Abstract
Mating system variation is profound in animals. In insects, female willingness to remate varies from mating with hundreds of males (extreme polyandry) to never remating (monandry). This variation in female behaviour is predicted to affect the pattern of selection on males, with intense pre-copulatory sexual selection under monandry compared to a mix of pre- and post-copulatory forces affecting fitness under polyandry. We tested the hypothesis that differences in female mating biology would be reflected in different costs of pre-copulatory competition between males. We observed that exposure to rival males early in life was highly costly for males of a monandrous species, but had lower costs in the polyandrous species. Males from the monandrous species housed with competitors showed reduced ability to obtain a mate and decreased longevity. These effects were specific to exposure to rivals compared with other types of social interactions (heterospecific male and mated female) and were either absent or weaker in males of the polyandrous species. We conclude that males in monandrous species suffer severe physiological costs from interactions with rivals and note the significance of male–male interactions as a source of stress in laboratory culture.
Keywords: monandry, polyandry, copulation duration, social interaction, costs, longevity
1. Introduction
Female mating behaviour varies widely among taxa [1]. The rate at which females remate is a fundamental parameter in evolution and ecology, impacting on disease transmission (e.g. [2]), male/female dimorphism (e.g. [3]), the degree of sexual conflict [4] and potentially the rate of evolution of a species (reviewed in [5]) and its propensity both to speciate (e.g. [6]) and go extinct (e.g. [7]). Within the genus Drosophila alone, species exist where females mate once in their lifetime, and others where females will mate with a different male within 30 minutes of completing copulation [8,9]. These patterns of mating behaviour influence the pattern of selection on males. Where females are monandrous, male reproductive fitness depends solely on pre-copulatory sexual selection (success in acquiring mates), which often involves male–male competition. By contrast, male fitness under female polyandry depends on both pre- and post-copulatory sexual selection, which involves sperm competition and cryptic female choice of sperm [1]. Post-copulatory sexual selection can be intense, driving the evolution of, for example, increased testes size and unusual ejaculate properties [10,11].
Recently, it has been recognized that female mating behaviour may profoundly affect male life history. In Antechinus marsupial mice, synchronous polyandry by females drives intense post-copulatory sexual selection in males, leading to the death of the males within the breeding season [12]. More recently, longevity effects associated with continued mate-seeking behaviour have been observed in Drosophila melanogaster [13]. Males maintained in the presence of female pheromones, but in the absence of a mate, died before males that were sexually satiated or maintained without the stimulus to seek mates.
In this paper, we examined whether monandry might likewise produce strong life-history impacts, where male–male interactions occur over a protracted period, rather than during a confined breeding bout. Our study was motivated by the recent observation that males in the monandrous species Drosophila subobscura mate for twice as long when previously housed with rival males [14]. Previous work has demonstrated that increases in copulation duration can represent an adaptive response to the risk of sperm competition (reviewed in [15]). However, this explanation is unlikely to apply to the generally monandrous D. subobscura [16]. It was proposed that the effect in D. subobscura may instead derive from poorer condition of males that had been subject to social interactions. For instance, increasing male age is known to be associated with increased copulation duration in flies [17]. We reasoned that social interaction may be producing a similar effect to ageing in male D. subobscura.
Because condition is an important determinant of male ability to provide nuptial gifts and gain matings in D. subobscura [18], we predicted that the impact of prior social interactions may go beyond copulation duration, and be reflected in an array of traits. To this end, we examined the impact of social environment on a male's ability to acquire matings and on longevity. We compared social environment effects across two Drosophila species: the monandrous D. subobscura, and the closely related polyandrous species Drosophila pseudoobscura [16,19,20]. As males of a monandrous species are only subjected to pre-copulatory sexual selection while males of polyandrous species are subjected to both pre- and post-copulatory sexual selection, we expected that the presence of rivals before copulation would have a more profound impact on the fitness (reproductive success and survival) of males of the monandrous species than the polyandrous one.
2. Material and methods
(a). Species examined and mating procedure
Multi-female lines of D. subobscura (initially established from nine inseminated wild females from British Columbia) and D. pseudoobscura (N = 100) were collected in 2008 from Vancouver Island (British Columbia, Canada) and Show Low (AZ, USA), respectively. Flies were maintained in large outbred populations in a humidified room at 21°C with a 12 L : 12 D photoperiod, on standard corn–sugar–yeast–agar medium (ASG medium). Experimental adult flies were raised at standard densities of 50 larvae per vial and isolated as virgins within 24 h of eclosion. Females were kept at a standard density of five per vial. Males were kept in a vial in the conditions described later in the methods. Experiments were performed at 21°C.
