Abstract
Migratory bird species that feed on air-borne insects are experiencing widespread regional declines, but these remain poorly understood. Agricultural intensification in the breeding range is often regarded as one of the main drivers of these declines. Here, we tested the hypothesis that body mass in breeding individuals should reflect habitat quality in an aerial insectivore, the tree swallow (Tachycineta bicolor), along a gradient of agricultural intensity. Our dataset was collected over 7 years (2005–2011) and included 2918 swallow captures and 1483 broods. Analyses revealed a substantial decline of the population over the course of the study (−19% occupancy rate), mirrored by decreasing body mass. This trend was especially severe in females, representing a total loss of 8% of their mass. Reproductive success was negatively influenced by intensive agriculture, but did not decrease over time. Interestingly, variation in body mass was independent of breeding habitat quality, leading us to suggest that this decline in body mass may result from carry-over effects from non-breeding areas and affect population dynamics through reduced survival. This work contributes to the growing body of evidence suggesting that declines in migratory aerial insectivores are driven by multiple, complex factors requiring better knowledge of year-round habitat use.
Keywords: agricultural intensification, aerial insectivores, body mass, breeding success, phenotypic plasticity, tree swallow
1. Introduction
In recent decades, global anthropogenic environmental changes, such as climate change and large-scale habitat destruction, have contributed to the decline or extinction of a wide range of animal species. For instance, migratory songbirds, especially those breeding in farmland habitats, are declining on a global scale [1,2]. Similarly, aerial insectivores, which comprise several farmland species, are often regarded as the most steeply declining bird guild in industrialized countries [3], particularly in northeastern North America [4]. While the reasons for this decline remain largely speculative, insectivory is a life-history trait shared by all species in this otherwise ecologically diverse guild, suggesting their decline is related to a parallel widespread decline in insect abundances [4,5]. Numerous studies have shown that agricultural intensification, most likely through habitat homogenization and pesticide use, negatively impacts insect diversity and abundance in agricultural landscapes [6,7], constraining farmland aerial insectivores to breed in depauperate habitats.
The quality of the breeding habitat is an important fitness determinant of birds, as it typically affects survival and reproductive success through resource availability and environmental conditions [8,9]. Poor habitat quality may hamper individual performance in a given season and also impact survivorship or subsequent reproduction through carry-over effects ([10]; see [11] for a review). Evaluation of habitat quality can be achieved through the quantification of food resources and the evaluation of available cover, nesting sites and predation risks [9]. An alternative approach is to investigate correlates of habitat quality directly in bird populations. Deterioration in habitat is often reflected in several demographic and individual parameters, including body mass and body condition [12,13]. Adult body mass and condition are important traits in evolutionary and ecological theory, as they are associated with fitness in many taxa through their effect on survival and reproductive success [14,15]. While most empirical data come from species typically described as capital breeders, such as waterfowl, even passerine birds, usually considered as income breeders, are known to benefit from larger mass (generally associated with greater fat reserves) and better nutritional condition prior to breeding [16,17]. Body mass in bird populations may fluctuate in response to breeding habitat quality and directly impact survival and reproductive success. Understanding the relationship among habitat, body mass and individual performance in declining populations may help elucidate the forces driving such population dynamics.
Here, we assess the change in adult body mass during reproduction over 7 years (2005–2011) in a declining population of tree swallows (Tachycineta bicolor), an aerial insectivore breeding along a gradient of agricultural intensity (AI) in southern Québec, Canada. Tree swallow populations in northeastern North America are experiencing a well-documented decline, similar to that noted in several aerial insectivores, but in sharp contrast with their stable or even increasing numbers in the western part of the continent [4,18]. Data from the Breeding Bird Survey (BBS) [19] for the province of Québec illustrate this regional decline (figure 1a). Our first objective was to assess whether there was an overall decline in both body mass and breeding success over time in this population, which would parallel the regional decline observed in tree swallow abundance. A temporal change in a trait can occur at two, non-mutually exclusive levels: the trait can change due to a plastic response to habitat quality and environmental conditions (i.e. phenotypic plasticity or ‘within-individual change’) or, alternatively, recruited individuals can be different on average from those from the previous years due to immigration or a microevolutionary response (‘between-individual change’). To evaluate the causes and consequences of change in a trait, one thus needs to test whether patterns of ‘within-individual change’ are similar to those of ‘between-individual change’ [20,21]. In this work, we tested whether the changes in mass observed at the population level result from changes within individuals or changes in the individuals that make up the population. Our second objective was to assess the effect of AI on spatial variation in body mass in T. bicolor and identify the main determinants of body mass during the breeding season. While the adverse effects of agricultural intensification on bird and insect communities have been documented in terms of richness and abundance [7,22], impacts on body mass or condition in aerial insectivores have not, to our knowledge, been assessed.
Figure 1.
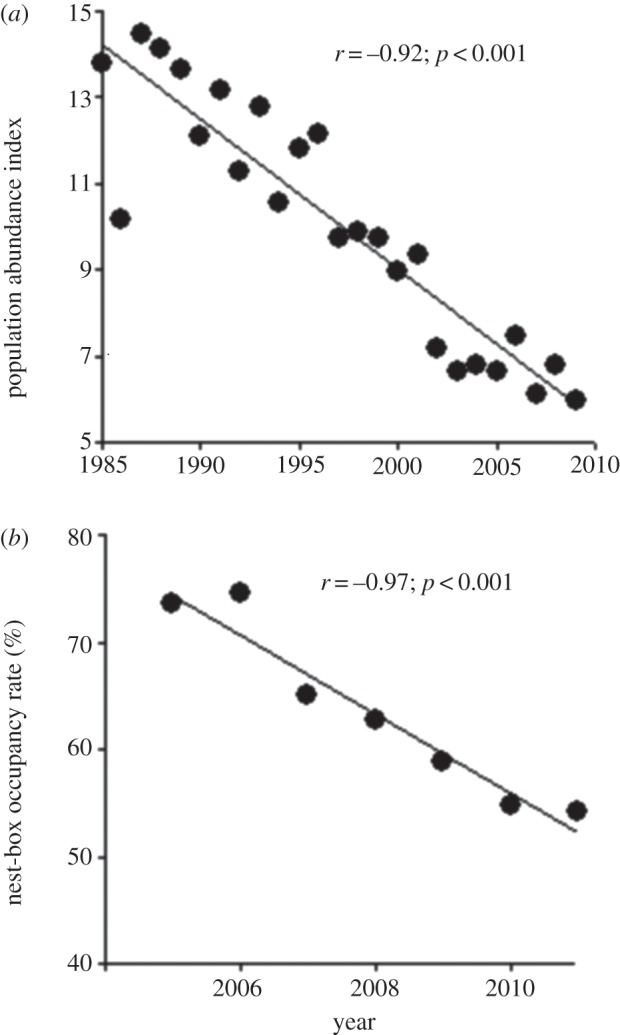
(a) Population abundance index for the tree swallow in the province of Québec, Canada, between 1985 and 2009 (data obtained from the Bird Breeding Survey [19]); (b) nest-box occupancy rate by tree swallows in our study area (southeastern Québec, Canada) between 2005 and 2011. Linear correlation coefficients (r) and their p-values are indicated on each panel.
