediate mf density levels are within the range of microfilaremia found in feline hosts of B. pahangi [36] and in human hosts of B. malayi [37] and W. bancrofti [38], and our worm counts from dissected mosquitoes are in the normal range for experimentally infected natural vectors of Brugia [39]. Thus, similar impacts of larval environment and infectious dose on survival and parasite load may occur in natural vector–parasite associations.
Crowded larvae yielded adults with a smaller proportion of total parasites in the head, a requisite for infectiousness. This suggests that, contrary to results from other mosquito–pathogen systems [10,11], adults from crowded larval densities somehow limit (e.g. physiologically) filarial worm growth, development and dissemination better than adults from uncrowded larval densities. There are, however, alternative hypotheses for our results. Most obviously, one of the effects of larval crowding is smaller adult size. Smaller adults may take smaller blood meals and thus may have reduced initial parasite load. However, for some mosquito species the relationship between blood meal volume and the number of mf ingested is nonlinear. For example, A. aegypti can exert a ‘concentrating’ effect, ingesting a greater abundance of mf than predicted based on blood volume and host mf density [39]. Hence, reduced blood intake by smaller mosquitoes may not sufficiently explain the reduced parasite load in our adults from crowded larval environments. Reduction in parasite success (=proportion in the head) could also be attributed to mosquito size if developing worms compete more intensely within smaller bodied individuals. A more intriguing alternative hypothesis for decreased parasite load and success is that adults from crowded larvae may be better able to limit or to suppress infection, perhaps by investing more heavily into parasite defences. Studies of other insects have shown that crowding can increase parasite resistance [40]; however, the outcome of mosquito larval crowding for adult filarial worm defence has yet to be tested.
We found no effect of larval crowding on flight capability, yet pronounced effects of both gerbil mf density and total parasite load. This result is consistent with other studies demonstrating mf density-dependent effects on flight [28]. Mobility is integral to both survival and fitness, enabling feeding on nectar and females' acquisition of blood to provide protein for vitellogenesis [41]. Critically, flight impairment decreases the ability to locate and to feed on blood hosts, which can potentially reduce or eliminate functional vector competence despite all other factors. In this way, high infectious dose and heavy parasite load appear to reduce the probability of transmission.
Results from our mosquito–filarial system contrast in some ways recent investigations of arboviruses showing that larval competition can enhance vector competence (reviewed by [29]). Some of these differences probably result from greater damage to the vector by large metazoan parasites like B. pahangi compared with arboviruses. Larval crowding seems to enhance those fitness costs, which is consistent with our hypotheses of a resource competition-mediated trade-off between larval growth and development and adult infection responses (i.e. stressed mosquitoes appear to be more harmed by infection). However, contrary to our prediction of greater physiological competence in stressed mosquitoes, our results indicate that mosquitoes from benign larval rearing environments (low density and ample food) are more physiologically capable vectors of B. pahangi. These individuals probably have greater longevity as adults and have greater parasite load and success. Investigations of environmental effects on physiological vector competence alone are unlikely to provide a clear picture of disease transmission risk, as interactions between the larval environment and adult infectious dose may produce counterintuitive outcomes for other important vector traits. Inter-individual physiological variation in vector competence, whatever the source, needs to be integrated with differences in mortality, flight ability and ultimately pathogen transfer to uninfected hosts, to better understand functional vector competence and heterogeneity of disease transmission itself. Results from this laboratory model system suggest that complex effects of environmental stress on vector–pathogen systems probably occur via multiple mechanisms. A better understanding of such effects may reveal important aspects of disease ecology, including population-level responses and trade-offs within individuals that may be mediated by immunity. These effects may also have implications for vector control and may help to refine current approaches targeting specific larval environments. In particular, investigations of trade-offs involving adult mosquito immune function could prove vital for our understanding of the ecology of vector–pathogen interactions.
Acknowledgements
We thank B. Noden for discussion, two anonymous referees for helpful comments and R. Bontz, C. Boyer, N. Vick and A. Schwenk for assistance in the laboratory. Mosquito eggs and parasite resources were supplied by the NIH/NIAID Filariasis Research Reagent Resource Centre (www.filariasiscenter.org).