(i). Experiment 1: male response to social environment
Previous work showed that the presence of a single rival affected copulation duration in both species [14,16,21]. Here, we examined the specificity of the male response to rivals by exposing males to various social environments.
For each species, males were placed either individually (treatment ‘single’), or with one rival male (treatment ‘one rival’), four rival males (treatment ‘four rivals’), one heterospecific male (treatment ‘heterospecific’) or one conspecific mated female (treatment ‘mated female’), from within 8 h of eclosion to 8 days old (N total D. subobscura: single = 126, one rival = 152, four rivals = 99, heterospecific = 57, mated female = 63; N total D. pseudoobscura: single = 58, one rival = 56, four rivals = 41, heterospecific = 19, mated female = 16). Numbers of trials were higher for D. subobscura to reduce the risk that the lower mating rate in this species would reduce the number of matings below which reasonable analysis of copulation duration and latency was difficult. It was also partly owing to the difficulty of synchronizing eclosion of the two species. ‘Heterospecifics’ corresponded to a D. pseudoobscura male for D. subobscura and vice versa. The females used to alter social environment in the ‘mated female’ treatment were mated a day before exposure; they were conspecific and the same age as the males tested. As D. subobscura females are monandrous [16], they were thus inaccessible to the tested male. However, D. pseudoobscura females typically remate after 5 days, and so it is likely that they will have mated with the experimental male. This means that the experimental D. pseudoobscura male for the ‘mated female’ treatments would not have been a virgin. Moreover, the relatively low sample size for the D. pseudoobscura trials investigating the impact of heterospecific males and mated females also means that the results need to be interpreted cautiously. Conspecific and heterospecific males were right or left wing-clipped at emergence to allow distinction (the clipped wing was randomized across replicates).
After the exposure period, the male was placed in a vial into which a single virgin female was then added. Where males were exposed to multiple conspecific males, only one male was randomly chosen from each vial to avoid pseudoreplication. Sexual partners had a 3 h window to copulate, mimicking their natural ecology. This procedure was followed for all treatments detailed above. Male responses to treatments were assessed by measuring their propensity to acquire a mate (proportion mating) (N mated males D. subobscura: single = 97, one rival = 64, four rivals = 26, heterospecific = 38, mated female = 39; N mated males D. pseudoobscura: single = 53, one rival = 53, four rivals = 39, heterospecific = 18, mated female = 14). For each mating observed, we also recorded copulation latency (time from male introduction into a female vial until mating occurred) and copulation duration.
(ii). Experiment 2: the effect of rivals on male ability to compete for a female
Males were either kept singly (‘single’ males) or exposed to ‘four rivals’ as described above, before being placed in competition for female access in the arena of mating. To this end one ‘single’ and one ‘four rival’ male were placed in each vial prior to adding one virgin female (ND. subobscura: 24; ND. pseudobscura: 40). The success of ‘four rivals’ and ‘single’ males in acquiring the female as a mate was then scored. Copulation latency and duration were also recorded for each mating.
(iii). Experiment 3: effect of exposure to rivals early in life on male longevity
Males were either kept singly (‘single’ males) or exposed to ‘four rivals’ as described above. After the exposure period, males were isolated (for the ‘four rivals’ males) and transferred to a new fresh vial (ND. subobscura: singlelongevity = 40, four rivalslongevity = 37; ND. pseudobscura: singlelongevity = 35, four rivalslongevity = 40). Every Monday, Wednesday and Friday, the number of dead males in each group for each species was recorded. Live males were transferred to a new fresh vial of food weekly until all males died. This experiment was run from mid June 2012 to mid November 2012. Longevity assessments were performed at 18°C for D. subobscura and 21°C for D. pseudoobscura.
(b). Relevance of the methods to the ecology of the species
Conditions used here are likely to approximate situations experienced by these flies in nature. The two species inhabit the same forests on Vancouver Island [22], almost certainly interacting on breeding sites. Our rival/no rival conditions are likely to occur to flies in nature as local density and sex ratio can be highly variable in both species (ranging from intense male–male interactions to almost complete absence of rivals) [23,24]. Mating trials in vials do increase the likelihood of copulation occurring during an interaction, but the impact is modest (female flies provided with a refuge have a 20% lower mating rate) [25]. Both species are crepuscular, with mating typically occurring at 21°C during a few hours around dusk and dawn [23,24]. However D. subobscura is less tolerant to high temperatures than D. pseudoobscura, so we used 18°C for the D. subobscura longevity trials.