Previous analyses of the breeding success of this population have revealed that the mean number of nestlings per brood surviving to fledging was lower in intensively managed habitats. This effect was hypothesized to be related to the negative impacts of intensive agriculture on air-borne insects, the main food resource for breeding tree swallows [23]. AI was the only consistently significant predictor of breeding success among several habitat characteristics included in that study [23]. Subsequent spatio-temporal analyses of Diptera abundance (i.e. the main prey of tree swallows) in these landscapes further reinforced that AI globally had a negative impact on prey abundance, although this effect also had a strong temporal component and varied during the breeding season [24]. Building on these previous findings, we used AI as an indicator of breeding habitat quality. Considering that females with greater body mass usually perform better during incubation and nesting stages in birds [15,17], we hypothesized that female body mass during reproduction would be lower in agro-intensive habitats and that it would negatively affect reproductive success.
2. Material and methods
(a). Study system and sampling
From 2005 to 2011, we monitored breeding activities of tree swallows in a network of 400 nest-boxes distributed among 40 farms (10 per farm) over an area of approximately 10 200 km2. These farms are located along a gradient of agricultural intensification, with a transition from intensive cultures (mostly maize, cereals and soya beans) in the western part to more extensive ones in the east (pastures, hayfields and fallows). More details on this system and landscape characterization around nest-boxes (proportion of intensive cultures around nest-boxes) are available in [23].
Nest-boxes were visited every 2 days throughout the breeding season (April to August). A nest-box was considered occupied when at least one egg was laid. Several breeding parameters for each box were noted: laying date (of the first egg), clutch size, number of eggs hatched (hatchlings) and number of chicks fledged (fledglings). Female tree swallows were captured at least once during incubation (around 12 days after laying date, which is 2–6 days before the end of incubation). Males were captured during the nesting period. All captured individuals were ringed and weighed (±0.01 g), and wing chord (±0.1 mm; a measure of non-flattened wing length, hereafter referred to simply as wing length) and tarsus length (±0.01 mm; starting in 2007) were measured. Females were aged according to plumage coloration (second year (SY) or after second year (ASY) [25]). Females were recaptured opportunistically during incubation and nesting phases and were weighed every time. No attempt was made to capture males or recapture females in 2004, thus we restricted our analyses to 2005–2011. Tree swallows typically lay a single clutch per breeding season, but may produce, albeit rarely at this latitude, a second clutch, especially if the first nesting attempt fails early in the season [25]. We did not include second clutches in our analyses. The dataset gathered for this work included 2918 individual mass measurements and 1483 broods.
(b). Statistical analyses
(i). Predictors of body mass
Body mass is intrinsically linked to body condition, a term generally employed to refer to energy reserves of an individual [26,27]. Although the use of body condition indices (e.g. ratio of mass on size or regression residuals) is pervasive in the ecological literature, recent studies suggested that body mass alone often outperforms condition indices as a measure of fat content in bird species [28,29]. Here, we included a size covariate (wing length) in all analyses involving mass, thus effectively correcting mass for size (‘mass adjusted for size’, e.g. [12]). Henceforth, body mass is employed as a proxy for condition.
We modelled body mass separately for each sex with linear mixed models (LMMs) computed with the R library lme4 [30]. Sexes were analysed separately because preliminary analyses showed highly significant differences between males and females (females being heavier by 0.89 g on average in raw data, t = 14.8, p < 0.001) and because the costs of reproduction at different stages of breeding in birds are also different between sexes [31], which may lead to sex-specific mass fluctuations during the breeding season. A ‘base’ model was first constructed to control for variations in the moment when individuals were weighed. This model included as fixed effects: Julian date, number of days since laying date, time of the day when sampling was performed (coded as a proportion, where noon = 0.5), wing length (in mm; tarsus length was not used since it was not measured in 2005–2006), age as a two-level factor for females only (SY/ASY), clutch size and the interaction between days since laying date and clutch size. Four additional models were then constructed to reflect ecological hypotheses on the effect of AI and year on body mass. They included the proportion of intensive cultures within 5 km of nest-boxes (this scale was chosen because it showed the largest effect sizes in previous models of breeding success [23]), the interaction between laying date and intensive cultures, the year of sampling and the interaction between year and intensive cultures (see the electronic supplementary material, tables S1–S2, for description of models). The first interaction was included to test whether intensive agriculture affects body mass during the breeding season, and the second to assess whether its impacts varied during the course of the study. In all models, farm identity was included as a random factor, as well as individual identity because swallows were recaptured within and among years. All variables (except year) were centred on their mean before analysis. Competing models (including a null model) were compared on the basis of ΔAICc following [32]. Weighted support of models and model averaging were calculated with the R package AICcmodavg [33].
(ii). Within- and between-individual mass variation in females
We applied the mixed-model framework described in [20] to distinguish within- and between-individual effects based on within-subject centring of the predictor of interest. Briefly, in a typical mixed model computed from a dataset that includes i observations made on j individuals, like the models computed above (i), the slope β of a fixed term predictor xij reflects the combined within- and between-individual effects. However, one can isolate the two effects if xij is replaced by two terms: one is the centred value of i with respect to the mean of individual j (xij − x̄j), while the other is simply the individual mean x̄j (see eqn (2) in [20]). These two terms allow for the estimation of the slopes of the within- (βW) and between-individual (βB) effects, respectively. Restricting the analysis to individuals recaptured during at least two different years (n = 1027 measurements, from 286 individuals), we fitted a LMM with the same covariates as above, but using within-subject centring for the predictor year. We then re-parametrized the model following ([20], eqn 3) to test the null hypothesis that βW and βB were equal. This analysis was not performed in males due to the smaller number of recaptures (only 63 individuals recaptured in more than 2 years).