All vertebrate animal use was in strict accordance with the Guide for the Care and Use of Laboratory Animals of the US National Institutes of Health. Protocols were approved by the Institutional Animal Care and Use Committee, Illinois State University (Institutional Animal Assurance no. A3762-01, protocols 01-2010, 13–2009, 11-2012).
Funding statement
This research was supported by grants from Illinois State University, NIAID grant nos. R15-AI075306-0, R15-AI094322-01A1, R01-AI044793 to S.A.J., Phi Sigma grants and Sigma Xi GIAR to J.A.B. and the Bradley University STEM research program.
References
- 1.Nowak MA, May RM. 1994. Superinfection and the evolution of parasite virulence. Proc. R. Soc. Lond. B 255, 81–89. ( 10.1098/rspb.1994.0012) [DOI] [PubMed] [Google Scholar]
- 2.Dawes EJ, Churcher TS, Zhuang S, Sinden RE, Basáñez M-G. 2009. Anopheles mortality is both age- and Plasmodium-density dependent: implications for malaria transmission. Malar. J. 8, 228 ( 10.1186/1475-2875-8-228) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ewald PW. 1995. The evolution of virulence: a unifying link between parasitology and ecology. J. Parasitol. 81, 659–669. ( 10.2307/3283951) [DOI] [PubMed] [Google Scholar]
- 4.Hurd H. 2003. Manipulation of medically important insect vectors by their parasites. Annu. Rev. Entomol. 48, 141–61. ( 10.1146/annurev.ento.48.091801.112722) [DOI] [PubMed] [Google Scholar]
- 5.Tripet F, Aboagye-Antwi F, Hurd H. 2008. Ecological immunology of mosquito–malaria interactions. Trends Parasitol. 24, 219–227. ( 10.1016/j.pt.2008.02.008) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lambrechts L, Chavatte J-M, Snounou G, Koella JC. 2006. Environmental influence on the genetic basis of mosquito resistance to malaria parasites. Proc. R. Soc. B 273, 1501–1506. ( 10.1098/rspb.2006.3483) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Juliano SA. 2009. Species interactions among larval mosquitoes: context dependence across habitat gradients. Annu. Rev. Entomol. 54, 37–56. ( 10.1146/annurev.ento.54.110807.090611) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Juliano SA, Lounibos LP. 2005. Ecology of invasive mosquitoes: effects on resident species and on human health. Ecol. Lett. 8, 558–574. ( 10.1111/j.1461-0248.2005.00755.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bevins SN. 2008. Invasive mosquitoes, larval competition, and indirect effects on the vector competence of native mosquito species (Diptera: Culicidae). Biol. Invasions 10, 1109–1117. ( 10.1007/s10530-007-9188-8) [DOI] [Google Scholar]
- 10.Alto BW, Lounibos LP, Christopher NM, Michael HR. 2008. Larval competition alters susceptibility of adult Aedes mosquitoes to dengue infection. Proc. R. Soc. B 275, 463–471. ( 10.1098/rspb.2007.1497) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alto BW, Lounibos LP, Higgs S, Juliano SA. 2005. Larval competition differentially affects arbovirus infection in Aedes mosquitoes. Ecology 86, 3279–3288. ( 10.1890/05-0209) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Lord CC. 1998. Density dependence in larval Aedes albopictus (Diptera: Culicidae). J. Med. Entomol. 35, 825–829. [DOI] [PubMed] [Google Scholar]
- 13.Gimnig JE, Ombok M, Otieno S, Kaufman MG, Vulule JM, Walker ED. 2002. Density-dependent development of Anopheles gambiae (Diptera: Culicidae) larvae in artificial habitats. J. Med. Entomol. 39, 162–172. ( 10.1603/0022-2585-39.1.162) [DOI] [PubMed] [Google Scholar]