Drosophila subobscura females mature at 8 days post eclosion, with males maturing at 2–3 days [26]. Drosophila pseudoobscura females mature at 3 days post eclosion, and males at 1–2 days [26]. Both species have a highly male-biased operational sex ratio, owing to monandry in D. subobscura [16], and a 3–5 day female remating latency in D. pseudoobscura [22]. Both species can live for three months in ideal laboratory conditions [27], but are much shorter lived as adults in nature [28,29]. Assuming an 8% death rate in nature as measured for D. pseudoobscura, 40% of flies will die within a week of eclosion, which is relevant to the mating ages we use for both species. Our longevity study probably overestimates longevity, but longevity is frequently used as an indicator of stress occurring at a younger age in Drosophila [30].
(c). Statistical analysis
All statistical analyses were performed using v. R 2.12.1 [31]. Data on the proportion of males copulating were analysed using a generalized linear model (GLM) procedure assuming a binomial error distribution with a logit link function. Copulation latencies and duration data were tested for normality using Shapiro tests, and for variance heteroscedasticity using Bartlett tests, and they were non-normally distributed. A range of transformations was tried, with copulation duration normalized by Ln transformation, and copulation latency normalized by log 10 transformation. The transformed data were then analysed using a GLM procedure assuming a normal distribution with an identity link function [32]. Differences in responses under different treatments were assessed by analysis of variance (ANOVA) followed by a Tukey HSD test. Mating success in two male mating trials was evaluated using an exact binomial test. Cox proportional-hazard regressions were used to assess variation in longevity for ‘single’ males and for ‘four rivals’ males. Survival analysis involves the modelling of time to event data, with death (the hazard function) being considered the ‘event’, so that each death corresponded to one ‘event’ modelled against time with treatments (‘single’ or ‘four rivals’) as factor (using the Survdiff function).
3. Results
(a). Experiment 1: male response to social environment
We first examined the effect of social environment on the ability to acquire a mate in the absence competition. We observed that exposure to ‘one rival’ or ‘four rivals’ significantly reduced male ability to acquire a mate in the monandrous species, D. subobscura (figure 1a; electronic supplementary material, table S1a). In this species, exposure to one or more rivals led to a nearly threefold reduction in male ability to acquire a mate compared with ‘single’ males. The other types of social environment had an effect comparable with the ‘single’ male treatment (electronic supplementary material, table S1a). By contrast, there was no difference among treatments in male ability to acquire a mate in D. pseudoobscura, to gain a mating (figure 1b; electronic supplementary material, table S1b).
Figure 1.
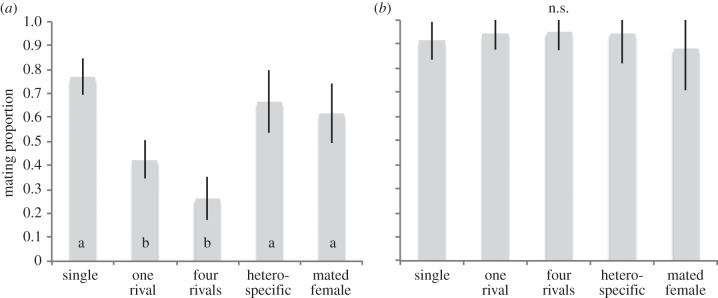
Probability of successfully mating when alone with a female for (a) D. subobscura and for (b) D. pseudoobscura, when males were kept singly (single) or with one rival (one rival), four rivals (four rivals), a heterospecific male (heterospecific) or a mated female (mated female) prior to copulation. Different letters represent significant differences within each graph. For statistical results, see the electronic supplementary material, table S1a,b.
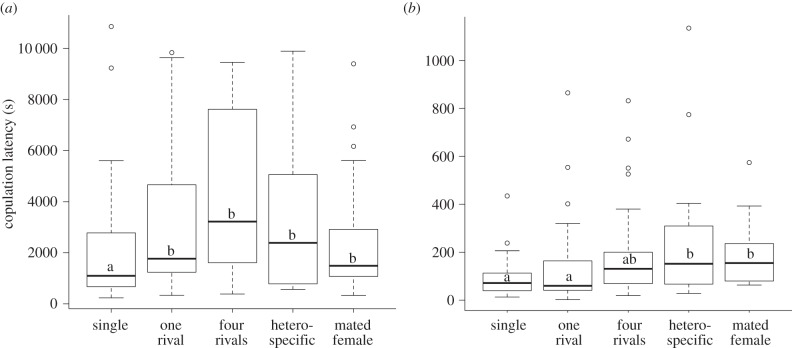
Any type of social environment increased copulation latency in D. subobscura compared with ‘single’ males (figure 2a; electronic supplementary material, table S2a). By contrast, exposure to a ‘heterospecific’ male or ‘mated female’ increased copulation latency in D. pseudoobscura compared with ‘single’ males and to ‘one rival’ males, while exposure to ‘four rivals’ had no effect (figure 2b; electronic supplementary material, table S2b).
