Abstract
Several plant traits are known to evolve in predictable ways on islands. For example, herbaceous species often evolve to become woody and species frequently evolve larger leaves, regardless of growth form. However, our understanding of how seed sizes might evolve on islands lags far behind other plant traits. Here, we conduct the first test for macroevolutionary patterns of seed size on islands. We tested for differences in seed size between 40 island–mainland taxonomic pairings from four island groups surrounding New Zealand. Seed size data were collected in the field and then augmented by published seed descriptions to produce a more comprehensive dataset. Seed sizes of insular plants were consistently larger than mainland relatives, even after accounting for differences in growth form, dispersal mode and evolutionary history. Selection may favour seed size increases on islands to reduce dispersibility, as long-distance dispersal may result in propagule mortality at sea. Alternatively, larger seeds tend to generate larger seedlings, which are more likely to establish and outcompete neighbours. Our results indicate there is a general tendency for the evolution of large seeds on islands, but the mechanisms responsible for this evolutionary pathway have yet to be fully resolved.
Keywords: insular evolution, island, New Zealand, plants, seed
1. Introduction
Seed size varies greatly among plant species, from tiny wind-dispersed orchid seeds to the massive double coconut (Lodoicea maldivica) [1], and has important consequences for reproductive success [2–4]. Seedling survival is directly influenced by seed size [4,5], and many functional traits covary with the size of seeds. These traits include dispersal mode, growth form, specific leaf area and seed number [6,7]. Many of these other traits evolve predictably on islands (e.g. growth form and leaf area; [8,9]); however, detailed quantitative investigations of how seed size is affected by insularity are lacking.
A reduction in the dispersal ability of seeds is a common evolutionary pathway for plants on islands [9,10]. For example, wind-dispersed members of the family Asteraceae typically display a reduction in pappus size relative to achene size on islands [9,10]. Animal-dispersed taxa (such as Bidens), which produce structures that promote ectozoochory, illustrate a similar pattern, with a reduction in the size of hooks and awns relative to achene size [9]. Furthermore, fleshy-fruited members of the family Araliaceae tend to produce larger fruits and seeds on islands [9]. The evolution of reduced dispersibility has also been detected in as few as five generations, suggesting strong selection pressures [10].
One explanation for potential changes in seed size on islands is that the small size and isolation of islands may select against dispersal to reduce propagule mortality at sea [9,11]. Selection acting to increase seed size may reduce wind dispersal distances in anemochorous species [12]. The same may be true for fleshy-fruited plants, although tests for directional changes in seed size of fleshy-fruited species have yet to be conducted.
Selection may favour larger seed size on islands for reasons other than dispersal ability. For example, most islands house fewer species than comparable communities on the mainland. Therefore, a germinating seedling on an island is more likely to be adjacent to a conspecific, leading to greater levels of intraspecific competition [13,14]. Larger seeds are more competitive than small seeds, all else being equal [2,4,5]. Therefore, higher levels of intraspecific competition on islands may also select for increases in seed size.
Here, we conduct the first macroevolutionary test for increased seed size on islands. By collecting specimens in the field and using published seed descriptions, we compiled a diverse dataset consisting of 40 island–mainland taxonomic pairings from four island systems surrounding mainland New Zealand. To test for overarching changes in seed size on islands, we first compared seed sizes on islands with seed sizes on the mainland using reduced major axis (RMA) regression. Second, we used a mixed effects modelling approach to test for effects of evolutionary history, growth form and dispersal mode on the island–mainland seed size relationship.
2. Methods
New Zealand has a long history of geological isolation. It separated from Gondwana 80 Ma and has been isolated in the southwest Pacific since. The three main islands (North, South and Stewart Islands) are encircled by numerous smaller islands [15], the flora of which consists mainly of taxa that have dispersed overwater from New Zealand. Although many of these islands were once connected by land bridges, we focused on four island groups that remained isolated from the main islands during the Pleistocene (Kermadec, Three Kings, Chatham and sub-Antarctic Islands; see [16]). In particular, we focused on the Chatham group (176° W, 44° S), situated 850 km east of the main islands (figure 1).
