Abstract
In recent years, natural and anthropogenic factors have increased aquatic hypoxia the world over. In most organisms, the cellular response to hypoxia is mediated by the master regulator hypoxia-inducible factor-1 (HIF-1). HIF-1 also plays a critical role in the normal development of the cardiovascular system of vertebrates. We tested the hypothesis that hypoxia exposures which resulted in HIF-1 induction during embryogenesis would be associated with enhanced hypoxia tolerance in subsequent developmental stages. We exposed zebrafish (Danio rerio) embryos to just 4 h of severe hypoxia or total anoxia at 18, 24 and 36 h post-fertilization (hpf). Of these, exposure to hypoxia at 24 and 36 hpf as well as anoxia at 36 hpf activated the HIF-1 cellular pathway. Zebrafish embryos that acutely upregulated the HIF-1 pathway had an increased hypoxia tolerance as larvae. The critical window for hypoxia sensitivity and HIF-1 signalling was 24 hpf. Adult male fish had a lower critical oxygen tension (Pcrit) compared with females. Early induction of HIF-1 correlated directly with an increased proportion of males in the population. We conclude that mounting a HIF-1 response during embryogenesis is associated with long-term impacts on the phenotype of later stages which could influence both individual hypoxia tolerance and population dynamics.
Keywords: Hypoxia-inducible factor-1 cellular pathway, developmental plasticity, hypoxia tolerance, sex ratio bias, zebrafish
1. Introduction
Low environmental oxygen (hypoxia) represents a major selective pressure in vertebrate evolution, particularly in aquatic ecosystems, driving morphological, physiological and behavioural adaptations [1]. In recent years, the prevalence and intensity of aquatic hypoxic episodes has increased worldwide [2]. Many systems that are particularly prone to both chronic and diurnal hypoxia (i.e. slow-moving, shallow and warm waters) are also ideal nursery habitats for aquatic vertebrates [3]. Developing organisms use their early environment to predict the environmental conditions they will most likely encounter as adults and adjust their developmental trajectory accordingly to produce traits best suited for those environments [4]. For example, there is some evidence that early exposure to low oxygen (O2) may increase hypoxia tolerance. When parental zebrafish (Danio rerio) are exposed to two to six weeks of low O2, their offspring maintain equilibrium longer in severe hypoxia [5]. Additionally, zebrafish reared chronically in mild hypoxia maintain resting metabolic rates at a lower level of dissolved O2 (DO; [6]). It appears that early exposure to hypoxia may prime aquatic animals to better cope with hypoxic environments they may face as adults.
Given the natural variation in both the frequency and duration of hypoxic episodes in aquatic ecosystems, developing fishes are likely to experience fluctuating levels of low DO [3]. It is well known that the level of low O2 that fishes can tolerate changes throughout embryogenesis [7–9]. Additionally, in mammals, birds and invertebrates, the downstream phenotypic effects of early hypoxia are developmental stage-specific [10,11]. However, the importance of the timing of early hypoxic events and the mechanisms driving developmental plasticity of hypoxia tolerance in fish embryos are poorly understood. It is particularly important to understand this dimension of hypoxia stress because frequent, acute bouts of hypoxia are thought to be more stressful and cause greater physiological impairment than chronic low O2 environments [3].
One potential mechanism that could mediate developmental plasticity of hypoxia tolerance lies in the highly conserved hypoxia stress response. In all animals, the cellular response to low O2 is thought to be mediated by hypoxia-inducible factors (HIFs; [12,13]). HIFs are heterodimeric compounds with hypoxia sensitive α-subunits [12]. HIF-1 is a master regulator transcription factor that controls the expression of many genes that act downstream to increase O2 uptake and decrease O2 demand. These include the growth-suppressing insulin-like growth-factor-binding protein-1 (igfbp-1; [14]), the haematopoietic regulating hormone erythropoietin (epo; [15]) and the angiogenesis-promoting cytokine vascular endothelial growth factor (vegf; [16]). In addition to its role in the cellular hypoxia response, HIF-1 is required for the regulation of many of its target genes during ontogeny [10,17] and is essential for proper development of vertebrate cardiovascular systems [17,18]. It is therefore likely that an induction of HIF-1 in response to hypoxia during development could have numerous long-term consequences.
In this study, we tested the hypothesis that zebrafish embryos exposed to acute low O2 during critical stages of development would exhibit enhanced hypoxia tolerance in subsequent developmental stages. Furthermore, we hypothesized that this enhanced tolerance would be associated with an induction of HIF-1 in response to low O2 during embryogenesis. To test these hypotheses, we exposed embryos to acute (4 h) episodes of severe hypoxia (5% DO) or total anoxia (less than 0.5% DO). We targeted three specific stages of embryogenesis (18, 24 and 36 h post-fertilization; hpf). At 18 hpf, zebrafish embryos are highly anoxia tolerant though their cardiovascular and nervous systems are just beginning to form [19]. By 24 hpf, the heart tube is developed and heart rate is initiated [20]. At this stage, anoxia tolerance declines and embryos are known to upregulate 148 genes in response to severe hypoxia (10% DO; [21]). Finally, by 36 hpf, the cardiovascular system is relatively well developed with circulating red blood cells [22], anoxia tolerance is diminished and embryos are hatching competent, though they generally will not hatch until 48 hpf [19].
2. Material and methods
(a). Animals
Fertilized eggs were collected from divided chamber breeding baskets with four adult males and four adult females from a breeding colony of zebrafish, housed at the University of Guelph, Hagen Aqualab. For each experiment, eggs from four to five breeding baskets were pooled to increase genetic diversity. Fish raised to adulthood originated from two to three separate breeding events. Adult fish were maintained at 26°C with a 14 L : 10 D photoperiod. Developmental staging was performed as previously described [23].