Figure 2.
Copulation latency (mean ± s.e) for (a) D. subobscura and (b) D. pseudoobscura when males were kept singly (single) with one rival (one rival), four rivals (four rivals), a heterospecific male (heterospecific) or a mated female (mated female) prior to copulation. Different letters represent significant differences within each graph. For statistical analysis, see the electronic supplementary material, table S2a,b.
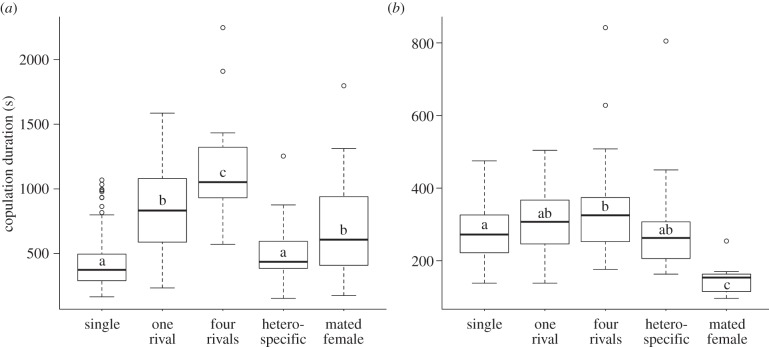
In D. subobscura, exposure to a rival increased copulation duration as previously reported, but this increase was even stronger following exposure to ‘four rivals’, whereas exposure to a ‘heterospecific’ male had no effect on copulation duration (figure 3a). However, exposure to a ‘mated female’ increased copulation duration to the same extent as exposure to ‘one rival’ did (electronic supplementary material, table S3a). By contrast, the effect of additional rivals was not profound in D. pseudoobscura (figure 3b). The null hypothesis that a single rival affected copulation duration was not rejected, while exposure to ‘four rivals’ did increase copulation duration. As in D. subobscura, exposure to a ‘heterospecific’ male had no effect on copulation duration, but exposure to a ‘mated female’ significantly decreased copulation duration (electronic supplementary material, table S3b).
Figure 3.
Copulation duration (mean ± s.e) for (a) D. subobscura and (b) D. pseudoobscura when males were kept singly (single), with one rival (one rival), four rivals (four rivals), a heterospecific male (heterospecific) or a mated female (mated female) prior to copulation. Different letters represent significant differences within each graph. For statistical analysis, see the electronic supplementary material, table S3a,b.
(b). Experiment 2: the effect of rivals on male ability to compete for a female
Competition assays revealed that males of both species which had been exposed to rivals before copulation were less successful in acquiring matings when in competition with ‘single’ males. In the monandrous species D. subobscura, the male exposed to ‘four rivals’ was successful in only two of 24 trials (8.33%, exact binomial test: p = 0.001). The male exposed to ‘four rivals’ were also less successful in the polyandrous species D. pseudoobscura, but the effect was smaller, with males exposed to ‘four rivals’ successfully mated in 11 of 40 trials (27.5%, exact binomial test: p = 0.006).
(c). Experiment 3: effect of exposure to rivals early in life on male longevity
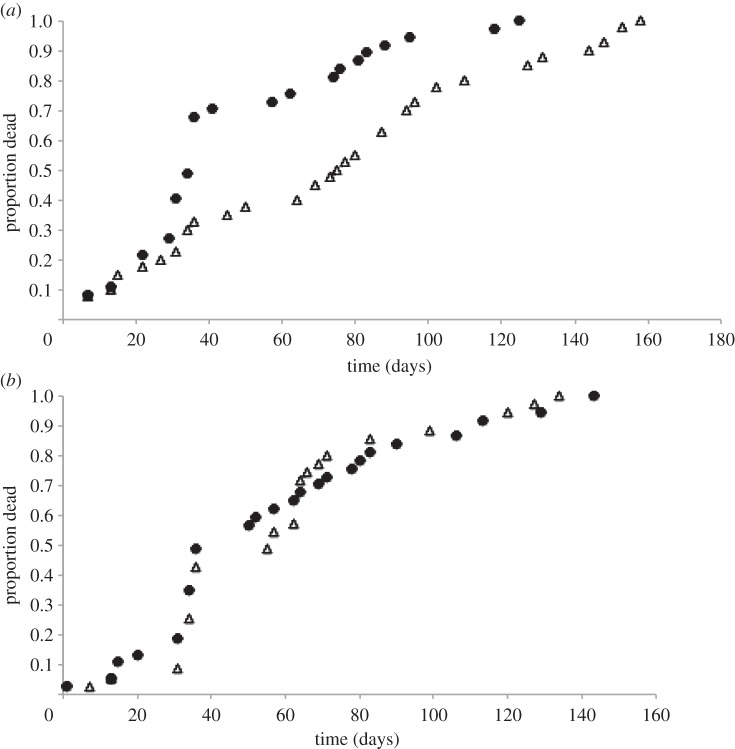
Exposure to rivals during the first 8 days of life significantly decreased the longevity of males of the monandrous species (D. subobscura) (Survdiff; 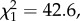 p = 6.84×10−11) (figure 4a) but not of males of the polyandrous species (D. pseudoobscura) (Survdiff;
p = 6.84×10−11) (figure 4a) but not of males of the polyandrous species (D. pseudoobscura) (Survdiff; 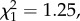 p = 0.264) (figure 4b).