Figure 1.
Map of study islands surrounding New Zealand.
To maximize the number of species in the dataset, we visited the Chatham Islands twice, at different times of year. This allowed the inclusion of species with different fruiting phenologies. Seed sizes were measured on Chatham Island taxa in January 2008 [8] and March 2012. Searches were made in Henga Scenic Reserve (43°51.0′ S, 176°33.2′ W), Nikau Forest Reserve (43°45.7′ S, 176°34.8′ W) and Rangaika Scenic Reserve (44°3′37.0182″ S, 176°26′6.0792″ W). Mainland samples were collected from Otari Walton's Bush (41°14′ S, 174°45′ E), Moa Point (41°20′ S, 174°49′ E) and Nelson Lakes National Park (41°48′ S, 172°50′ E). Fruits were randomly selected from each individual, collecting five or more from at least five individuals (following [17]). This was not always achievable; therefore, a variable number of seeds were used to characterize seed size (table 1). Seed size was estimated as the product of seed length (length of the longest axis) × seed width (maximum distance perpendicular to the length measurement at the widest point of the seed).
Table 1.
Average seed area for insular species and mainland relatives (mm2). Numbers in parentheses refer to the numbers of individuals and seeds sampled, respectively. Italicized letters in parentheses indicate data acquired from published sources (a ,[18]; h, [19]).
| insular taxa | seed size (mm2) | growth form | dispersal mode | mainland taxa | seed size (mm2) |
|---|---|---|---|---|---|
| Olearia chathamica | 19.94 (5, 25)a | tree | wind | Pleurophyllum criniferum | 14.56 (a)a |
| Olearia traversiorum | 3.26 (6, 30)a | tree | wind | Olearia virgata | 1.27 (a)a |
| Rhipogonum scandens | 71.78 (7, 48) | vine | fleshy fruit | Rhipogonum scandens | 55.40 (3, 22) |
| Myrsine chathamica | 39.30 (7, 67) | shrub | fleshy fruit | Myrsine argentea | 8.70 (a) |
| Myrsine divaricata | 8.10 (a) | ||||
| Corokia macrocarpa | 40.44 (8, 66) | shrub | fleshy fruit | Corokia cotoneaster | 18.01 (3, 16) |
| Leptecophylla robusta | 18.63 (6, 49) | shrub | fleshy fruit | Leptecophylla juniperina | 5.93 (6, 46) |
| Coprosma propinqua var. martinii | 13.93 (8, 84) | shrub | fleshy fruit | Coprosma propinqua var. propinqua | 11.75 (1, 30) |
| Melicytus chathamicus | 20.43 (7, 118) | shrub | fleshy fruit | Melicytus aff. alpinus | 9.04 (2, 12) |
| Coprosma acerosa | 9.87 (5, 58) | shrub | fleshy fruit | Coprosma acerosa | 3.26 (1, 30) |
| Macropiper excelsum | 3.94 (3, 32) | shrub | fleshy fruit | Macropiper excelsum | 3.59 (3, 30) |
| Apium prostratum subsp. denticulatum | 2.93 (5, 38) | herb | water | Apium prostratum subsp. prostratum | 4.05 (3, 30) |
| Rhopalostylis aff. sapida | 175.83 (3, 24) | tree | fleshy fruit | Rhopalostylis sapida | 80.79 (3, 30) |