(b). Experimental design
Embryos at 18, 24 or 36 hpf were exposed to one of three O2 regimes for 4 h: a control treatment (normoxia; DO: 95% or approx. 7.2 mg l−1), hypoxic treatment (DO: 5% or 0.4 mg l−1) and anoxic treatment (DO: less than 0.5% or 0.04 mg l−1). Preliminary experiments showed that low O2 treatments caused 2–4 h developmental delays (electronic supplementary material, figure S1) so an additional developmentally matched control group (DO: 95%) was included. Water O2 was controlled by regulating delivery of N2 gas or air through ceramic air stones into 20 l tanks and monitored using an O2 electrode (Liquid Dissolved O2 Field Probe, Hach, Loveland, CO, USA). Embryos were sampled immediately after exposure or allowed to recover in normoxia for an additional 4 h and stored at −80°C for later analysis. Finally, embryos exposed to the same O2 conditions described above were reared in normoxia to 4 dpf (larvae) or sexual maturity (six months post-fertilization), when their hypoxia tolerance was determined. Briefly, larvae were transferred to 1 l flow-through tanks at 4 dpf. At 5 dpf, larvae were fed 30 μm rotifer replacement supplements (Argent Chemical Laboratories, Redmond, WA, USA) twice a day and maintained on this diet until 20 dpf when they were fed a mixture of newly hatched Artemia and TetraMin fish flakes (Tetra, Blacksburg, VA, USA). Animal density was maintained between tanks.
(c). Protein extraction
To quantify whole embryo levels of HIF-1α protein, groups of 20 embryos (n = 6) were thawed in radio immune-precipitation assay lysis buffer with protease inhibitors (1 mM PMSF; 2 mM EDTA) and dispersed with a 1 ml Pasteur pipette to disrupt the chorion and yolk. Samples were briefly centrifuged (8000g × 2 min) to separate the yolk proteins and protein concentration was determined using a Bradford assay (BioRad Protein Assay Dye Reagent, BioRad, Hercules, CA, USA).
(d). Hypoxia-inducible factor-1α western blots
Forty micrograms of total protein was separated by 12% SDS-poly-acrylamide gel electrophoresis and transferred to PVDF membranes. Blots were probed with an affinity-purified polyclonal antibody generated in rabbits against full-length zebrafish HIF-1α (1 : 500; [24]) and HRP-conjugated goat anti-rabbit IgG secondary antibody (1 : 2000, Santa Cruz Biotechnology, Santa Cruz, CA, USA). Blots were imaged colorimetrically with the ChemiDocXRS + system (BioRad) ensuring that only non-saturated bands were quantified. Band density was determined via densitometry with ImageJ software (US National Institute of Health, Bethesda, MD, USA) and was expressed relative to total protein which was determined by subsequently staining blots with GelCode Blue Safe Stain Reagent (Thermo Scientific, Waltham, MA, USA; [25]). To compare between blots, all samples were normalized to a single sample (hypoxia exposed embryos at 24 hpf) ran on every gel. Western blot analysis of zebrafish fibroblast cells [26], 36 hpf zebrafish larvae and full-length recombinant zebrafish HIF-1α using anti-zebrafish HIF-1α antibody revealed specific binding at the expected molecular mass of 86 kDa as well as cross reaction to non-specific proteins (electronic supplementary material, figure S2). Densiometric analysis of the non-specific bands showed that the expression of these proteins was not affected by the low O2 treatments. Similarly, inclusion of a 100 kDa non-specific band (figures 1–3) with the 86 kDa HIF-1α band in a separate densiometric analysis did not affect the overall differences in HIF-1α signal between the different treatments. Therefore, only the 86 kDa band was used for quantification of HIF-1α levels at all developmental stages. The increase in HIF-1α band density and width in response to low O2 (figures 1–3; electronic supplementary material, figure S2) is probably a result of post-translational modifications of the stabilized protein [27,28].
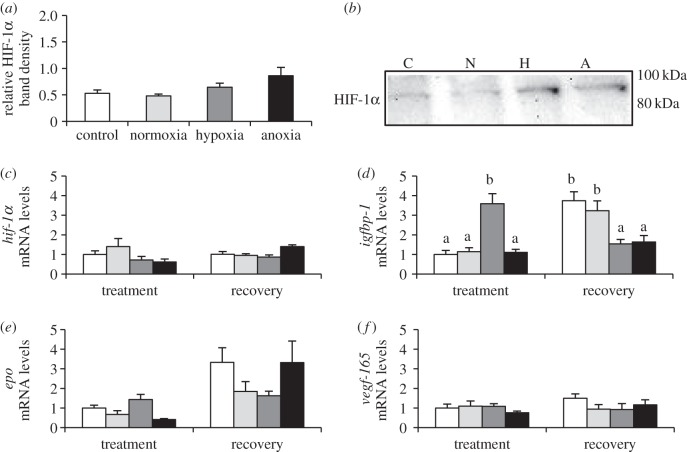
Figure 1.
HIF-1 cellular response of zebrafish (D. rerio) embryos to 4 h of hypoxia (5% DO; H) or anoxia (less than 0.5% DO; A) at 18 hpf. (a) HIF-1α levels relative to total protein immediately following treatment. (b) Representative western blot of HIF-1α protein band at 86 kDa. (c) Hif-1α mRNA and (d–f) HIF-1 target gene mRNA immediately following treatment and after 4 h of recovery at 95% DO. To account for developmental delays caused by low O2 both developmentally matched (control; C) and time-matched (normoxia; N) control groups are included. Values are mean ± s.e.m. (n = 6). Significant differences are indicated by dissimilar letters (one- or two-way ANOVA and Tukey's post hoc test, p < 0.05).
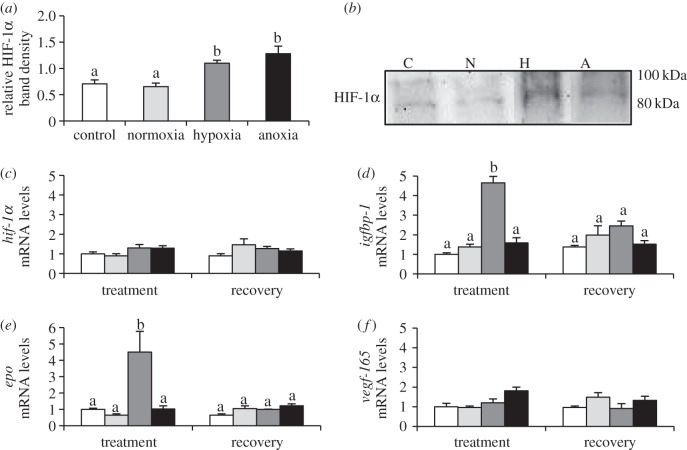
Figure 2.
HIF-1 cellular response of zebrafish (D. rerio) embryos to 4 h of hypoxia (5% DO; H) or anoxia (less than 0.5% DO; A) at 24 hpf. See figure 1 for legend.