p = 0.264) (figure 4b).
Figure 4.
Longevity represented as proportion dead of males over time (in days) for (a) D. subobscura and for (b) D. pseudoobscura, when males were kept singly (single: triangles) or with four rivals (four rivals: circles) for 8 days after having reached sexual maturity.
4. Discussion
For males in a monandrous species, the fitness benefit of a single mating is very high. This generates strong competition for mating opportunities, and high level of investment in obtaining matings, as reflected in adaptations such as provision of nuptial gifts. We predicted that presence of one or more rival males in monandrous species would lead to costs associated with this competition. Our results demonstrated that these costs are profound, with prior exposure to rivals associated with decreased ability to obtain matings, and reduced longevity. Furthermore, these costs are greater in the monandrous D. subobscura than in the polyandrous D. pseudoobscura. Although based on a comparison of only two species, the result is consistent with the hypothesis that pre-copulatory male competition should be vital to male success in monandrous species, and so be associated with significant effort and costs.
Previous studies in Drosophila have demonstrated a cost of sex, in that individuals that mate more frequently die younger (e.g. [30,33]). In D. melanogaster, there is also a longevity impact in males associated with mate-seeking and courtship activity [13]. Our results demonstrate that there is also a direct cost of intrasexual social interaction in the monandrous species D. subobscura, even in the absence of mate finding, courtship and sex. Thus, intrasexual social interaction intensity should be accounted for as one of the major factors associated with reproduction that affect longevity.
Sexual selection leading to ‘suicidal reproduction’ in males has been demonstrated in the Australian redback spider, where cannibalized males manipulate female behaviour to increase paternity [34]. Very recently, this has also been observed in semelparous marsupials, where females manipulate male behaviour to increase their own reproductive success [12]. Males in these species increase mating effort at the expense of survival, not because adult male or female survival is low for environmental reasons or because males are altruistic, but ultimately because females profit from sperm competition and their remating behaviour selects for males that invest all their reserves into mating effort [35]. In these two examples, the reproductive system is polyandrous, with females remating with several males. Our results demonstrate that even in monandrous species, pre-copulatory sexual selection may lead to male behaviours that severely impact upon their longevity.
There are two possible explanations for why males of monandrous species would have their reproductive ability heavily reduced when exposed to rivals. First, pre-copulatory male–male interactions may be more intense in monandrous species. The increased effort involved in this competition may be reflected in more profound effects on physiological condition, reducing their ability to seek matings. Second, monandry is associated with increased choosiness of females in mating, and increased probability of a virgin female rejecting a male [10,30]. If exposure to rivals impacts on male condition, this reduction in condition may drastically reduce his mating success in the face of highly selective monandrous females. Evidence for increased effects of rivals on condition derives from the profound longevity impact of housing with rivals. Evidence for differential choosiness is suggested by the requirement for female D. subobscura to be provided with nuptial gifts [18], and the lower acceptance rate by D. subobscura females than D. pseudoobscura females even for males kept alone (77 versus 91%, respectively).
Previous work on polyandrous species highlighted the adaptive benefits of plasticity in mating duration following exposure to a rival (reviewed in [15]). We observed that plasticity in mating duration was most profoundly observed in D. subobscura, a monandrous species where there is little or no sperm competition [14,16]. The general deterioration in male condition following housing with rivals provides the most parsimonious explanation for the prolonged copulation duration following exposure to rivals in D. subobscura. In other words, exhausted D. subobscura males mate for longer and die earlier. Fatigue also increases the length of copulation in the wolf spider [36]. Our favoured hypothesis is that rival presence may impact on sleep patterns. Sleep is essential for longevity in D. melanogaster [37]. In our experiment, exposure to rivals led to increased aging rate in this monandrous species, which could indirectly be caused by shortened periods of sleeping. To our knowledge, the results presented in this study are the first to demonstrate a link between exhaustion induced by social interactions, reproductive deficiency and decreased longevity. Our results also imply that plasticity in mating duration can have both adaptive (sperm competitive advantage) and non-adaptive (exhaustion) causes. Thus, selection for plasticity in mating duration may be driven by more complex forces than simply for increasing male success in sperm competition.