(iii). Effect of female body mass and year on breeding success
To evaluate whether female body mass is a significant predictor of breeding success, the numbers of fledglings from each brood were modelled with mixed models. Body mass (the measurement closest to day 12 after laying date was used for each female), wing length, age, laying date, proportion of intensive cultures within 5 km of nest-box and year, treated as a linear term to parallel analyses in (i), were included as fixed effects, with farm and female identity as random factors. While the number of fledglings per brood was normally distributed for broods that fledged one chick or more, there was a substantial proportion of zeros when including all broods, mainly due to a high incidence of complete nest failure (24% of nests were predated and/or abandoned). We thus fitted two models to make the distinction between factors explaining complete nest failure (related to predation) and those explaining partial failure (related to parental care and chick growth). The first included all broods and the number of fledglings was recoded as a binary variable (all numbers more than 0 were coded as 1). This model had a binomial error distribution and logit link function. The second model was a LMM (Gaussian distribution) fitted by restricting the analyses to broods that produced at least one fledgling. Models using the number of hatchlings per brood instead of fledglings were very similar and thus, only models for fledglings were retained.
3. Results
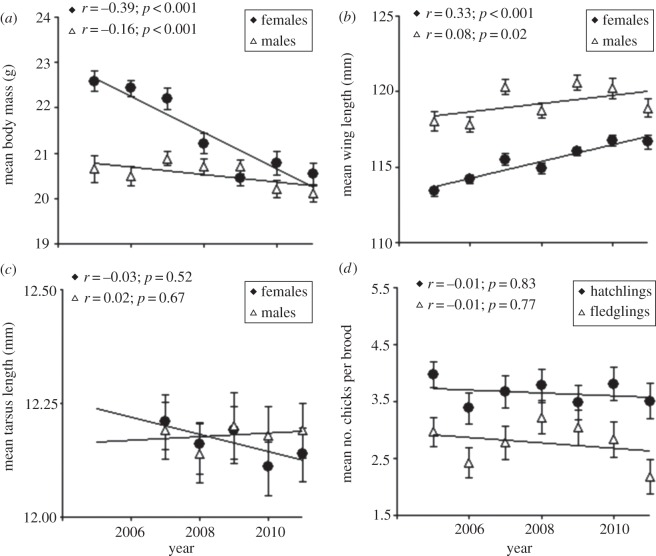
The nest-box occupancy rate in the population decreased steadily from 73.5% in 2005 to 54.3% in 2011 (figure 1b), paralleling, albeit on a much shorter time period, the trend observed in the BBS data (figure 1a). Inspection of the raw data showed that mean body mass declined through time, especially in females, while mean wing length showed the opposite trend (figure 2a,b). Tarsus length remained stable over time (figure 2c). The mean number of hatchlings and fledglings per brood did not change through time (figure 2d). Also, there was a small but highly significant advance in mean laying date (corresponding to 1.7 days over the course of the study, or approximately 0.3 days yr−1; figure 3a), while the proportion of young females did not vary (figure 3b). Finally, the proportion of intensive cultures around occupied nest-boxes was similar across years (figure 3c), suggesting that swallows did not choose nest-boxes in more extensive landscapes as more nest-boxes became available over the years. A generalized LMM (binomial error) of occupancy also confirmed this result (see the electronic supplementary material, table S3, for details).
Figure 2.
Variation in phenotypic traits and breeding success in our tree swallow population in southeastern Québec from 2005 to 2011: (a) mean body mass; (b) mean wing length; (c) mean tarsus length and (d) mean number of hatched and fledged chicks per brood. The dataset includes 1017 females (2070 captures), 556 males (848 captures) and 1483 broods. Error bars indicate 95% CIs of mean values and linear correlations (r) were calculated on the raw data.
Figure 3.
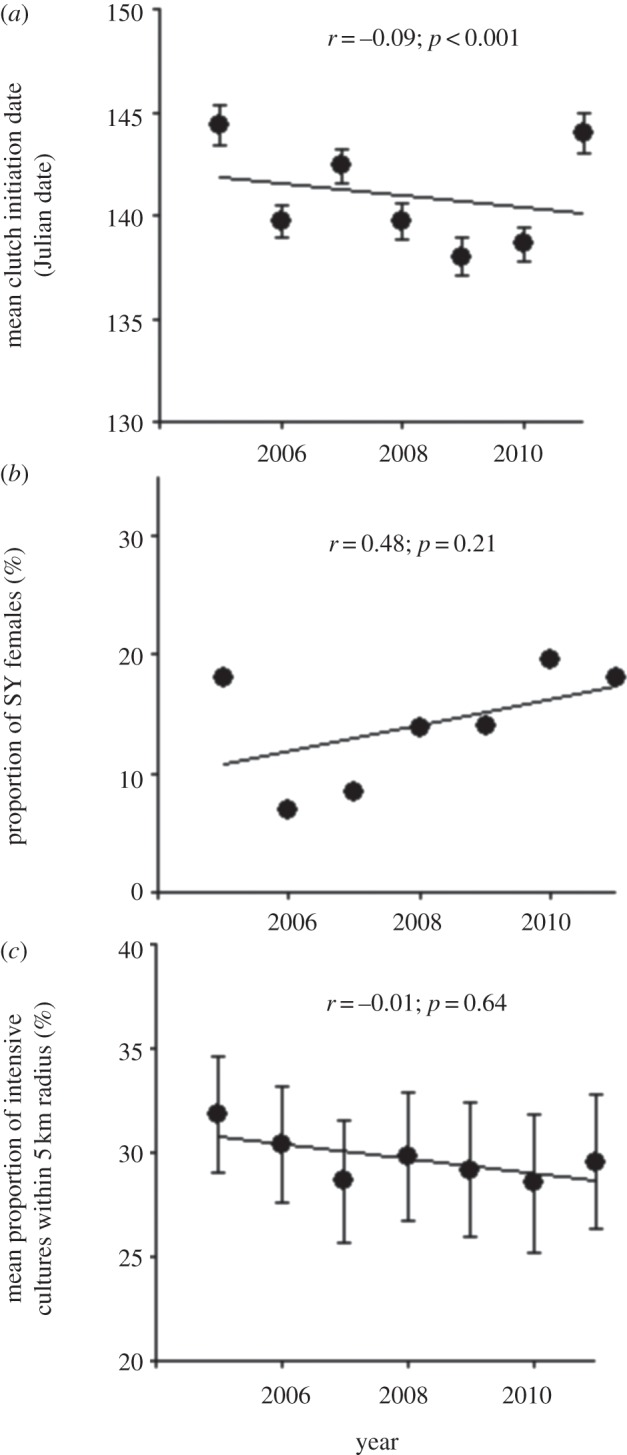
Variation in other covariates of tree swallow body mass considered in this study: (a) mean laying date; (b) proportion of SY, as opposed to ASY females in the adult female population and (c) mean proportion of intensive cultures within 5-km radius of occupied nest-boxes. Error bars indicate 95% CIs of mean values and linear correlations (r) were calculated on the raw data except for the proportion of SY females (b), for which it was calculated on annual population means.