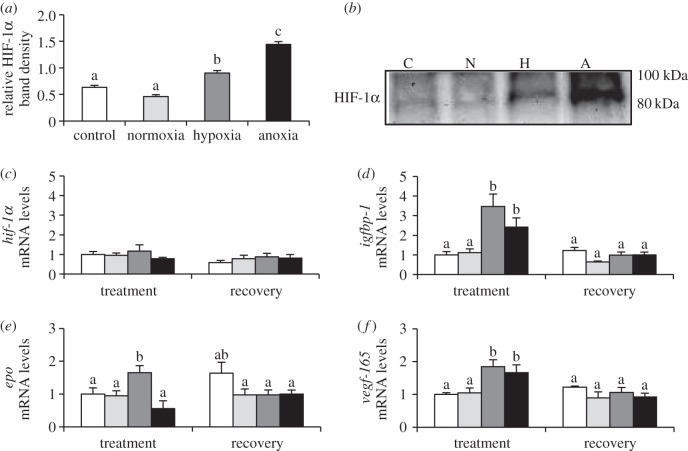
Figure 3.
HIF-1 cellular response of zebrafish (D. rerio) embryos to 4 h of hypoxia (5% DO; H) or anoxia (less than 0.5% DO; A) at 36 hpf. See figure 1 for legend.
(e). Quantification of mRNA levels of hypoxia-inducible factor-1 target genes
Total RNA was extracted from pools of 20 embryos using QIAzol Lysis Reagent (QIAGEN, Toronto, Ontario, Canada). Whole embryo mRNA levels of igfbp-1, epo, hif-1α, ef1α and the embryonic isoform of vegf (vegf-165; [24]) were quantified using quantitative real time PCR as previously described [29]. For epo, 2× TaqMan Gene Expression Master Mix (Applied BioSystems, Foster City, CA, USA) was used. For cycling conditions, see the electronic supplementary material, figure S3. Gene expression was normalized to the housekeeping gene ef1-α and quantified as previously described. All primer sets had an amplification efficiency more than 80% (electronic supplementary material, table S1).
(f). l-lactate concentrations
Lactate concentrations (pools of 10 embryos) were determined spectrophotometrically [30].
(g). Routine metabolic rate, regulation index, O2 uptake limit and Pcrit
Flow-through glass respirometry chambers were used to measure routine metabolic rate (Mr), regulation index (RI; [31]) and the % DO at which O2 consumption ceases (O2 uptake limit; [32]) in 4 dpf larvae, and to measure Mr and the critical O2 tension (Pcrit) in single adult fish. Chambers were sealed following a 1 h (larvae) or 2 h (adults) acclimatization period, and O2 consumption was recorded using a 25 μm tip DO microelectrode (larvae; Unisense, Aarhus, Denmark) or a Clark-type DO probe and Logger Pro software (adults; Vernier, Beaverton, OR, USA) for 3 h. O2 consumption rates were calculated using the slope of DO versus time at 100, 75, 50, 40, 30, 20, 15 10 and 5% DO. Mr was calculated using the slope between 100 and 70% DO. For adults, Pcrit was calculated using Physiological Regulation and Conformation Regress software (Wake Forest University, NC, USA; [33]). For larvae, we used RI, a measure of the degree to which an organism conforms to environmental O2 levels, as an indicator of hypoxia tolerance. Briefly, RI = 1 for a perfect regulator and RI = 0 for a perfect conformer [34]. Finally, quadratic equations were fitted to O2 consumption rate versus DO curves (mean r2 = 0.95) and the O2 uptake limit values were calculated as the level of DO where O2 consumption ceases [32].
(h). Loss of equilibrium
Individual adult zebrafish were placed in a 1 l airtight, plastic container, filled with hypoxic water (5% DO). This insured that fish were unable to perform aquatic surface respiration. Time to loss of equilibrium (LOE) was determined as previously described [5].
(i). Sex determination
All adult fish were sexed based on external morphology. The accuracy of this method was confirmed by dissecting a random subset of fish (n = 133–140 embryonic stage−1), fixed in 4% paraformaldehyde in phosphate buffered saline (0.2% Tween20).
(j). Statistical analysis
All data are presented as mean ± s.e.m. Significant differences between O2 treatments within a developmental stage were analysed by one-way ANOVA for HIF-1α protein, lactate, larval Mr, RI and O2 uptake limit. Larval O2 consumption versus environmental DO was analysed with two-way repeated measures ANOVAs. Two-way ANOVAs were used to determine the effects of time and O2 treatment on mRNA expression. At 18 and 36 hpf, igfbp-1 gene expression data were log-transformed to meet assumptions of normality. Two-way ANOVAs were also used to determine the effects of embryonic O2 treatment and sex on adult Mr, Pcrit and time to LOE. Adult population sex ratios were compared against an expected outcome of 50% male using χ2 analysis. If significant main effects were determined, then Tukey's post hoc test was used for further analysis.
3. Results
(a). Cellular response
At 18 hpf, both hypoxia and anoxia treatments caused a small non-significant induction of HIF-1α protein (figure 1a; p = 0.054) with no changes in hif-1α mRNA (figure 1c). Only igfbp-1 was induced during hypoxia exposure (p = 0.002, p < 0.001) and was lower than either of the control levels following 4 h of recovery (figure 1d; p = 0.048, p = 0.027). Note that treated groups differed from both a time-matched (normoxia) and developmental control (control), and the p-values are given in that order.
At 24 hpf, both hypoxia and anoxia caused a twofold increase in HIF-1α protein relative to controls (figure 2a; hypoxia, p = 0.013, p = 0.040; anoxia, p < 0.001, p = 0.002); however, there was no change in hif-1α mRNA expression (figure 2c). Hypoxia exposure caused a fivefold induction of igfbp-1 (figure 2d, p < 0.001, p < 0.001) and epo (figure 2e, p < 0.001, p < 0.001), both of which returned to control levels after 4 h of recovery.
Embryos exposed to both hypoxia and anoxia at 36 hpf accumulated significant levels of HIF-1α protein, 1.5- to 2.5-fold of both controls (figure 3a; hypoxia, p < 0.001, p = 0.024; anoxia, p < 0.001, p < 0.001). Again, neither of these low O2 treatments caused hif-1α expression levels to change (figure 3c). At 36 hpf, both hypoxia and anoxia exposure resulted in an increased expression of multiple HIF-1 target genes (figure 3d–f). Hypoxia exposure caused a two- to fourfold induction of igfbp-1, epo and vegf-165 (p < 0.001, p < 0.001; p = 0.005, p = 0.034; p = 0.003, p = 0.006), while embryos exposed to anoxia upregulated igfbp-1 and vegf-165 (p = 0.018, p = 0.034; p = 0.044, p = 0.027) by twofold. Anoxia exposure also resulted in a significant twofold accumulation of tissue lactate (table 1; p = 0.010, p = 0.013).