In this study, the results for D. pseudoobscura are similar to those in previous published studies, with exposure to a rival increasing latency to mating and copulation duration [21]. However, we found little evidence that exposure to four males had a greater impact on male mating behaviour than exposure to a single rival. This has also been observed in D. melanogaster [38] and is predicted by theory on risk and intensity of sperm competition [39]. The data on response by males to exposure to a previously mated female has to be interpreted cautiously, because focal males are likely to have remated with this female. Drosophila pseudoobscura females typically remate after 4 days [20], and so are likely to have mated with the focal male at least once. Males of this species can mate with three females a day, each day for several days without any loss of ability to mate [40], so the impact of this may have been minor. More detailed experiments would be needed to fully understand the impact of the presence of females on subsequent male mating behaviour in this species.
Our data provide support for the notion that social environment (presence of rivals) has a large impact on condition in a monandrous species. The data are notable because D. subobscura is commonly used as a model of stress tolerance in nature [41–43]. However, stress tolerance studies in this species have focused on thermal effects on genetic and phenotypic polymorphisms of wings and desiccation resistance [43–45]. Our data suggest that experimental analysis of stress impacts should both control and incorporate social environment in their design.
In conclusion, we find remarkably strong impacts of social environment on the ability of males to obtain mates, on their ability to compete with rivals, and on their longevity. These impacts are far stronger in a monandrous species than in a polyandrous relative, which is likely to reflect the importance of pre-copulatory success in monandrous species. We suggest that mating system can be used to predict the costs of social interactions in a species, and this could be useful for the design of effective laboratory studies, and potentially for animal husbandry for conservation or human use.
Acknowledgements
We are grateful to Prof. Michael Ritchie for providing D. subobscura, and to Dr Hope Klug, Dr Hanne Løvlie and one anonymous reviewer for their useful comments on this manuscript.
Data accessibility
The datasets supporting this article have been deposited with Dryad: doi:10.5061/dryad.3nv8n.
Funding statement
This work was funded by a Marie Curie Fellowship to A.L. (FP7 PEOPLE/235645) and a NERC Fellowship (NE/H015604/1) to T.P.
References
- 1.Simmons LW. 2001. Sperm competition and its evolutionary consequences in the insects. Princeton, NJ: Princeton University Press. [Google Scholar]
- 2.Webberley KM, Buszko J, Isham V, Hurst GDD. 2006. Sexually transmitted disease epidemics in a natural insect population. J. Anim. Ecol. 75, 33–43. ( 10.1111/j.1365-2656.2005.01020.x) [DOI] [PubMed] [Google Scholar]
- 3.Holland B, Rice WR. 1999. Experimental removal of sexual selection reverses intersexual antagonistic coevolution and removes reproductive load. Proc. Natl Acad. Sci. USA 96, 5083–5088. ( 10.1073/pnas.96.9.5083) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Chapman T, Arnqvist G, Bangham J, Rowe L. 2003. Sexual conflict. Trends Ecol. Evol. 18, 41–47. ( 10.1016/S0169-5347(02)00004-6) [DOI] [Google Scholar]
- 5.Swanson WJ, Vacquier VD. 2002. The rapid evolution of reproductive proteins. Nat. Rev. Genet. 3, 137–144. ( 10.1038/nrg733) [DOI] [PubMed] [Google Scholar]
- 6.Arnqvist G, Nilsson T. 2000. The evolution of polyandry: multiple mating and female fitness in insects. Anim. Behav. 60, 145–164. ( 10.1006/anbe.2000.1446) [DOI] [PubMed] [Google Scholar]
- 7.Price TAR, Hurst GDD, Wedell N. 2010. Polyandry prevents extinction. Curr. Biol. 9, 471–475. ( 10.1016/j.cub.2010.01.050) [DOI] [PubMed] [Google Scholar]
- 8.Singh SR, Singh BN, Hoenigsberg HF. 2002. Female remating, sperm competition and sexual selection in Drosophila. Genet. Mol. Res. 1, 178–215. [PubMed] [Google Scholar]