(a). Predictors of body mass
In both males (n = 848) and females (n = 2070), there was a significant decline in body mass over time (table 1). For males, body mass was on average 0.06 g lower per year, representing a decrease of approximately 2% between 2005 and 2011. The annual effect was much greater in females, for which body mass decreased by 0.26 g per year, corresponding to a body mass reduction of 8%. The proportion of intensive cultures did not influence body mass in either sex (table 1). Only models that included year as a predictor had a non-null contribution to the final model-averaged results in females (see the electronic supplementary material, tables S1 and S2, for model statistics).
Table 1.
Predictors of body mass in female and male tree swallows during breeding seasons from 2005 to 2011. These model-averaged estimates were obtained from LMMs with individual and farm identities as random effects and fixed effects centred on their mean. Predictors in italic are statistically significant (i.e. their 95% confidence interval (CI) does not include zero).
| fixed effects | females (n = 2070; 1017 individuals) |
males (n = 848; 556 individuals) |
||||||
|---|---|---|---|---|---|---|---|---|
| estimate | s.e. | lower CI | upper CI | estimate | s.e. | lower CI | upper CI | |
| intercept | 22.6 | 0.1 | 22.3 | 22.8 | 20.7 | 0.08 | 20.5 | 20.9 |
| year | −0.264 | 0.025 | −0.312 | −0.215 | −0.056 | 0.018 | −0.091 | −0.021 |
| Julian date | −0.009 | 0.006 | −0.021 | 0.003 | −0.038 | 0.005 | −0.048 | −0.028 |
| no. days since laying date | −0.082 | 0.008 | −0.097 | −0.067 | −0.022 | 0.007 | −0.035 | −0.008 |
| time of day | 1.366 | 0.320 | 0.740 | 1.990 | 0.758 | 0.246 | 0.277 | 1.240 |
| proportion of intensive cultures within 5 km radius (intensive) | 0.005 | 0.006 | −0.006 | 0.016 | 0.007 | 0.005 | −0.002 | 0.016 |
| wing length (mm) | 0.061 | 0.014 | 0.034 | 0.089 | 0.023 | 0.006 | 0.010 | 0.036 |
| age (SY) | −0.296 | 0.130 | −0.550 | −0.042 | — | — | — | — |
| clutch size | 0.200 | 0.049 | 0.104 | 0.296 | −0.021 | 0.039 | −0.091 | 0.055 |
| days since laying × intensive | −0.0002 | −0.0002 | −0.0006 | 0.0002 | −0.0002 | 0.0002 | −0.0006 | 0.0003 |
| days since laying × clutch size | −0.0050 | 0.0040 | −0.0129 | 0.0029 | 0.0081 | 0.0056 | −0.0029 | 0.0191 |
| year × intensive | −0.0005 | 0.0009 | −0.0023 | 0.0013 | −0.0012 | 0.0007 | −0.0026 | 0.0002 |
(b). Within- and between-individual mass variation in females
Fitting a mixed model on the restricted dataset (recaptured females only) with within-subject centring revealed that there were both a significant within-individual (βW: −0.161 ± 0.058) decline in mass and a significant between-individual (βB: −0.244 ± 0.048) decline in mass across years (electronic supplementary material, equation (2) in table S4). However, the slope of the within-individual effect of year on body mass was equivalent to the slope of the between-individual effect of year on mass, as the difference βB − βW (−0.084 ± 0.071) did not differ from zero (electronic supplementary material, equation (3) in table S4). This indicates that the change in female body mass from 2005 to 2011 is mainly the result of within-individual change (phenotypic plasticity).
(c). Effect of body mass and year on breeding success
The logistic model describing the probability of brood success (where 0 corresponds to not fledging a single chick and 1 to having at least one fledgling; n = 1483 broods) revealed a highly significant positive effect of female body mass (electronic supplementary material, table S5). Both laying date and age had a marginally significant negative effect on brood success, with earlier clutches and ASY females having greater success. After discarding complete brood failures (n = 1134 broods), laying date was the most important predictor of the number of fledglings, with earlier clutches producing more fledglings. AI around nest-boxes also had a significant negative effect on the number of fledglings (electronic supplementary material, table S5). Female body mass still had a positive effect on fledging success, but it was not significant. There was no significant effect of year on breeding success, either in the binomial failure/success model or the LMM for the number of chicks fledged.
4. Discussion
We have revealed that nest-box occupancy in a tree swallow population in southern Québec has declined by 19.2% between 2005 and 2011. More importantly, this decline was paralleled by a substantial reduction of body mass in breeding individuals. This decline in mass appears to be mainly a plastic response that is especially important in females, which have lost the equivalent of approximately 8% of their mean body mass during the course of the study. Variation in body mass does not appear to be related to AI, the proxy we used for habitat quality in the breeding area. In combination with the fact that the number of breeding pairs in the area has decreased steadily, while mean reproductive success has remained constant, these results lead us to suggest that this trend may result from non-breeding area carry-over effects.
(a). Predictors of body mass
Our analyses found significant effects of covariates on tree swallow body mass in accordance with current knowledge of determinants of bird body condition. For instance, mass decreased daily after laying throughout incubation [31], but linearly increased during the day from morning to evening [34]. Older females were also heavier than younger ones [35], although previous results on this aspect are more equivocal, with several studies finding no effect of age on body mass or condition [36]. Previous work on the population studied here highlighted the negative effects of agriculturally intensive landscapes on tree swallow breeding success [23], so we also expected to find a negative relationship between AI and body mass during the breeding season. Our results suggest otherwise; AI does not appear to influence adult swallow body mass. Investigation of the abundance of Diptera, the main food resource of tree swallows, in our study area revealed that AI does significantly reduce prey availability [24]. However, there is a strong temporal component to this relationship, whence the difference between habitats increases as the swallow breeding season progresses. In the early stages of laying and nesting, there is virtually no negative impact of AI on Diptera abundance [24], thus providing a possible explanation for the lack of relationship between AI and body mass revealed here. Yet, extending findings from [23], we did detect a negative effect of AI on the number of chicks fledged (electronic supplementary material, table S5), suggesting that, if food abundance is not involved, other factors such as food quality and availability [37] or pesticide use [38] may affect the quality of breeding habitats in agricultural landscapes and explain variation in breeding success. Similarly, it is possible that the same variables (e.g. food quality or pesticides) or other unmeasured variables may explain variation in body mass in the studied population. Until conclusive evidence is acquired to explain the trend in body mass, this option cannot be completely discarded. Nevertheless, the indirect indicator that we used for habitat quality (AI), which is a predictor of breeding success and prey abundance in this population [23,24], simply has no significant effect on adult tree swallow body mass. Additionally, AI did not modulate the loss in body mass during the breeding season, as the interaction between AI and days since laying was not significant. One possible confounding factor preventing us from detecting an effect of AI on body mass is potential movement of individuals among farms between years. In a separate study on survival and dispersal in this population, the probability of dispersing was estimated at 0.15 in females and 0.02 in males [39] and the majority of breeding dispersal events occurred to the closest or second-closest farm (74%; S. Rioux Paquette and M. Bélisle 2013, unpublished data), so that estimates of AI at the 5 km scale are rather similar between these farms. Thus, it is unlikely that this process explains the lack of relationship between body mass and AI.