Table 1.
Whole zebrafish embryo lactate concentration (μmol g−1) in response to 4 h of normoxia (control), hypoxia (5% DO) or anoxia (less than 0.5% DO). (Within a developmental stage, treatments that do not share a common letter are statistically different as determined by one-way ANOVA and Tukey's post hoc test (p < 0.05). Data are mean ± s.e.m. (n = 8).)
| developmental stage (hpf) | developmental control | time-matched control | hypoxia | anoxia |
|---|---|---|---|---|
| 18 | 11.85 ± 1.70a | 14.97 ± 1.59a | 12.72 ± 2.01a | 12.60 ± 1.65a |
| 24 | 10.40 ± 2.15a | 12.53 ± 2.11a | 17.42 ± 1.57a | 13.75 ± 2.48a |
| 36 | 9.09 ± 2.12a | 11.09 ± 1.43a | 15.95 ± 1.24ab | 18.42 ± 1.83b |
(b). Larval hypoxia tolerance
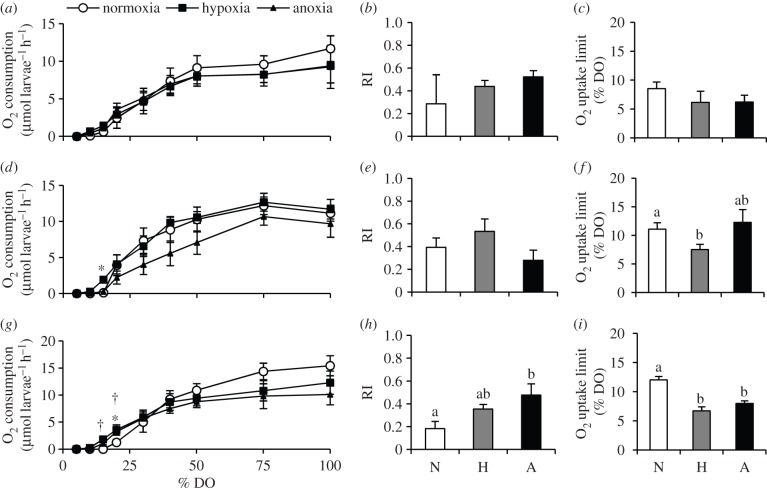
The Mr of 4 dpf larvae was similar across all groups regardless of prior low O2 treatment as embryos and they were able to regulate Mr to a certain degree until approximately 40–50% DO (figure 4a,d,g). Below this point, larvae were O2 conformers (figure 4b,e,h) and O2 uptake generally ceased below 15% DO (figure 4c,f,i). Larvae exposed to hypoxia or anoxia at 18 hpf had similar O2 consumption patterns to control larvae (figure 4a–c). However, those larvae that had been previously exposed to hypoxia at 24 hpf had greater rates of O2 consumption at 15% DO than either anoxia or control (figure 4d) and were able to maintain O2 uptake at significantly lower levels of DO (figure 4f; p = 0.047). Both anoxia and hypoxia exposure at 36 hpf resulted in higher rates of O2 consumption than the control at both 20 and 15% DO (figure 4g) and these larvae were able to maintain O2 uptake at significantly lower environmental O2 levels than controls (figure 4i, p < 0.001). Anoxia exposure at 36 hpf also resulted in a significantly higher RI (better O2 regulator; p = 0.028) than controls (figure 4h).
Figure 4.
O2 consumption, RI and O2 uptake limit of zebrafish (D. rerio) larvae (4 dpf) exposed as embryos at 18 (a–c), 24 (d–f) or 36 (g–i) hpf to 4 h of normoxia (N), hypoxia (5% DO; H), or anoxia (less than 0.5% DO; A). Values are mean ± s.e.m. (n = 6) and are representative of measurements from pools of 20 larvae. The entire O2 consumption curves were used to calculate the RI values. Patterns were analysed with two-way RM ANOVA. Asterisks and dagger symbols indicate that hypoxia and anoxia treatments differs from normoxia, respectively. For RI and O2 uptake limit values, significant differences are indicated by dissimilar letters (one-way ANOVA and Tukey post hoc test, p < 0.05).
(c). Adult hypoxia tolerance
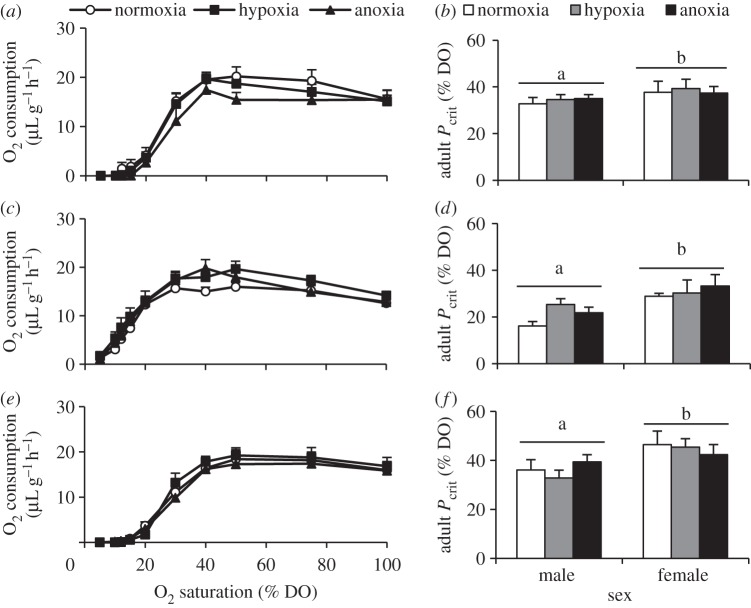
Survival rates were similar across all groups (40.1% ± 3.3%, data not shown) and mortalities primarily occurred during larval development in all treatments. Adult fish, exposed as embryos to hypoxia or anoxia, had similar Mr to control fish regardless of the developmental stage of their exposure (figure 5a,c,e). As with the larvae, adult Pcrit was not affected by exposure to low O2 at 18 hpf (figure 5b). In contrast to larvae, there was no effect of hypoxia or anoxia exposure on Pcrit of adult fish exposed at 24 or 36 hpf (figure 5d,f). There was a significant effect of sex on hypoxia tolerance where, regardless of embryonic O2 treatment, female fish always had a higher Pcrit than males (figure 5b–f; 18 hpf, p = 0.022; 24 hpf, p = 0.003; 36 hpf, p = 0.017). There was no effect of embryonic exposure to low O2 on LOE regardless of the timing of the exposure (electronic supplementary material, table S2).