- 14.Scott TW, Takken W. 2012. Feeding strategies of anthropophilic mosquitoes result in increased risk of pathogen transmission. Trends Parasitol. 28, 114–121. ( 10.1016/j.pt.2012.01.001) [DOI] [PubMed] [Google Scholar]
- 15.Telang A, Qayum AA, Parker A, Sacchetta BR, Byrnes GR. 2012. Larval nutritional stress affects vector immune traits in adult yellow fever mosquito Aedes aegypti (Stegomyia aegypti). Med. Vet. Entomol. 26, 271–281. ( 10.1111/j.1365-2915.2011.00993.x) [DOI] [PubMed] [Google Scholar]
- 16.Suwanchaichinda C, Paskewitz SM. 1998. Effects of larval nutrition, adult body size, and adult temperature on the ability of Anopheles gambiae (Diptera: Culicidae) to melanize Sephadex beads. J. Med. Entomol. 35, 157–161. [DOI] [PubMed] [Google Scholar]
- 17.Zera AJ, Harshman LG. 2001. The physiology of life history trade-offs in animals. Annu. Rev. Ecol. Syst. 32, 95–126. ( 10.1146/annurev.ecolsys.32.081501.114006) [DOI] [Google Scholar]
- 18.Sisterson MS. 2009. Transmission of insect-vectored pathogens: effects of vector fitness as a function of infectivity status. Environ. Entomol. 38, 345–355. ( 10.1603/022.038.0206) [DOI] [PubMed] [Google Scholar]
- 19.Woolhouse MEJ, et al. 1997. Heterogeneities in the transmission of infectious agents: implications for the design of control programs. Proc. Natl Acad. Sci. USA 94, 338–342. ( 10.1073/pnas.94.1.338) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Pollitt L, Churcher TS, Dawes EJ, Khan SM, Sajid M, Basáñez MG, Colgrave N, Reece SE. 2013. Costs of crowding for the transmission of malaria parasites. Evol. Appl. 6, 617–629. ( 10.1111/eva.12048) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bockarie MJ, Pedersen EM, White GB. 2009. Role of vector control in the global program to eliminate lymphatic filariasis. Annu. Rev. Entomol. 54, 469–487. ( 10.1146/annurev.ento.54.110807.090626) [DOI] [PubMed] [Google Scholar]
- 22.Erickson SM, Zhiyong X, Mayhew GF, Ramirez JL, Aliota MT, Christensen BM, Dimopoulos G. 2009. Mosquito infection responses to developing filarial worms. PLoS Neglected Trop. Dis. 3, 1–11. ( 10.1371/journal.pntd.0000529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Aliota MT, Fuchs JF, Rocheleau TA, Clark AK, Hillyer JF, Chen CC, Christensen BM. 2010. Mosquito transcriptome profiles and filarial worm susceptibility in Armigeres subalbatus. PLoS Neglected Trop. Dis. 4, e666 ( 10.1371/journal.pntd.0000666) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Javadian E, Macdonald WW. 1974. The effect of infection with Brugia pahangi and Dirofilaria repens on the egg-production of Aedes aegypti. Ann. Trop. Med. Parasitol. 68, 477–481. [DOI] [PubMed] [Google Scholar]
- 25.Hurd H. 2001. Host fecundity reduction: a strategy for damage limitation? Trends Parasitol. 17, 363–368. ( 10.1016/S1471-4922(01)01927-4) [DOI] [PubMed] [Google Scholar]
- 26.Ferdig MT, Beerntsen BT, Spray FJ, Li J, Christensen B. 1993. Reproductive costs associated with resistance in a mosquito–filarial worm system. Am. J. Trop. Med. Hyg. 49, 756–762. [DOI] [PubMed] [Google Scholar]
- 27.Vézilier J, Nicot A, Gandon S, Rivero A. 2012. Plasmodium infection decreases fecundity and increases survival of mosquitoes. Proc. R. Soc. B 279, 4033–4041. ( 10.1098/rspb.2012.1394) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hockmeyer WT, Schiefer BA, Redington BC, Eldridge BF. 1974. Brugia pahangi: effects upon the flight capability of Aedes aegypti. Exp. Parasitol. 38, 1–5. ( 10.1016/0014-4894(75)90031-4) [DOI] [PubMed] [Google Scholar]