This finding, coupled with declining nest-box occupancy but constant reproductive success, suggests that the severe decline in body mass observed here may not reflect deterioration of breeding habitats, but of wintering grounds and migration conditions instead. Recent and rapid morphological changes in bird populations are often attributed to climate change [40], but the magnitude and even the direction of these changes vary extensively among species and regions ([41,42]; see [43] and references therein for review in homeotherms). For instance, wing length in bird species nesting or migrating through Pennsylvania has decreased between 1961 and 2006 for a majority of species [41], but has steadily increased for most bird species in California between 1983 and 2009 [42]. While some researchers have proposed that decrease in body size may be an universal response to global warming [44], meta-analyses suggest that evidence for this hypothesis is equivocal at best [43], as shown by the diverging results obtained in North American birds. Here, we found that wing length actually increased at a rate corresponding to 0.5% per year in females and 0.2% in males. Goodman et al. [42] postulated that longer wing length might be a response to greater climatic variability or changes in primary productivity, both often described as consequences of global change that vary among biomes. Conversely, a recent study reported that cliff swallows (Petrochelidon pyrrhonota) nesting alongside a highway had reduced their wing length in a period of 30 years in order to favour agility and avoid vehicles [45], showing that selective pressures can also be local and come from unexpected sources. At this point, it remains unclear why the studied population showed a different trend from that of tree swallow spring migrants captured in Pennsylvania [41], and the causes of this change remain highly speculative. Nonetheless, the mechanisms proposed by Goodman et al. [42] (e.g. to better resist extreme weather events such as severe storms during migration) constitute an avenue for further research.
The only consensus on the effects of climate change on birds is the phenological shift towards earlier breeding [46]. We detected a modest but significant shift in laying date, suggesting that the swallow population is responsive to global climatic patterns, but this change alone (less than 2 days between 2005 and 2011) is unlikely to explain the decline in body mass of swallows on their breeding grounds. In some species, these shifts may cause a phenological mismatch between bird populations and their arthropod prey [47], but this mismatch does not necessarily impede population growth [48]. In addition, the mismatch hypothesis has been refuted in tree swallows, because their prey do not exhibit a distinctive peak and generally increase in abundance throughout the main stages of the swallow breeding season [49], which is also the case in our study area [24]. On the other hand, there is strong evidence that body condition of migratory songbirds does respond, sometimes very quickly, to wintering habitat quality and that it does affect condition after arrival at breeding sites [12,13,50]. We propose that carry-over effects from wintering habitats or migration are responsible for the gradual mass loss observed in this population. A reduction in prey availability, likely through intensification of agricultural practices in the breeding area, has often been viewed as the most parsimonious explanation for the global declines of aerial insectivores. Very little is known about habitat use by tree swallows in their wintering grounds in the southern US or Central America, but deteriorating habitat at wintering and stopover sites could also explain decreasing adult body mass on arrival at their breeding sites. Interestingly, a preliminary study using geolocator data from 11 tree swallow individuals from three breeding sites revealed that individuals wintered in three regions: Florida, Mexico (Yucatan peninsula) and the Bahamas [51]. In all of these locations, mean pesticide use is greater than in Canada, but there is also important variation among regions (2.2 kg ha−1 of arable land in the USA, 4.5 kg ha−1 in Mexico and 59.4 kg ha−1 in the Bahamas between 2005 and 2009 [52]). Such differences in agricultural practices are likely to affect insect abundance to different levels and ultimately help in understanding the variation in demographic trends observed not only among aerial insectivores, but even within species. Geographical variation in the consequences of global change may also play a role. As a growing number of studies suggest that survival and performance in non-breeding seasons may have the greatest importance in the population dynamics of migratory species [11,53], we believe that non-breeding habitat quality may represent a key aspect in understanding the current decline of the tree swallow population studied in this work, and possibly aerial insectivores in general.
(b). Within- and between-individual mass variation in females
The decline in female body mass found in our study is similar, albeit much more pronounced, to the trend documented in a Spanish population of barn swallows Hirundo rustica [21]. Despite establishing a significant positive relationship between temperature and body condition (as warmer springs were associated with better body condition upon arrival at the breeding grounds), Balbontin et al. [21] observed a decrease in body condition of breeding female swallows between 1991 and 2007, and concluded that other environmental variables missing from their models likely explained this temporal trend. They also found that female body condition responded plastically to environmental conditions during migration but that there was a between-individual response of female condition in wintering habitats owing to selection against individuals in poor condition [21]. Here, we determined that the temporal decline in female body mass was mainly due to phenotypic plasticity, instead of being caused by a genetic microevolutionary response to changing conditions (see also [54,55]).
The fourfold difference in mass decline between females and males also mirrors results from [21] and leaves little doubt that this difference is biologically relevant. In species with uniparental incubation, the costs of reproduction are greater in females ([31]; T. bicolor is a female uniparental incubator in which males do not feed incubating females [56]) and indeed, we found that daily mass loss was four times greater in females during incubation (table 1). It is possible that these higher sex-specific costs exacerbate potential carry-over effects from non-breeding periods. This is also reflected in differential survival in the studied population, as female tree swallows exhibit lower year-round survival [39].