Figure 5.
O2 consumption and critical O2 tension (Pcrit) of zebrafish (D. rerio) adults exposed as embryos at 18 (a,b), 24 (c,d) or 36 (e,f) hpf to 4 h of hypoxia (5% DO) or anoxia (less than 0.5% DO). Values are mean ± s.e.m. (O2 consumption, n = 8, Pcrit, n = 4). Significant differences are indicated by dissimilar letters (one-way ANOVA and Tukey post hoc test, p < 0.05).
(d). Sex ratios
Fish that had been previously exposed to hypoxia at 24 hpf were 72.5% male (p < 0.01) compared with control or anoxia-exposed fish which were 47% and 55% male, respectively (table 2). Fish exposed to anoxia at 36 hpf were also male-biased at 71% compared with 41% males in the control group (p < 0.05). There were no changes in sex ratios of adult fish treated at 18 hpf.
Table 2.
Population sex ratios of adult zebrafish exposed as embryos to 4 h of normoxia (control), hypoxia (5% DO) or anoxia (less than 5% DO). (Asterisk (*) indicates significantly different from an expected χ2 value of 50% (p < 0.05).)
| embryonic stage when treated (hpf) | control |
hypoxia |
anoxia |
|||
|---|---|---|---|---|---|---|
| male (%) | female (%) | male (%) | female (%) | male (%) | female (%) | |
| 18 (N = 140) | 47 | 53 | 50 | 50 | 44 | 56 |
| 24 (N = 133) | 47 | 53 | 73* | 27* | 55 | 45 |
| 36 (N = 133) | 41 | 59 | 61 | 39 | 71* | 29* |
4. Discussion
We have shown that acute hypoxia exposure causes zebrafish embryos to mount differential cellular responses based on developmental stage and severity of O2 deprivation. Early in development (18 hpf), exposure to low O2 did not elicit a HIF-1 response, however as zebrafish age and become increasingly more sensitive (36 hpf), they are HIF-1 responsive to low O2. Our study has identified 24 hpf as the start of the critical window for hypoxia sensitivity as embryos at this stage initiate HIF-1 signalling to hypoxia (5% DO) but not anoxia (less than 0.5% DO). These HIF-1 responsive fish display marked increases in hypoxia tolerance as free-swimming larvae and had a greater propensity to become males. To our knowledge, this is the only study to directly compare the effects of HIF-1 induction at different developmental stages in any vertebrate. These data also indicate that activation of HIF-1 during early development corresponds with altered larval and adult phenotypes of zebrafish.
(a). Embryonic cellular hypoxia response
In order for a stressor to alter the developmental trajectory of an organism, it must: (i) occur within a specific critical window of development, and (ii) meet the innate response threshold of a system [35]. We have identified both the critical developmental window and the response threshold for HIF-1 by exposing zebrafish embryos at different developmental stages to different severities of low O2. We have strong evidence of HIF-1 activity in response to hypoxia at 24 hpf and both hypoxia and anoxia at 36 hpf. These embryos accumulated significant amounts of HIF-1α protein which corresponded with two- to fivefold increases in the expression of multiple HIF-1 target genes. Twenty-four hours post-fertilization occurs just prior to the onset of the first heart beat [20]. In mammalian systems, HIF-1 is required for the vascularization of the heart and inhibition of HIF-1 during cardiac hypertrophy leads to heart failure [36]. The onset of HIF-1 responsiveness at this stage could be a cardio-protective mechanism.
We have also demonstrated that the response threshold for HIF-1 activation changes with development. At 24 hpf, anoxia exposure does not appear to result in a HIF-1-mediated cellular response, although there is a clear response by 36 hpf. We did observe accumulation of HIF-1α protein in anoxia-exposed embryos at 24 hpf but there were no corresponding changes in any of the HIF-1 target genes measured. Interestingly, roughly half of the zebrafish embryos exposed to anoxia at 36 hpf hatched during the treatment (electronic supplementary material, figure S4). At 36 hpf, embryos exposed to anoxia also accumulate significant amounts of lactate, probably as a result of their active efforts to hatch. Embryos at 24 hpf are not hatching competent and therefore cannot use this strategy to avoid anoxia. These data suggest that until hatching competency is reached, there is an energetic trade-off below a DO limit where mounting a HIF-1 response is not beneficial. Mechanistically, this trade-off could be mediated by other low O2-sensitive transcription factors such as HIF-2 or p53 which can both out-compete HIF-1 for its required cofactors and targets [37–39].
Differential hypoxia dosage thresholds within a critical window have been previously identified in chicken embryos [40] where different levels of hypoxia were required to alter different physiological traits. This is particularly noteworthy as our results suggest that HIF-1 regulates different genes based on the level of DO and developmental timing.
(b). Plasticity of larval hypoxia tolerance
In general, zebrafish larvae show a higher degree of O2 conformation than adults within their tolerable range of environmental DO. This is particularly true below 40–50% DO; however, we have shown that there is a level of plasticity in the degree to which they conform under severely hypoxic conditions. Regardless of O2 level or developmental stage of treatment, acute induction of the HIF-1 pathway was correlated with an increased ability to regulate and maintain O2 uptake in hypoxia in larvae at 4 dpf. Conversely, larvae that did not have a HIF-1 response to low O2 during development had similar capacity to regulate O2 uptake to normoxic controls. These findings support our hypothesis that the plasticity of hypoxia tolerance is associated with the induction of HIF-1 during critical developmental windows.
There are several factors that can influence hypoxia tolerance in fishes which could explain the differences observed in our study. Mandic et al. [41] found that 70% of variation in Pcrit in intertidal sculpin can be explained by differences in Mr, haemoglobin–O2 (Hb–O2) binding affinity or gill-surface area. We found that all previously exposed larvae had similar routine Mr suggesting that routine Mr is not a driving factor in this case. Alternatively, HIF-1 regulates the expression of Hb isoforms. However, young larvae may not use Hb to transport O2 and instead rely on passive diffusion [42]. Therefore, while altered Hb–O2 binding may contribute to increased hypoxia tolerance at 4 dpf, it seems unlikely to be the sole mechanism.