- 9.Markow TA, O'Grady P. 2005. Evolutionary genetics of reproductive behavior in Drosophila: connecting the dots. Annu. Rev. Genet. 39, 263–291. ( 10.1146/annurev.genet.39.073003.112454) [DOI] [PubMed] [Google Scholar]
- 10.Harcourt AH, Harvey PH, Larsen SG, Short RV. 1981. Testis size, body weight and breeding system in primates. Nature 293, 55–57. ( 10.1038/293055a0) [DOI] [PubMed] [Google Scholar]
- 11.Wigby S, Sirot LK, Linklater JR, Buehner N, Calboli FCF, Bretman A, Wolfner MF, Chapman T. 2009. Seminal fluid protein allocation and male reproductive success. Curr. Biol. 19, 751–757. ( 10.1016/j.cub.2009.03.036) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fisher DO, Dickman CR, Jones ME, Blomberg SP. 2013. Sperm competition drives the evolution of suicidal reproduction in mammals. Proc. Natl Acad. Sci. USA 110, 17910–17914. ( 10.1073/pnas.1310691110) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gendron CM, Kuo TH, Harvanek ZM, Chung BY, Yew JY, Dierick HA, Pletcher SD. 2013. Drosophila life span and physiology are modulated by sexual perception and reward. Science 343, 544–548. ( 10.1126/science.1243339) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lizé A, Doff RJ, Smaller EA, Lewis Z, Hurst GDD. 2012. Perception of male-male competition influences Drosophila copulation behaviour even in species where females rarely remate. Biol. Lett. 8, 35–38. ( 10.1098/rsbl.2011.0544) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bretman A, Gage MJG, Chapman T. 2011. Quick-change artists: male plastic behavioural responses to rivals. Trends Ecol. Evol. 26, 467–473. ( 10.1016/j.tree.2011.05.002) [DOI] [PubMed] [Google Scholar]
- 16.Fisher DN, Doff RJ, Price TAR. 2013. True polyandry and pseudopolyandry: why does a monandrous fly remate? BMC Evol. Ecol. 13, 157 ( 10.1186/1471-2148-13-157) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bretman A, Westmancoat JD, Gage MJG, Chapman T. 2013. Costs and benefits of lifetime exposure to mating rivals in male Drosophila melanogaster. Evolution 67, 2413–2422. ( 10.1111/evo.12125) [DOI] [PubMed] [Google Scholar]
- 18.Immonen E, Hoikkala A, Kazem AJN, Ritchie MG. 2009. When are vomiting males attractive? Sexual selection on condition-dependent nuptial feeding in Drosophila subobscura. Behav. Ecol. 20, 289–295. ( 10.1093/beheco/arp008) [DOI] [Google Scholar]
- 19.Maynard-Smith J. 1956. Fertility, mating behaviour and sexual selection in Drosophila subobscura. J. Genet. 54, 261–279. ( 10.1007/BF02982781) [DOI] [PubMed] [Google Scholar]
- 20.Price TAR, Hodgson DJ, Lewis Z, Hurst GDD, Wedell N. 2008. Selfish genetic elements promote polyandry in a fly. Science 322, 1241–1243. ( 10.1126/science.1163766) [DOI] [PubMed] [Google Scholar]
- 21.Price TAR, Lizé A, Marcello M, Bretman A. 2012. Experience of mating rivals causes males to modulate sperm transfer, in the fly Drosophila pseudoobscura . J. Insect Physiol. 58, 1669–1675. ( 10.1016/j.jinsphys.2012.10.008) [DOI] [PubMed] [Google Scholar]
- 22.Prevosti A, Ribo G, Serra L, Aguadé M, Balanyà J, Monclus M, Mestres F. 1988. Colonization of America by Drosophila subobscura: experiment in natural populations that supports the adaptive role of chromosomal-inversion polymorphism. Proc. Natl Acad. Sci. USA 85, 5597–5600. ( 10.1073/pnas.85.15.5597) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dobzhansky T, Epling C. 1944. Contributions to the genetics, taxonomy, and ecology of Drosophila pseudoobscura and its relatives. Washington, DC: Carnegie Institution of Washington. [Google Scholar]
- 24.Krimbas C. 1993. Drosophila subobscura: biology, genetics and inversion polymorphism. Hamburg, Germany: Kovac. [Google Scholar]
- 25.Rice WR, Stewart AD, Morrow EH, Linder JE, Orteiza N, Byrne PG. 2006. Assessing sexual conflict in the Drosophila melanogaster laboratory model system. Phil. Trans. R. Soc. B 361, 287–299. ( 10.1098/rstb.2005.1787) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Holman L, Freckleton RP, Snook RR. 2008. What use is an infertile sperm? A comparative study of sperm-heteromorphic Drosophila. Evolution 62, 374–385. ( 10.1111/j.1558-5646.2007.00280.x) [DOI] [PubMed] [Google Scholar]