(c). Effect of body mass and year on breeding success
Female body mass was a significant positive predictor of the probability of brood success (i.e. producing at least one fledgling), in line with previous studies that have found that female body condition can influence parental defence and the return to the nest after encountering predators [57]. Yet, there was no temporal effect on breeding success and the mean number of fledglings per brood did not decline between 2005 and 2011 (figure 2d). Thus, while heavier females generally have greater brood success within a given year, declining body mass is not affecting breeding success over time. As the nest-box occupancy rate decreased substantially during the study, breeders could have chosen better nesting sites on average at lower population densities to alleviate the effects of poorer body condition, but this does not appear to be the case, as landscape composition around selected nest-boxes remained the same (figure 3c). Breeding tree swallows may be able to compensate for poorer body condition, but this may be at the cost of increased mortality. We suggest that declining body mass through carry-over effects from non-breeding habitats may reduce survival rates of adult individuals and ultimately play a part in the current decline of this population, and perhaps of other aerial insectivore populations. We are not aware of studies that have tried to link demographic trends with body mass or condition in aerial insectivores, but the link between condition and survival rate has been found in other avian taxa (e.g. [58,59]). While individual breeding success appears to be stable in the studied population (although the fate of juveniles after fledging remains largely unknown and is difficult to investigate with a natal return rate below 1%), one cannot exclude the possibility that further deterioration of female body condition could eventually have significant impacts on breeding success if the limits of parental compensation are reached.
5. Conclusion
The decline of aerial insectivores nesting in farmlands has mostly been investigated from a breeding habitat perspective (e.g. [3,4]). Because of the diversity and geographical distance between the wintering ranges of species in this ecological guild, it could be argued that factors in these ranges are unlikely to simultaneously affect these species, while forces affecting arthropod populations in their breeding range (e.g. AI and changing agricultural practices) are more likely to explain this guild-wide decline [5]. Nevertheless, in UK-breeding birds, breeding habitat (farmland versus wood) was a very strong predictor of historical declines in the 1970s and 1980s but did not explain more recent demographic trends [60], whereas current declines of migrant birds breeding in Europe are best explained by characteristics of wintering habitats [61]. While agricultural practices in North America have most likely affected arthropod and bird populations in the past, they may not be the sole, or even the main, driver of current aerial insectivore declines.
We have shown that the dynamics of a declining aerial insectivore population are mirrored by a similar trend in adult body mass, and argued that this may result from non-breeding area carry-over effects. Present knowledge of tree swallow wintering and stopover habitats is minimal [51]; this is a recurring admission of failure when it comes to migratory bird declines, as we simply do not know enough about wintering and migration stopover habitats to understand the processes driving these declines [62]. These gaps in knowledge are exacerbated by the complexity of the multifaceted effects of human-driven environmental changes. The use of geolocators has recently proved extremely useful in identifying non-breeding ranges and migratory connectivity in another declining North American aerial insectivore, the purple martin (Progne subis) [63]. Similar projects are underway in tree swallows, and a single year of geolocator data has so far allowed the identification of an important stopover site in Louisiana [51]. Our work provides further incentives for such logistically challenging endeavours, as understanding population decline in aerial insectivores will involve disentangling complex interactions between multiple environmental factors, especially for migratory species occupying diverse habitats during their lifecycle.
Acknowledgements
We wish to thank the 40 farm owners who give us access to their lands and all graduate students and field assistants who have worked on this project.
Data accessibility
The datasets used in this work have been uploaded to the Dryad Digital Repository: (doi:10.5061/dryad.67t23).
Funding statement
This work was supported by a Fonds de Recherche du Québec—Nature et Technologies (FRQNT) new researcher grant to F.P. and by Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grants to M.B., D.G. and F.P., Canada Research Chairs to M.B. and F.P., as well as a grant from the Canadian Wildlife Service (thanks to J.-P. L. Savard) to M.B.
References
- 1.Donald PF, Green RE, Heath MF. 2001. Agricultural intensification and the collapse of Europe's farmland bird populations. Proc. R. Soc. Lond. B 268, 25–29. ( 10.1098/rspb.2000.1325) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Murphy MT. 2003. Avian population trends within the evolving agricultural landscape of eastern and central United States. Auk 120, 20–34. ( 10.1642/0004-8038(2003)120[0020:APTWTE]2.0.CO;2) [DOI] [Google Scholar]
- 3.Benton TG, Bryant DM, Cole L, Crick HPQ. 2002. Linking agricultural practice to insect and bird populations: a historical study over three decades. J. Appl. Ecol. 39, 673–687. ( 10.1046/j.1365-2664.2002.00745.x) [DOI] [Google Scholar]
- 4.Nebel S, Mills A, McCracken JD, Taylor PD. 2010. Declines of aerial insectivores in North America follow a geographic gradient. Avian Conserv. Ecol. 5, 1 ( 10.5751/ACE-00391-050201) [DOI] [Google Scholar]
- 5.Nocera JJ, et al. 2012. Historical pesticide applications coincided with an altered diet of aerially foraging insectivorous chimney swifts. Proc. R. Soc. B 279, 3114–3120. ( 10.1098/rspb.2012.0445) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Vickery JA, Tallowin JR, Feber RE, Asteraki EJ, Atkinson PW, Fuller RJ, Brown VK. 2001. The management of lowland neutral grasslands in Britain: effects of agricultural practices on birds and their food resources. J. Appl. Ecol. 38, 647–664. ( 10.1046/j.1365-2664.2001.00626.x) [DOI] [Google Scholar]