| Muehlenbeckia australis | 9.10 (3, 35) | vine | fleshy fruit | Muehlenbeckia australis | 6.98 (3, 30) |
| Tetragonia implexicoma | 28.85 (2, 24) | herb | fleshy fruit | Tetragonia implexicoma | 14.26 (3, 30) |
| Coprosma chathamica | 38.76 (a) | tree | fleshy fruit | Coprosma repens | 21.61 (a) |
| Hebe dieffenbachii | 1.17 (a)a | shrub | wind | Hebe elliptica | 1.83 (a)a |
| Psuedopanax chathamicus | 19.92 (a)a | tree | fleshy fruit | Psuedopanax crassifolius | 6.74 (a)a |
| Psuedopanax kermadecensis | 12.03 (a)a | tree | fleshy fruit | Psuedopanax arboreus | 11.14 (a)a |
| Alectryon excelsus subsp. grandis | 55.40 (a)a | tree | fleshy fruit | Alectryon excelsus | 45.52 (a)a |
| Streblus smithii | 43.52 (a)a | tree | fleshy fruit | Streblus banksii | 26.96 (a)a |
| Myoporum kermadecense | 31.57 (a)a | tree | fleshy fruit | Myoporum laetum | 35.52 (a)a |
| Metrosideros kermdecensis | 11.44 (a)a | tree | wind | Metrosideros excelsum | 12.95 (a)a |
| Macropiper melchior | 5.23 (a)a | shrub | fleshy fruit | Macropiper excelsum | 4.42 (a)a |
| Ascarina lucida var. lanceolata | 1.60 (a)a | tree | fleshy fruit | Ascarina lucida var. lucida | 2.35 (a)a |
| Coprosma acutifolia | 15.93 (a) | shrub | fleshy fruit | Coprosma tenuifolia | 17.39 (a) |
| Stilbocarpa polaris | 4.27 (a)a | herb | fleshy fruit | Stilbocarpa lyalii | 5.03 (a)a |
| Olearia lyallii | 38.63 (a)a | shrub | wind | Olearia colensoi | 21.88 (a)a |
| Myosotis capitata | 2.87 (a) | herb | wind | Myosotis australis | 1.44 (a) |
| Gentianella cerina | 0.51 (a)a | herb | wind | Gentianella saxosa | 0.89 (a)a |
| Leptinella plumosa | 3.57 (a)a | herb | wind | Leptinella nana | 0.73 (a)a |
| Leptinella minor | 1.07 (a)a | ||||
| Leptinella filiformis | 0.73 (a)a | ||||
| Leptinella lanata | 3.40 (a)a | herb | wind | Leptinella nana | 0.73 (a)a |
| Leptinella minor | 1.07 (a)a | ||||
| Leptinella filiformis | 0.73 (a)a | ||||
| Abrotanella rosulata | 2.08 (a)a | herb | wind | Abrotanella rostrata | 4.27 (a)a |
| Abrotanella spathulata | 2.78 (a)a | herb | wind | Abrotanella rostrata | 4.27 (a)a |
| Pennantia baylisiana | 43.52 (a)a | tree | fleshy fruit | Pennantia corymbosa | 23.93 (a)a |
| Embergeria grandifolia | 17.32 (a)a | herb | wind | Kirkianella novae-zelandiae | 12.64 (a)a |
| Hebe chathamica | 1.54 (h) | shrub | wind | Hebe elliptica | 1.74 (a) |
| Hebe barkeri | 1.86 (h) | tree | wind | Hebe elliptica | 1.74 (a) |
| Leptinella featherstonii | 1.27 (a)a | herb | wind | Leptinella serrulata | 1.48 (a)a |
| Geranium traversii | 5.23 (a)a | herb | wind | Geranium brevicaule | 3.08 (a)a |
| Brachyglottis huntii | 5.23 (a)a | tree | wind | Brachyglottis stewartiae | 7.92 (a)a |
aCases where seed area was estimated from length measurements (see §2).