Finally, respiratory surface area may be a factor in increased hypoxia tolerance in larvae. The skin is the main respiratory surface for larval zebrafish before 14 dpf [43], and an increase in the capacity for epidermal O2 uptake would be analogous to increased gill-surface area in adult fish. Hypoxia exposure remodels larval amphibian skin to be thinner and more highly vascularized, increasing gas exchange efficiency [44]. It is possible that increased hypoxia tolerance in zebrafish larvae is partially a result of HIF-1-mediated skin remodelling. These changes could confer a competitive advantage for larvae that forage for food and actively avoid predators in hypoxic waters.
(c). Adult hypoxia tolerance
Although we have shown that larval differences in hypoxia tolerance were not sustained to adulthood, we were surprised to find a sex difference in adult Pcrit. To our knowledge, this is the first study to report that male zebrafish have a higher hypoxia tolerance (i.e. lower Pcrit) than females. Rees et al. [45] and colleagues found that female zebrafish had slightly higher survival in lethal hypoxia than males, however this reversed following acclimatization to mild hypoxia. Reardon & Chapman found similar sex differences in Pcrit of Pseudocrenilabrus multicolor that corresponded with differences in Mr, whereas exposure to chronic hypoxia during development eliminated this sex effect [46]. In our study, differences in Pcrit were not driven by resting Mr which was similar between sexes. Correspondingly, we found that populations of fish that mounted a HIF-1 response to low O2 during development had a higher proportion of males as adults. Therefore, induction of the cellular hypoxia response during early development results in an improved hypoxia tolerance at the population level.
Hypoxia-induced, male-biased populations of fishes have been observed in laboratory and wild populations of fishes living in chronic hypoxic dead-zones [47–50]. In every case, this has been linked to increased testosterone production as a result of impaired aromatase activity which has been observed [47–50]. Interestingly, aromatase activity is regulated by HIF-1β, therefore it is possible that the activation of HIF-1 could indirectly disrupt steroid hormone production during embryogenesis [51]. Although the low O2 exposures in this study occurred long before sexual differentiation in zebrafish (approx. 10 dpf, [52]), in rainbow trout differences in brain aromatase activity are present in males and females long before gonadal development [53]. Exposure to environmental oestrogens prior to hatching is also known to affect zebrafish sex determination [54]. Therefore, it is possible that even acute disruption of oestrogen production during embryogenesis could alter sex determination. Alternatively, differential survivorship between sexes during larval development may have resulted in the biased sex ratios observed in this study, but this is unknown.
Given that males had a lower Pcrit, a male-biased zebrafish population would have an advantage in acute hypoxic conditions. In this respect, hypoxia-induced sex ratio imbalances could be adaptive in fluctuating O2 environments. However, reproductive output of a population is limited by the number of females. Therefore, male-biased populations may prove mal-adaptive for long-term reproductive fitness in chronically hypoxic dead-zones caused by eutrophication [46].
5. Conclusion
The embryonic cellular response to low O2 can be influenced by both developmental stage and the severity of O2 deprivation that they encounter. We have shown evidence for the induction of the HIF-1 pathway in embryos exposed to low O2 at around the timing of cardiac maturation. Our data suggest that very acute induction of the HIF-1 pathway during embryogenesis alters larval hypoxia tolerance. Additionally, our data indicate that a secondary effect of HIF-1 may alter sex determination. These gender biases could improve population hypoxia tolerance while limiting reproductive output, only adding to the importance of understanding the many aspects of hypoxia-induced developmental plasticity.
Acknowledgements
We wish to thank Dr Andreas Heyland for his valuable comments on experimental design and Dr Nina Jones for the use of the ChemiDocXRS + blot-imaging system.
All animal care and experimentation was conducted according to the University of Guelph animal care protocol no. 1256.
Data accessibility
All data used in this paper have been archived in the dryad repository (doi:10.5061/dryad.3ks4h).
Funding statement
This work was financially supported by a Natural Sciences and Engineering Research Council (NSERC) of Canada postgraduate scholarship (C.E.R.), by Tiroler Wissenschaftsfond (L.K.) and by NSERC Discovery grants (P.A.W., N.J.B.).
References
- 1.Romer AS. 1958. Tetrapod limbs and early tetrapod life. Evolution 12, 365–369. ( 10.2307/2405858) [DOI] [Google Scholar]
- 2.Diaz RJ, Rosenberg R. 1995. Marine benthic hypoxia: a review of its ecological effects and the behavioural responses of benthic macrofauna. Oceanogr. Mar. Biol. 33, 245–303. [Google Scholar]
- 3.Diaz RJ, Breitburg DL. 2009. The hypoxic environment. In Hypoxia (eds Richards JG, Farrell AP, Brauner CJ.), pp. 2–17. London, UK: Academic Press. [Google Scholar]
- 4.West-Eberhard MJ. 2005. Developmental plasticity and the origin of species differences. Proc. Natl Acad. Sci. USA 102, 6543–6549. ( 10.1073/pnas.0501844102) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ho H, Burggren WW. 2012. Parental hypoxic exposure confers offspring hypoxia resistance in zebrafish (Danio rerio). J. Exp. Biol. 215, 4208–4216. ( 10.1242/jeb.074781) [DOI] [PubMed] [Google Scholar]