- 7.Attwood SJ, Maron M, House APN, Zammit C. 2008. Do arthropod assemblages display globally consistent responses to intensified agricultural land use and management? Glob. Ecol. Biogeogr. 17, 585–599. ( 10.1111/j.1466-8238.2008.00399.x) [DOI] [Google Scholar]
- 8.Przybylo R, Wiggins DA, Merilä J. 2001. Breeding success in blue tits: good territories or good parents? J. Avian Biol. 32, 214–218. ( 10.1111/j.0908-8857.2001.320302.x) [DOI] [Google Scholar]
- 9.Johnson MD. 2007. Measuring habitat quality: a review. Condor 109, 489–504. ( 10.1650/8347.1) [DOI] [Google Scholar]
- 10.Norris DR, Marra PP, Kyser TK, Sherry TW, Ratcliffe LM. 2004. Tropical winter habitat limits reproductive success on the temperate breeding grounds in a migratory bird. Proc. R. Soc. Lond. B 271, 59–64. ( 10.1098/rspb.2003.2569) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Harrison X, Blount AJD, Inger R, Norris DR, Bearhop S. 2011. Carry-over effects as drivers of fitness differences in animals. J. Anim. Ecol. 80, 4–18. ( 10.1111/j.1365-2656.2010.01740.x) [DOI] [PubMed] [Google Scholar]
- 12.Johnson MD, Sherry TW, Holmes RT, Marra PP. 2006. Assessing habitat quality for a migratory songbird wintering in natural and agricultural habitats. Conserv. Biol. 20, 1433–1444. ( 10.1111/j.1523-1739.2006.00490.x) [DOI] [PubMed] [Google Scholar]
- 13.Angelier F, Tonra CM, Holberton RL, Marra PP. 2011. Short-term changes in body condition in relation to habitat and rainfall abundance in American redstarts Setophaga ruticilla during the non-breeding season. J. Avian Biol. 42, 335–341. ( 10.1111/j.1600-048X.2011.05369.x) [DOI] [Google Scholar]
- 14.Møller AP, Szep T. 2002. Survival rate of adult barn swallows Hirundo rustica in relation to sexual selection and reproduction. Ecology 83, 2220–2228. ( 10.1890/0012-9658(2002)083[2220:SROABS]2.0.CO;2) [DOI] [Google Scholar]
- 15.Blums P, Clark R. 2002. Patterns of reproductive effort and success in birds: path analyses of long-term data from European ducks. J. Anim. Ecol. 71, 280–295. ( 10.1046/j.1365-2656.2002.00598.x) [DOI] [Google Scholar]
- 16.Møller AP. 1994. Sexual selection and the barn swallow. New York, NY: Oxford University Press. [Google Scholar]
- 17.Smith RJ, Moore FR. 2003. Arrival fat and reproductive performance in a long-distance passerine migrant. Oecologia 134, 325–331. ( 10.1007/s00442-002-1152-9) [DOI] [PubMed] [Google Scholar]
- 18.Shutler D, et al. 2012. Spatiotemporal patterns in nest box occupancy by tree swallows across North America. Avian Conserv. Ecol. 7, 3 ( 10.5751/ACE-00517-070103) [DOI] [Google Scholar]
- 19.Sauer JR, Hines JE, Fallon JE, Pardieck KL, Ziolkowski DJ, Jr, Link DA. 2011. The North American breeding bird survey, results and analysis 1966–2011, version 12.13.2011 Laurel, MD: USGS Patuxent Wildlife Research Center. [Google Scholar]
- 20.Van de Pol M, Wright J. 2009. A simple method for distinguishing within- versus between-subject effects using mixed models . Anim. Behav. 77, 753–758. ( 10.1016/j.anbehav.2008.11.006) [DOI] [Google Scholar]
- 21.Balbontin J, Møller AP, Hermosell IG, Marzal A, Reviriego M, de Lope F. 2012. Lifetime individual plasticity in body condition of a migratory bird. Biol. J. Linn. Soc. 105, 420–434. ( 10.1111/j.1095-8312.2011.01800.x) [DOI] [Google Scholar]
- 22.Fischer C, Flohre A, Clement LW, Batáry P, Weisser WW, Tscharntke T, Thies C. 2011. Mixed effects of landscape structure and farming practice on bird diversity. Agric. Ecosyst. Environ. 141, 119–125. ( 10.1016/j.agee.2011.02.021) [DOI] [Google Scholar]
- 23.Ghilain A, Bélisle M. 2008. Breeding success of tree swallows along a gradient of agricultural intensification. Ecol. Appl. 18, 1140–1154. ( 10.1890/07-1107.1) [DOI] [PubMed] [Google Scholar]
- 24.Rioux Paquette S, Garant D, Pelletier F, Bélisle M. 2013. Seasonal patterns in tree swallow prey (Diptera) abundance are affected by agricultural intensification. Ecol. Appl. 23, 122–133. ( 10.1890/12-0068.1) [DOI] [PubMed] [Google Scholar]
- 25.Winkler DW, Hallinger KK, Ardia DR, Robertson RJ, Stutchbury BJ, Cohen RR. 2011. Tree swallow (Tachycineta bicolor). In The birds of North America online (ed. Poole A.). Ithaca, NY: Cornell Lab of Ornithology. [Google Scholar]
- 26.Bromley RG, Jarvis RL. 1993. The energetics of migration and reproduction of dusky Canada geese. Condor 95, 193–210. ( 10.2307/1369400) [DOI] [Google Scholar]
- 27.Battley PF, Piersma T, Dietz MW, Tang S, Dekinga A, Hulsman K. 2000. Empirical evidence for differential organ reductions during trans-oceanic bird flight. Proc. R. Soc. Lond. B 267, 191–195. ( 10.1098/rspb.2000.0986) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schamber JL, Esler D, Flint PL. 2009. Evaluating the validity of using unverified indices of body condition. J. Avian Biol. 40, 49–56. ( 10.1111/j.1600-048X.2008.04462.x) [DOI] [Google Scholar]
- 29.Labocha MK, Hayes JP. 2012. Morphometric indices of body condition in birds: a review. J. Ornithol. 153, 1–22. ( 10.1007/s10336-011-0706-1) [DOI] [Google Scholar]
- 30.Bates D, Maechler M. 2010. lme4: linear mixed-effects models using S4 classes. R package, v. 0.999375-37 See http://cran.r-project.org/web/packages/lme4. [Google Scholar]
- 31.Moreno J. 1989. Strategies of mass change in breeding birds. Biol. J. Linn. Soc. 37, 297–310. ( 10.1111/j.1095-8312.1989.tb01907.x) [DOI] [Google Scholar]
- 32.Burnham KP, Anderson DR. 2002. Model selection and multimodel inference: a practical information-theoretic approach, 2nd edn Berlin, Germany: Springer. [Google Scholar]
- 33.Mazerolle M. 2011. AICcmodavg: model selection and multimodel inference based on (Q)AIC(c). R Package, v. 1.14 See http://cran.r-project.org/web/packages/AICcmodavg. [Google Scholar]
- 34.Schaub M, Jenni L. 2000. Body mass of six long-distance migrant passerine species along the autumn migration route. J. Ornithol. 141, 441–460. ( 10.1046/j.1439-0361.2000.00037.x) [DOI] [Google Scholar]
- 35.Blem CR, Blem LB. 2006. Variation in mass of female prothonotary warblers during nesting. Wilson J. Ornithol. 118, 3–12. ( 10.1676/1559-4491(2006)118) [DOI] [Google Scholar]