To allow for the greatest possible number of island–mainland comparisons, we supplemented field data (25 taxa) with seed measurements contained in Seeds of New Zealand gymnosperms and dicotyledons (hereafter ‘seed atlas’; [18]). Seed descriptions in the seed atlas result from the examination of at least 10 seeds from each of 10 collections of fruiting material for each species. We obtained the median value from seed dimension ranges when compiling data from within the seed atlas. To justify the use of seed atlas data and to promote accuracy, we ran ordinary least-squares (OLS) regression of field measures against data from the seed atlas. Measurements for two Hebe species were not contained in the seed atlas, and seed sizes were obtained from An Illustrated Guide to New Zealand Hebes [19]. In many cases (42 taxa), the literature provided only length dimensions for seed size. We therefore ran OLS regression of area (length × width, as described above) against length, for the 36 taxa where both length and width parameters were available. Regression parameters were then used to estimate area for those taxa where only a length dimension was available. ANOVA of observed versus predicted values was carried out to test the robustness of seed area predictions. Variables were log-transformed prior to analysis.
Determining mainland relatives for insular species was simple when taxa were undifferentiated (table 1). For Chatham Island endemics, the recent molecular analysis by Heenan et al. [20] was used where possible. In this study, DNA sequence data were used to identify the closest relatives for 35 taxa endemic to the Chatham Islands. For taxa from other island systems, phylogenetic analyses were used where available [21–26]. When multiple mainland taxa were identified as being equally related to an island endemic, the average seed size of the mainland taxa was used (Leptinella plumosa and L. lanata—see [22] and Myrsine chathamica—see [27]). Where taxa were differentiated at species, but not genus level the mainland taxon chosen is the most likely relative based on morphological similarities (e.g. Macropiper melchior and M. excelsum; Coprosma acutifolia and C. tenuifola—see [28]). In other cases, insular taxa were a variety or subspecies of well-known mainland species.
To test for differences in seed size among island and mainland taxa, a variety of statistical methods could be used. Regressing mean values for insular taxa against mainland taxa is one option. The slope and intercept parameters providing information on the relationship (slope > 1 and intercept > 0 = island taxa with larger seeds; slope < 1 and intercept < 0 = mainland seed size larger). However, the use of OLS regression minimizes the sum of squared variation in the Y (in this case, island) direction and is not appropriate when measurement error in X and Y variables is likely. To avoid these confounding sources of bias, we ran RMA regression. RMA was used to obtain slope and intercept parameters along with 95% confidence intervals.
Phylogenetic relatedness between island–mainland taxonomic pairs creates a lack of independence in our dataset. To overcome this, we ran a mixed effects model treating seed size as the dependent variable, location (island or mainland) as a fixed factor, and ‘species pair’ (island–mainland taxonomic pairing) as a random factor. Many of the taxonomic pairings are separated at species level, potentially indicating more evolutionary divergence than conspecific pairings. We therefore included a second random effect of nested taxonomy (with two levels: undifferentiated at species level or differentiated at species level) in the model structure. Seed dispersal mode and plant growth form were included as fixed factors to test for effects they may have on the island–mainland seed size relationship.
The majority of taxa in our dataset were either wind-dispersed or fleshy-fruited plants. We therefore defined dispersal modes as either ‘dry-fruited’ (wind, water, ballistic/wind) or ‘fleshy-fruited’. Only two island–mainland taxonomic pairings were vines, and we therefore removed these taxa from the growth form analysis. We are specifically interested in whether dispersal mode or growth form influences seed size differences between ‘locations’ (island or mainland). Therefore, the two-way interactions between location and these two factors were maintained in the model structure. We calculated the ratio of island seed size to mainland seed size to visualize whether seeds tended to be larger on islands for each level of dispersal mode and growth form. Ratios greater than one indicate a tendency for larger island seeds. Kruskal–Wallis tests were then used to compare ratios between dispersal modes and growth forms. Robust tests for the effect of island system were not possible owing to uneven sample sizes between islands.
All analyses were conducted in the R environment for statistical computing [29]. RMA analyses were conducted with the SMATR package [30], and the CAR package [31] was used to carry out a likelihood ratio test for the mixed effects model. Seed size data were logarithm transformed to conform to normality assumptions.