- 27.Maynard-Smith J. 1955. Fertility, mating behavior and sexual selection in Drosophila subobscura. J. Genet. 84, 261–279. [DOI] [PubMed] [Google Scholar]
- 28.Dobzhansky T, Wright S. 1943. The genetics of natural populations. X. Dispersion rates in Drosophila pseudoobscura. Genetics 28, 304–340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rosewell J, Shorrocks B. 1987. The implication of survival rates in natural populations of Drosophila: capture–recapture experiments on domestic speceis. Bio. J. Linn. Soc. 32, 373–384. ( 10.1111/j.1095-8312.1987.tb00438.x) [DOI] [Google Scholar]
- 30.Chapman T, Liddle L, Kalb J, Wolfner MF, Partridge L. 1995. Cost of mating in Drosophila melanogaster is mediated by male accessory gland products. Nature 373, 241–244. ( 10.1038/373241a0) [DOI] [PubMed] [Google Scholar]
- 31.Ihaka R, Gentleman R. 1996. R: a language for data analysis and graphics. J. Comp. Graph. Stat. 5, 299–314. ( 10.2307/1390807) [DOI] [Google Scholar]
- 32.McCullagh P, Nelder JA. 1989. Generalized linear models, 2nd edn London, UK: Chapman and Hall. [Google Scholar]
- 33.Fowler K, Partridge L. 1989. A cost of mating in female fruitflies. Nature 338, 760–761. ( 10.1038/338760a0) [DOI] [Google Scholar]
- 34.Andrade MCB. 1996. Sexual selection for male sacrifice in the Australian redback spider. Science 271, 70–72. ( 10.1126/science.271.5245.70) [DOI] [Google Scholar]
- 35.Fisher DO, Double MC, Blomberg SP, Jennions MD, Cockburn A. 2006. Post-mating sexual selection increases lifetime fitness of polyandrous females in the wild. Nature 444, 89–92. ( 10.1038/nature05206) [DOI] [PubMed] [Google Scholar]
- 36.Rovner JS, Wright EE. 1975. Copulation in spiders: experimental evidence for fatigue effects and bilateral control of palpal insertions. Anim. Behav. 23, 233–236. ( 10.1016/0003-3472(75)90069-X) [DOI] [PubMed] [Google Scholar]
- 37.Bushey D, Hughes KA, Tononi G, Cirelli C. 2010. Sleep, aging, and lifespan in Drosophila. BMC Neurosci. 11, 56–74. ( 10.1186/1471-2202-11-56) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Bretman A, Fricke C, Hetherington P, Stone R, Chapman T. 2010. Exposure to rivals and plastic responses to sperm competition in Drosophila melanogaster. Behav. Ecol. 21, 317–321. ( 10.1093/beheco/arp189) [DOI] [Google Scholar]
- 39.Parker GA, Ball MA, Stockley P, Gage MJG. 1996. Sperm competition games: individual assessment of sperm competition intensity by group spawners. Proc. R. Soc. Lond. B 263, 1291–1297. ( 10.1098/rspb.1996.0189) [DOI] [Google Scholar]
- 40.Wu CI. 1983. Virility deficiency and the sex-ratio trait in Drosophila pseudoobscura. I. Sperm displacement and sexual selection. Genetics 105, 651–662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hoffmann AA. 2010. Physiological climatic limits in Drosophila: patterns and implications. J. Exp. Biol. 213, 870–880. ( 10.1242/jeb.037630) [DOI] [PubMed] [Google Scholar]
- 42.Rego C, Balanyà J, Fragata I, Matos M, Rezende EL, Santos M. 2010. Clinal patterns of chromosomal inversion polymorphisms in Drosophila subobscura are partly associated with thermal preferences and heat stress resistance. Evolution 64, 385–397. ( 10.1111/j.1558-5646.2009.00835) [DOI] [PubMed] [Google Scholar]
- 43.Huey RB, Gilchrist GW, Carlson ML, Berrigan D, Serra L. 2000. Rapid evolution of a geographical cline in size in an introduced fly. Science 287, 308–309. ( 10.1126/science.287.5451.308) [DOI] [PubMed] [Google Scholar]
- 44.Gilchrist GW, Huey RB, Serra L. 2001. Rapid evolution of wing size clines in Drosophila subobscura. Genetica 112–113, 273–286. ( 10.1023/A:1013358931816) [DOI] [PubMed] [Google Scholar]
- 45.Gilchrist G, Jeffers L, West B, Folk D, Suess J, Huey RB. 2008. Clinal patterns of desiccation and starvation resistance in ancestral and invading populations of Drosophila subobscura. Evol. Appl. 1, 513–523. ( 10.1111/j.1752-4571.2008.00040.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets supporting this article have been deposited with Dryad: doi:10.5061/dryad.3nv8n.