- 6.Barrionuevo WR, Fernandes MN, Rocha O. 2010. Aerobic and anaerobic metabolism for the zebrafish, Danio rerio, reared under normoxia and hypoxic conditions and exposed to acute hypoxia during development. Braz. J. Biol. 70, 425–434. ( 10.1590/S1519-69842010000200027) [DOI] [PubMed] [Google Scholar]
- 7.Alderice DF, Wickett WP, Brett JR. 1958. Some effects of temporary exposure to low dissolved oxygen in Pacific salmon eggs. J. Fish. Res. Board Can. 15, 229–250. ( 10.1139/f58-013) [DOI] [Google Scholar]
- 8.Rombough PJ. 1988. Growth, aerobic metabolism, and dissolved oxygen requirements of embryos and alevins of steelhead, Salmo gairdneri . Can. J. Zool. 66, 651–660. ( 10.1139/z88-097) [DOI] [Google Scholar]
- 9.Barrionuevo WR, Burggren WW. 1999. O2 consumption and heart rate in developing zebrafish (Danio rerio): influence of temperature and ambient O2. Am. J. Physiol. 276, R505–R513. [DOI] [PubMed] [Google Scholar]
- 10.Dunwoodie SL. 2009. The role of hypoxia in development of the mammalian embryo. Dev. Cell. 17, 755–773. ( 10.1016/j.devcel.2009.11.008) [DOI] [PubMed] [Google Scholar]
- 11.Heinrich EC, Farzin M, Klok CJ, Harrison JF. 2011. The effect of developmental stage on the sensitivity of cell and body size to hypoxia in Drosophila melanogaster. J. Exp. Biol. 214, 1419–1427. ( 10.1242/jeb.051904) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Semenza GL, Wang GL. 1992. A nuclear factor induced by hypoxia via de novo protein synthesis binds to the human erythropoietin gene enhancer at a site required for transcriptional activation. Mol. Cell Biol. 12, 5447–5454. ( 10.1128/MCB.12.12.5447) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rytkonen KT, Williams TA, Renshaw GM, Primmer CR, Nikinmaa M. 2011. Molecular evolution of the metazoan PHD-HIF oxygen sensing system. Mol. Biol. Evol. 28, 1913–1926. ( 10.1093/molbev/msr012) [DOI] [PubMed] [Google Scholar]
- 14.Kajimura S, Aida K, Duan C. 2006. Understanding hypoxia-induced gene expression in early development: in vitro and in vivo analysis of hypoxia-inducible factor 1- regulated zebra fish insulin-like growth factor binding protein 1 gene expression. Mol. Cell Biol. 26, 1142–1155. ( 10.1128/MCB.26.3.1142-1155.2006) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wang GL, Semenza GL. 1998. Molecular basis of hypoxia-induced erythropoietin expression. Curr. Opin. Hematol. 3, 156–162. ( 10.1097/00062752-199603020-00009) [DOI] [PubMed] [Google Scholar]
- 16.Iyer NV, et al. 1998. Cellular and developmental control of O2 homeostasis by hypoxia-inducible factor 1α. Genes Dev. 12, 149–162. ( 10.1101/gad.12.2.149) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ryan HE, Lo J, Johnson RS. 1998. HIF-1alpha is required for solid tumor formation and embryonic vascularisation. EMBO J. 17, 3005–3015. ( 10.1093/emboj/17.11.3005) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nagao K, Taniyama Y, Kietzmann T, Doi T, Komuro I, Morishita R. 2008. HIF-1alpha signaling upstream of NKX2.5 is required for cardiac development in Xenopus . J. Biol. Chem. 283, 11 841–11 849. ( 10.1074/jbc.M7025663200) [DOI] [PubMed] [Google Scholar]
- 19.Padilla PA, Roth MB. 2001. Oxygen deprivation causes suspended animation in the zebrafish embryo. Proc Natl Acad. Sci. USA 98 7331–7335. ( 10.1073/pnas.131213198) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stainier DYR, Lee RK, Fishman MC. 1993. Cardiovascular development in zebrafish. I. Myocardial fate map and hear tube formation. Development 119, 31–40. [DOI] [PubMed] [Google Scholar]
- 21.Ton C, Stamtiou D, Liew CC. 2003. Gene expression profile of zebrafish exposed to hypoxia during development. Physiol. Genomics 13, 97–106. ( 10.1152/pysiolgenomics.00128.2002) [DOI] [PubMed] [Google Scholar]
- 22.Davidson AJ, Zon LI. 2004. The ‘definitive’ (and ‘primitive’) guide to zebrafish hematopoiesis. Oncogene 23, 7233–7246. ( 10.1038/sj.onc.1207943) [DOI] [PubMed] [Google Scholar]
- 23.Kimmel CS, Ballard WW, Kimmel SR, Ullmann B, Schilling TF. 1995. Stages of embryonic development of the zebrafish. Dev. Dyn. 203, 253–310. ( 10.1002/aja.1002030302) [DOI] [PubMed] [Google Scholar]
- 24.Kopp R, Köblitz L, Egg M, Pelster B. 2011. HIF signalling and overall gene expression changes during hypoxia and prolonged exercise differ considerably. Physiol. Genomics 43, 506–516. ( 10.1152/physiolgenomics.0020.2010) [DOI] [PubMed] [Google Scholar]
- 25.Aldridge GM, Podrebarac DM, Greenough WT, Weiler IJ. 2008. The use of total protein stains as loading controls: an alternative to high-abundance single protein controls in semi-quantitative immunoblotting. J. Neurosci. Methods 17, 250–254. ( 10.1016/j.jneumeth.2008.05.003) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Egg M, et al. 2013. Linking oxygen to time: the bidirectional interaction between the hypoxic signaling pathway and the circadian clock. Chronobiol. Int. 30, 510–529. ( 10.3109/07420528.2012.754447) [DOI] [PubMed] [Google Scholar]
- 27.Nikinmaa M, Pursiheimo S, Soitamo AJ. 2004. Redox state regulates HIF-1α and its DNA binding and phosphorylation in salmonid cells. J. Cell Sci. 117, 3201–3206. ( 10.1242/jcs0.1192) [DOI] [PubMed] [Google Scholar]
- 28.Brahimi-Horn C, Mazure N, Pouysségur J. 2005. Signalling via the hypoxia-inducible factor-1α requires multiple posttranslational modifications. Cell Signal 17, 1–9. ( 10.1016/j.cellsig.2004.04.010) [DOI] [PubMed] [Google Scholar]
- 29.Bernier NJ, Craig PM. 2005. CRF-related peptides contribute to stress response and regulation of appetite in hypoxic rainbow trout. Am. J. Physiol. 289, R982–R990. ( 10.1152/ajpreg.00668.204) [DOI] [PubMed] [Google Scholar]
- 30.Bergmeyer HU, Bernt E. 1974. Lactate dehydrogenase. In Methods of enzymatic analysis (ed. Bergemeyer HU.), pp. 574–579, 2nd edn New York, NY: Academic Press. [Google Scholar]