- 29.Alto B, Lounibos LP. 2013. Vector competence for arboviruses in relation to the larval environment of mosquitoes. In Ecology of parasite–vector interactions (eds Takken D, Koenraadt CJM.), pp. 81–101. Wageningen, The Netherlands: Wageningen Academic Publishers. [Google Scholar]
- 30.Cator LJ, George J, Blanford S, Murdock CC, Baker TC, Read AF, Thomas MB. 2013. ‘Manipulation’ without the parasite: altered feeding behaviour of mosquitoes is not dependent on infection with malaria parasites. Proc. R. Soc. B 280, 20130711 ( 10.1098/rspb.2013.0711) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lima CA, Almeida WR, Hurd H, Albuquerque CMR. 2003. Reproductive aspects of the mosquito Culex quinquefasciatus (Diptera: Culicidae) infected with Wuchereria bancrofti (Spirurida: Onchocercidae). Mem. Inst. Oswaldo Cruz 98, 217–222. ( 10.1590/S0074-02762003000200009) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Frankino WA, Juliano SA. 1999. Costs of reproduction and geographic variation in the reproductive tactics of the mosquito Aedes triseriatus. Oecologia 120, 59–68. ( 10.1007/s004420050833) [DOI] [PubMed] [Google Scholar]
- 33.Reiter P. 2007. Oviposition, dispersal, and survival in Aedes aegypti: implications for the efficacy of control strategies. Vector Borne Zoonotic Dis. 7, 261–273. ( 10.1089/vbz.2006.0630) [DOI] [PubMed] [Google Scholar]
- 34.Araujo M, Gil LH, e-Silva A. 2012. Larval food quantity affects development time, survival and adult biological traits that influence the vectorial capacity of Anopheles darlingi under laboratory conditions. Malar. J. 11, 261 ( 10.1186/1475-2875-11-261) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Castillo JC, Reynolds SE, Eleftherianos I. 2011. Insect immune responses to nematode parasites. Trends Parasitol. 27, 537–547. ( 10.1016/j.pt.2011.09.001) [DOI] [PubMed] [Google Scholar]
- 36.Palmieri JR, Masbar S, Purnomo N, Marwoto HA, Tirtokusumo S, Darwis F. 1985. The domestic cat as a host for Brugian filariasis in South Kalimantan (Borneo), Indonesia. J. Helminthol. 59, 277–281. ( 10.1017/S0022149X00008099) [DOI] [PubMed] [Google Scholar]
- 37.Kim JS, Lee WY, Chun SL. 1973. Ecology of filariasis on Che Ju island. Korean J. Parasitol. 11, 33–53. ( 10.3347/kjp.1973.11.1.33) [DOI] [PubMed] [Google Scholar]
- 38.McGreevy PB, Kolstrup N, Tao J, McGreevy MM, de C. Marshall TF. 1982. Ingestion and development of Wuchereria bancrofti in Culex quinquefasciatus, Anopheles gambiae and Aedes aegypti after feeding on humans with varying densities of microfilariae in Tanzania. Trans. R. Soc. Trop. Med. Hyg. 76, 288–296. ( 10.1016/0035-9203(82)90170-5) [DOI] [PubMed] [Google Scholar]
- 39.Albuquerque CM, Cavalcanti V, Melo MAV, Verçosa P, Regis LN, Hurd H. 1999. Bloodmeal microfilariae density and the uptake and establishment of Wuchereria bancrofti infections in Culex quinquefasciatus and Aedes aegypti. Mem. Inst. Oswaldo Cruz 94, 591–596. ( 10.1590/S0074-02761999000500005) [DOI] [PubMed] [Google Scholar]
- 40.Barnes AI, Siva-Jothy MT. 2000. Density-dependent prophylaxis in the mealworm beetle Tenebrio molitor L. (Coleoptera: Tenebrionidae): cuticular melanization is an indicator of investment in immunity. Proc. R. Soc. Lond. B 267, 177–182. ( 10.1098/rspb.2000.0984) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Briegel H. 1985. Mosquito reproduction: incomplete utilization of the blood meal protein for ovogenesis. J. Insect Physiol. 31, 15–21. ( 10.1016/0022-1910(85)90036-8) [DOI] [Google Scholar]