3. Results
Field measurements scaled positively with those contained in the seed atlas (OLS regression: R2 = 0.912; p < 0.001). Slope and intercept parameters were not significantly different from one and zero, respectively (slope: 0.971, 95% CI = 0.811–1.131, p = 0.698; intercept: −0.054, 95% CI = −0.373–0.266, p = 0.721), indicating that published seed sizes accurately reflect field measurements. OLS regression showed that seed lengths scaled strongly with seed surface area (R2 = 0.928; p < 0.001; see the electronic supplementary material, figure S1). Seed area estimates calculated using the regression equation did not differ significantly from observed values (F1,70 = 0.086, p = 0.770), indicating that estimates accurately reflected real seed sizes.
Island seed sizes scaled positively with mainland seed sizes (RMA analysis: R2 = 0.854, p < 0.001; figure 2). However, the island–mainland seed size relationship had a slope greater than one (1.18; 95% CI = 1.041–1.338) and an intercept marginally less than zero (−0.021; 95% CI = −0.166 to 0.123). This indicates a tendency for insular taxa to produce larger seeds than mainland relatives, particularly at the larger end of the seed size spectrum.
Figure 2.
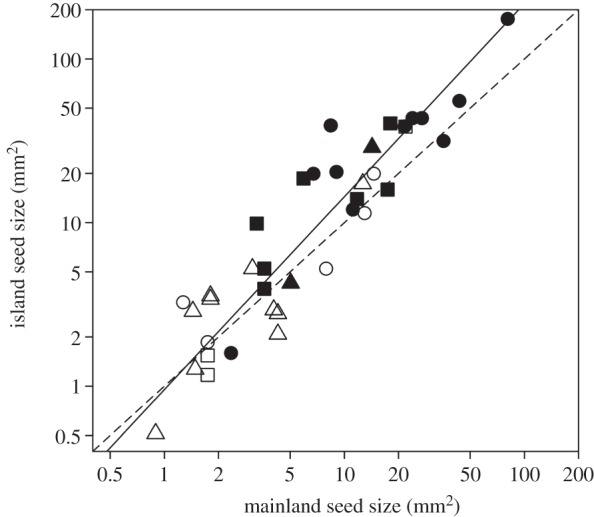
Relationship between island and mainland seed size. Each point represents a taxonomic pairing between an insular taxon and its corresponding mainland relative. Circles represent trees, squares are shrubs, and triangles are herbs. Closed symbols indicate fleshy-fruited taxa and open symbols dry-fruited. The dashed line represents isometry and the solid line is the result of RMA regression (y = 1.180x − 0.021). Both axes are logarithm transformed.
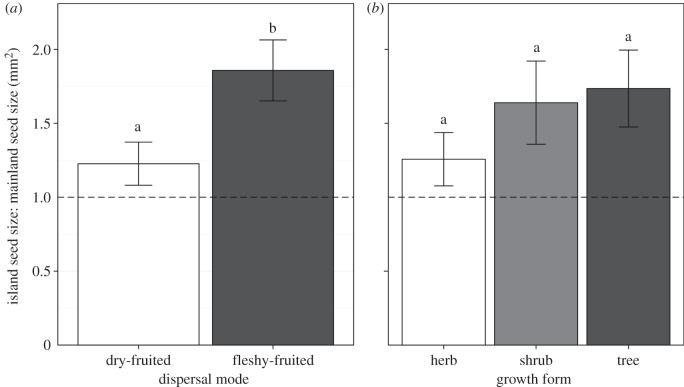
The likelihood ratio test from the mixed effects model showed no significant effect of plant growth form on the island-to-mainland seed size relationship (χ2 = 7.32, d.f. = 4, p = 0.120). However, there was a significant effect of dispersal mode (χ2 = 14.328, d.f. = 2, p < 0.001). Seed sizes did differ significantly by location (island or mainland; χ2 = 12.562, d.f. = 1, p < 0.001), consistent with results of the RMA analysis, and all dispersal modes and growth forms displayed a tendency for larger seed sizes on islands (figure 3a,b).