- 31.Mueller CA, Seymour RS. 2011. The regulation index: a new method for assessing the relationship between oxygen consumption and environmental oxygen. Physiol. Biochem. Zool. 84, 522–532. ( 10.1086/661953) [DOI] [PubMed] [Google Scholar]
- 32.Mangum C, van Winkle W. 1973. Responses of aquatic invertebrates to declining oxygen conditions. Am. Zool. 13, 529–541. [Google Scholar]
- 33.Yeager DP, Ultsch GR. 1989. Physiological regulation and conformation: a BASIC program for the determination of critical points. Physiol. Zool. 62, 888–907. [Google Scholar]
- 34.Terova G, Rimoldi S, Cora S, Bernardini G, Gornati R, Saroglia M. 2008. Acute and chronic hypoxia affects HIF-1α levels in sea bass (Dicentrarchus labrax). Aquaculture 279, 150–159. ( 10.1016/j.aquaculture.2008.03.041) [DOI] [Google Scholar]
- 35.Burggren WW, Reyna KS. 2011. Developmental trajectories, critical windows and phenotypic alteration during cardio-respiratory development. Resp. Physiol. Neurobiol. 178, 13–21. ( 10.1016/j.resp.2011.05.001) [DOI] [PubMed] [Google Scholar]
- 36.Sano M, et al. 2007. p53-induced inhibition of HIF-1 causes cardiac dysfunction during pressure overload. Nature 446, 444–448. ( 10.1038/nature05602) [DOI] [PubMed] [Google Scholar]
- 37.Blagosklonny MV, An WG, Romanova LY, Trepel J, Fojo T, Neckers L. 1998. p53 inhibits hypoxia-inducible factor-stimulated transcription. J. Biol. Chem. 273, 11 995–11 998. ( 10.1074/jbc.273.20.11995) [DOI] [PubMed] [Google Scholar]
- 38.Sermeus A, Michiels C. 2011. Reciprocal influences of the p53 and the hypoxic pathways. Cell Death Dis. 2, e164 ( 10.1038/cddis.2011.48) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rojas DA, Perez-Munizaga DA, Centanin L, Antonelli M, Wappner P, Allende ML, Reyes AE. 2007. Cloning hif-1α and hif-2α and mRNA expression pattern during development in zebrafish. Gene Expr. Patterns 7, 339–345. ( 10.1016/j.modgep.2006.08.002) [DOI] [PubMed] [Google Scholar]
- 40.Dzialowski EM, von Plettenberg D, Elmonoufy NA, Burggren WW. 2002. Chronic hypoxia alters the physiological and morphological trajectories of developing chicken embryos. Comp. Biochem. Physiol. A Mol. Integr. Physiol. 131, 713–724. ( 10.1016/S1095-6433(02)00009-0) [DOI] [PubMed] [Google Scholar]
- 41.Mandic M, Todgham AE, Richards JG. 2009. Mechanisms and evolution of hypoxia tolerance in fish. Proc. R. Soc. B 276, 735–744. ( 10.1098/rspb.2008.1235) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rombough P, Drader H. 2008. Hemoglobin enhances oxygen uptake in larval zebrafish (Danio rerio) but only under conditions of extreme hypoxia. J. Exp. Biol. 212, 778–784. ( 10.1242/jeb.026575) [DOI] [PubMed] [Google Scholar]
- 43.Rombough P. 2002. Gills are needed for ionoregulation before they are needed for O2 uptake in developing zebrafish, Danio rerio . J. Exp. Biol. 205, 1787–1794. [DOI] [PubMed] [Google Scholar]
- 44.Burggren W, Mwalukoma A. 1983. Respiration during chronic hypoxia and hyperoxia in larval and adult bullfrogs (Rana catesbeiana). I. Morphological responses of lungs, skin and gills. J. Exp. Biol. 105, 191–203. [DOI] [PubMed] [Google Scholar]
- 45.Rees BB, Sudradjat FA, Love JW. 2001. Acclimation to hypoxia increases survival time of zebrafish, Danio rerio, during lethal hypoxia. J. Exp. Zool. 289, 266–272. ( 10.1002/1097-010X) [DOI] [PubMed] [Google Scholar]
- 46.Reardon EE, Chapman LJ. 2010. Energetics of hypoxia in a mouth-brooding cichlid: evidence for interdemic and developmental effects. Physiol. Biochem. Zool. 83, 414–423. ( 10.1086/651100) [DOI] [PubMed] [Google Scholar]
- 47.Shang EHH, Yu RMK, Wu RSS. 2006. Hypoxia effects sex differentiation and development, leading to a male-dominated population in zebrafish (Danio rerio). Environ. Sci. Technol. 40, 3118–3122. ( 10.1021/es0522579) [DOI] [PubMed] [Google Scholar]
- 48.Shang EHH, Wu RSS. 2004. Aquatic hypoxia is a teratogen and affects embryonic development. Environ. Sci. Technol. 38, 4763–4767. ( 10.1021/es0496423) [DOI] [PubMed] [Google Scholar]
- 49.Thomas P, Rahman MS. 2012. Extensive reproductive disruption, ovarian masculinization and aromatase disruption in Atlantic croaker in northern Gulf of Mexico hypoxic zone. Proc. R. Soc. B 279, 28–38. ( 10.1098/rspb.2011.0529) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Friesen CN, Aubin-Horth N, Chapman LJ. 2012. The effect of hypoxia on sex hormones in an African cichlid Pseudocrenilabrus multicolor victoriae . Comp. Biochem. Physiol. A 162, 22–30. ( 10.1016/j.cbpa.2012.01.019) [DOI] [PubMed] [Google Scholar]
- 51.Kazeto Y, Iijiri S, Place AR, Zohar Y, Trant JM. 2001. The 5’-flanking regions of CYP19A1 and CYP19A2 in zebrafish. Biochem. Biophys. Res. Commun. 288, 503–508. ( 10.1006/bbrc.2001.5796) [DOI] [PubMed] [Google Scholar]
- 52.Liew WC, Bartfai R, Lim Z, Sreenivasan R, Siegfried KR. 2012. Polygenic sex determination system in zebrafish. PLoS ONE 7, e34397 ( 10.1371/journal.pone.0034397) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Vizziano-Cantonnet D, Anglade I, Pellegrini E, Gueguen MM, Fostier A, Guiguen Y, Kah O. 2011. Sexual dimorphism in the brain aromatase expression and activity, and in the central expression of other steroidogenic enzyme during the period of sex differentiation in monosex rainbow trout populations. Gen. Comp. Endocrinol. 170, 346–355. ( 10.1016/j.ygcen.2010.10.009) [DOI] [PubMed] [Google Scholar]
- 54.Andersen L, Holbech H, Gessbo A, Norrgren L, Petersen GI. 2003. Effects of exposure to 17alpha-ethinylestradiol during early development on sexual differentiation and induction of vitellogenin in zebrafish (Danio rerio). Comp. Biochem. Phsyiol. C 40, 519–530. ( 10.1016/S1532-0456(03)00006-1) [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
All data used in this paper have been archived in the dryad repository (doi:10.5061/dryad.3ks4h).