- 36.Whittingham LA, Dunn PO. 2000. Offspring sex ratios in tree swallows: females in better condition produce more sons. Mol. Ecol. 9, 1123–1129. ( 10.1046/j.1365-294X.2000.00980.x) [DOI] [PubMed] [Google Scholar]
- 37.Kleijn D, Schekkerman H, Dimmers WJ, Van Kats RJM, Melman D, Teunissen WA. 2010. Adverse effects of agricultural intensification and climate change on breeding habitat quality of black-tailed godwits Limosa l. limosa in The Netherlands. Ibis 152, 475–486. ( 10.1111/j.1474-919X.2010.01025.x) [DOI] [Google Scholar]
- 38.Mineau P, Whiteside M. 2013. Pesticide acute toxicity is a better correlate of U.S. grassland bird declines than agricultural intensification. PLoS ONE 8, e57457 ( 10.1371/journal.pone.0057457) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lagrange P, Pradel R, Bélisle M, Gimenez O. 2014. Estimating dispersal between numerous sites using capture–recapture data. Ecology, in press ( 10.1890/13-1564.1) [DOI] [PubMed] [Google Scholar]
- 40.Yom-Tov Y, Geffen E. 2011. Recent spatial and temporal changes in body size of terrestrial vertebrates: probable causes and pitfalls. Biol. Rev. 86, 531–541. ( 10.1111/j.1469-185X.2010.00168.x) [DOI] [PubMed] [Google Scholar]
- 41.Van Buskirk J, Mulvihill RS, Leberman RC. 2010. Declining body sizes in North American birds associated with climate change. Oikos 119, 1047–1055. ( 10.1111/j.1600-0706.2009.18349.x) [DOI] [Google Scholar]
- 42.Goodman RE, Lebuhn G, Seavy NE, Gardali T, Bluso-Demers JD. 2012. Avian body size changes and climate change: warming or increasing variability? Glob. Change Biol. 18, 63–73. ( 10.1111/j.1365-2486.2011.02538.x) [DOI] [Google Scholar]
- 43.Teplitsky C, Millien V. 2014. Climate warming and Bergmann's rule through time: is there any evidence? Evol. Appl. 7, 156–168. ( 10.1111/eva.12129) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gardner JL, Peters A, Kearney M, Joseph L, Heinsohn R. 2011. Declining body size: a third universal response to warming? Trends Ecol. Evol. 26, 285–291. ( 10.1016/j.tree.2011.03.005) [DOI] [PubMed] [Google Scholar]
- 45.Brown CR, Bomberger Brown M. 2013. Where has all the road kill gone? Curr. Biol. 23, R233–R234. ( 10.1016/j.cub.2013.02.023) [DOI] [PubMed] [Google Scholar]
- 46.Knudsen E, et al. 2011. Challenging claims in the study of migratory birds and climate change. Biol. Rev. 86, 928–946. ( 10.1111/j.1469-185X.2011.00179.x) [DOI] [PubMed] [Google Scholar]
- 47.Durant JM, Hjermann DØ, Ottersen G, Stenseth NC. 2007. Climate and the match or mismatch between predator requirements and resource availability. Clim. Res. 33, 271–283. ( 10.3354/cr033271) [DOI] [Google Scholar]
- 48.Reed TE, Grøtan V, Jenouvrier S, Saether B-E, Visser ME. 2013. Population growth in a wild bird is buffered against phenological mismatch. Science 340, 488–491. ( 10.1126/science.1232870) [DOI] [PubMed] [Google Scholar]
- 49.Dunn PO, Winkler DW, Whittingham LA, Hannon SJ, Robertson RJ. 2011. A test of the mismatch hypothesis: how is timing of reproduction related to food abundance in an aerial insectivore? Ecology 92, 450–461. ( 10.1890/10-0478.1) [DOI] [PubMed] [Google Scholar]
- 50.Marra PP, Hobson KA, Holmes RT. 1998. Linking winter and summer events in a migratory bird by using stable-carbon isotopes. Science 282, 1884–1886. ( 10.1126/science.282.5395.1884) [DOI] [PubMed] [Google Scholar]
- 51.Laughlin AJ, et al. 2013. Integrating information from geolocators, weather radar, and citizen science to uncover a key stopover area of an aerial insectivore. Auk 130, 230–239. ( 10.1525/auk.2013.12229) [DOI] [Google Scholar]
- 52.FAO Statistical Yearbook. 2013. World food and agriculture. Rome, Italy: FAO; (www.fao.org/publications) [Google Scholar]
- 53.Calvert AM, Walde SJ, Taylor PD. 2009. Non-breeding drivers of population dynamics in seasonal migrants: conservation parallels across taxa. Avian Conserv. Ecol. 4, 5. [Google Scholar]
- 54.Teplitsky C, Mills JA, Alho JS, Yarrall JW, Merilä J. 2008. Bergmann's rule and climate change revisited: disentangling environmental and genetic responses in a wild bird population. Proc. Natl Acad. Sci. USA 105, 13 492–13 496. ( 10.1073/pnas.0800999105) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Husby A, Hille SM, Visser ME. 2011. Testing mechanisms of Bergmann's rule: phenotypic decline but no genetic change in body size in three passerine bird populations. Am. Nat. 178, 202–213. ( 10.1086/660834) [DOI] [PubMed] [Google Scholar]
- 56.Ardia DR, Pérez JH, Chad EK, Voss MA, Clotfelter ED. 2009. Temperature and life history: experimental heating leads female tree swallows to modulate egg temperature and incubation behaviour. J. Anim. Ecol. 78, 4–13. ( 10.1111/j.1365-2656.2008.01453.x) [DOI] [PubMed] [Google Scholar]
- 57.Winkler DW. 1992. Causes and consequences of variation in parental defense behavior by tree swallows. Condor 94, 502–520. ( 10.2307/1369222) [DOI] [Google Scholar]
- 58.Conroy MJ, Costanzo GR, Stotts DB. 1989. Winter survival of female American black ducks on the Atlantic Coast. J. Wildl. Manag. 53, 99–109. ( 10.2307/3801314) [DOI] [Google Scholar]
- 59.Bergan JF, Smith LM. 1993. Survival rates of female mallards wintering in the Playa Lakes. J. Wildl. Manag. 57, 570–577. ( 10.2307/3809284) [DOI] [Google Scholar]
- 60.Thaxter CB, Joys A, Gregory R, Baillie SR, Noble DN. 2010. Hypotheses to explain patterns of population change among breeding bird species in England. Biol. Conserv. 143, 2006–2019. ( 10.1016/j.biocon.2010.05.004) [DOI] [Google Scholar]
- 61.Sanderson FJ, Donald PF, Pain DJ, Burfield IJ, van Bommel FPJ. 2006. Long-term population declines in Afro-Palearctic migrant birds. Biol. Conserv. 131, 93–105. ( 10.1016/j.biocon.2006.02.008) [DOI] [Google Scholar]
- 62.Faaborg J, et al. 2010. Conserving migratory land birds in the New World: do we know enough? Ecol. Appl. 20, 398–418. ( 10.1890/09-0397.1) [DOI] [PubMed] [Google Scholar]
- 63.Fraser KC, et al. 2012. Continent-wide tracking to determine migratory connectivity and tropical habitat associations of a declining aerial insectivore. Proc. R. Soc. B 279, 4901–4906. ( 10.1098/rspb.2012.2207) [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The datasets used in this work have been uploaded to the Dryad Digital Repository: (doi:10.5061/dryad.67t23).