Figure 3.
Average island seed size to mainland seed size (mm2) ratio by (a) dispersal mode and (b) growth form. The dashed horizontal line represents equal island and mainland seed size (ratio = 1; ratio > 1 indicates larger seeds on islands, ratio < 1 indicates larger mainland seeds). Error bars are standard error. Letters indicate whether average island to mainland seed size ratios are different between dispersal modes and growth forms (Kruskal–Wallis test: (a) χ2 = 5.61, d.f. = 1, p = 0.018; (b) χ2 = 2.28, d.f. = 2, p = 0.321).
4. Discussion
Seeds of insular taxa were consistently larger than those of their mainland relatives. Furthermore, this result was consistent, regardless of dispersal mode and growth form. Increasing seed size reduces dispersibility in anemochorous species, which is intuitive, as large seeds are likely to disperse over shorter distances than small seeds [1,10,12,32,33]. In the case of fleshy-fruited plants, this is the first time, to the best of our knowledge, that an increase in seed size on islands has been demonstrated quantitatively. Larger seeds in fleshy-fruits may limit the range of dispersal vectors available to a plant, as well as reducing the number of fruits eaten during a feeding period [34,35]. Furthermore, seed number scales negatively with seed size [36,37] and producing fewer seeds reduces the likelihood of long-distance dispersal [38].
RMA regression of island seed size against mainland seed size produced a scaling relationship that differed from isometry. In particular, a slope parameter of greater than one suggests that the size of insular seeds increases disproportionately with increasing seed size. The majority of small-seeded taxa in the dataset rely on wind dispersal, while species with larger seeds generally produced fleshy fruits. Anemochorous species may experience stronger constraints on maximum seed size owing to the aerodynamics of wind dispersal [12], while in fleshy-fruited species (and zoochorous species in general) seed size may be less tightly constrained. Birds often display size increases on islands and size coupling between fruits and frugivores is common [39,40]. Therefore, putative selection pressures for increased seed size may be less constrained in fleshy-fruited taxa. Analysis of dispersal mode reflected this, as fleshy-fruited taxa showed greater size increases on islands than dry-fruited taxa. However, dry-fruited taxa still displayed a tendency for larger seeds on islands. Furthermore, patterns in seed size persisted even after accounting for differences in taxonomic distance and phylogeny between island–mainland pairings, suggesting that selection for increased seed size is strong on islands (see [10]).
Work on animals suggests the depauperate nature of island communities may increase intraspecific competition and promote insular size changes [13,14,41]. This situation may also apply to plants. Larger seed sizes may promote a competitive advantage owing to the increased nutrient reserves, which produce larger, more competitive seedlings [2,4]. Evidence also suggests that larger seed sizes increase plant survival at later-life stages (e.g. sapling stage; [4]).
Selection early in ontogeny may also influence size patterns evident at later-life stages. In animals, adult body size is strongly influenced by size at birth and a recent investigation suggests that body size patterns on islands may reflect selection acting on birth size [42]. A parallel situation may be occurring in plants. The probability of seedling establishment increases with seed size [4]. Seed size is strongly correlated with traits evident later in ontogeny, such as plant height and stem size [2,7,43]. Many herbaceous lineages develop woodiness on islands, and recent research suggests that an increase in leaf size is common [8,9]. As a result, it could be that selection first acting on seeds may facilitate evolutionary changes at later-life-history stages.
Our results suggest that selection favours increased seed size on islands, regardless of dispersal mode, growth form and evolutionary history. Several processes may explain this macroevolutionary trend. First, increasing seed size may reduce propagule mortality associated with unfavourable dispersal into the ocean (see [9,11]). Second, it may provide competitive advantages post-dispersal and increase the likelihood of establishment. The sizes of plant traits are also known to scale allometrically with one another [37], so selection acting on seeds may facilitate size changes in other traits and this deserves further attention. Direct investigations of potential processes are now needed, in addition to global analyses of seed size to establish whether the observed pattern is universal.
Acknowledgements
We thank the Hokotehi Moriori Trust for granting ethical approval for data collection on Chatham Island. We also thank Rafael Barbieri for statistical advice and two anonymous referees for their comments on previous versions of this manuscript.
Data accessibility
For data accessibility, see the electronic supplementary material, S2.
Funding statement
The Wellington Botanical Society and the Arnold and Ruth Dench New Zealand Botanical Award provided funding.
References
- 1.Harper JL, Lovell PH, Moore KG. 1970. The shapes and sizes of seeds. Annu. Rev. Ecol. Syst. 1, 327–356. ( 10.1146/annurev.es.01.110170.001551) [DOI] [Google Scholar]
- 2.Leishman MR, Westoby M, Jurado E. 1995. Correlates of seed size variation: a comparison among 5 temperate floras. J. Ecol. 83, 517–529. ( 10.2307/2261604) [DOI] [Google Scholar]
- 3.Lord J, Westoby M, Leishman M. 1995. Seed size and phylogeny in 6 temperate floras: constraints, niche conservatism, and adaptation. Am. Nat. 146, 349–364. ( 10.1086/285804) [DOI] [Google Scholar]
- 4.Moles AT, Westoby M. 2004. Seedling survival and seed size: a synthesis of the literature. J. Ecol. 92, 372–383. ( 10.1111/J.0022-0477.2004.00884.x) [DOI] [Google Scholar]
- 5.Leishman MR, Westoby M. 1994. The role of seed size in seedling establishment in dry soil conditions: experimental–evidence from semiarid species. J. Ecol. 82, 249–258. ( 10.2307/2261293) [DOI] [Google Scholar]
- 6.Westoby M, Leishman M, Lord J. 1996. Comparative ecology of seed size and dispersal. Phil. Trans. R. Soc. Lond. B 351, 1309–1317. ( 10.1098/rstb.1996.0114) [DOI] [Google Scholar]
- 7.Corner EJH. 1949. The durian theory or the origin of the modern tree. Ann. Bot. Lond. 13, 367–414. [Google Scholar]
- 8.Burns KC, Herold N, Wallace B. 2012. Evolutionary size changes in plants of the southwest Pacific. Glob. Ecol. Biogeogr. 21, 819–828. ( 10.1111/J.1466-8238.2011.00730.x) [DOI] [Google Scholar]
- 9.Carlquist SJ. 1974. Island biology. New York, NY: Columbia University Press. [Google Scholar]
- 10.Cody ML, Overton JM. 1996. Short-term evolution of reduced dispersal in island plant populations. J. Ecol. 84, 53–61. ( 10.2307/2261699) [DOI] [Google Scholar]
- 11.Darwin C. 1859. On the origin of species by means of natural selection, or the preservation of favoured races in the struggle for life. London, UK: John Murray. [PMC free article] [PubMed] [Google Scholar]
- 12.Greene DF, Johnson EA. 1993. Seed mass and dispersal capacity in wind-dispersed diaspores. Oikos 67, 69–74. ( 10.2307/3545096) [DOI] [Google Scholar]
- 13.Case TJ. 1978. General explanation for insular body size trends in terrestrial vertebrates. Ecology 59, 1–18. ( 10.2307/1936628) [DOI] [Google Scholar]
- 14.Grant PR. 1965. The adaptive significance of some size trends in island birds. Evolution 19, 355–367. ( 10.2307/2406446) [DOI] [Google Scholar]
- 15.Gibbs G. 2006. Ghosts of Gondwana. Nelson, New Zealand: Craig Potton Publishers. [Google Scholar]
- 16.Wallis GP, Trewick SA. 2009. New Zealand phylogeography: evolution on a small continent. Mol. Ecol. 18, 3548–3580. ( 10.1111/j.1365-294X.2009.04294.x) [DOI] [PubMed] [Google Scholar]
- 17.Cornelissen JHC, et al. 2003. A handbook of protocols for standardised and easy measurement of plant functional traits worldwide. Aust. J. Bot. 51, 335–380. ( 10.1071/bt02124). [DOI] [Google Scholar]
- 18.Webb CJ, Simpson MJA. 2001. Seeds of New Zealand gymnosperms and dicotyledons. Christchurch, New Zealand: Manuka Press. [Google Scholar]
- 19.Bayly M, Kellow A. 2006. An illustrated guide to New Zealand hebes. Wellington, New Zealand: Te Papa Press. [Google Scholar]
- 20.Heenan PB, Mitchell AD, de Lange PJ, Keeling J, Paterson AM. 2010. Late-Cenozoic origin and diversification of Chatham Islands endemic plant species revealed by analyses of DNA sequence data. N.Z. J. Bot. 48, 83–136. ( 10.1080/0028825X.2010.494337) [DOI] [Google Scholar]
- 21.Glenny D. 2004. A revision of the genus Gentianella in New Zealand. N.Z. J. Bot. 42, 361–530. ( 10.1080/0028825X.2004.9512910) [DOI] [Google Scholar]
- 22.Himmelreich S, Breitwieser I, Oberprieler C. 2012. Phylogeny, biogeography, and evolution of sex expression in the southern hemisphere genus Leptinella (Compositae, Anthemideae). Mol. Phylogenet. Evol. 65, 464–481. ( 10.1016/j.ympev.2012.07.001) [DOI] [PubMed] [Google Scholar]
- 23.Perrie LR, Shepherd LD. 2009. Reconstructing the species phylogeny of Pseudopanax (Araliaceae), a genus of hybridising trees. Mol. Phylogenet. Evol. 52, 774–783. ( 10.1016/j.ympev.2009.05.030) [DOI] [PubMed] [Google Scholar]
- 24.Wagstaff SJ, Breitwieser I, Ito M. 2011. Evolution and biogeography of Pleurophyllum (Astereae, Asteraceae): a small genus of megaherbs endemic to the Subantarctic Islands. Am. J. Bot. 98, 62–75. ( 10.3732/ajb.1000238) [DOI] [PubMed] [Google Scholar]
- 25.Wagstaff SJ, Breitwieser I, Swenson U. 2006. Origin and relationships of the austral genus Abrotanella (Asteraceae) inferred from DNA sequences. Taxon 55, 95–106. ( 10.2307/25065531) [DOI] [Google Scholar]
- 26.Winkworth RC, Grau J, Robertson AW, Lockhart PJ. 2002. The origins and evolution of the genus Myosotis L. (Boraginaceae). Mol. Phylogenet. Evol. 24, 180–193. ( 10.1016/S1055-7903(02)00210-5) [DOI] [PubMed] [Google Scholar]
- 27.Stockler K. 2001. Origins and evolution of the New Zealand forest flora: a molecular phylogenetic approach, Ph.D. thesis. Massey University, Palmerston North, New Zealand. [Google Scholar]
- 28.Dawson J, Lucas R. 2011. New Zealand‘s native trees. Nelson, New Zealand: Craig Potton Publishers. [Google Scholar]
- 29.R Development Core Team. 2011. R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. (http://www.R-project.org/) [Google Scholar]
- 30.Warton DI, Duursma RA, Falster DS, Taskinen S. 2012. smatr 3: an R package for estimation and inference about allometric lines. Methods Ecol. Evol. 3, 257–259. ( 10.1111/j.2041-210X.2011.00153.x) [DOI] [Google Scholar]
- 31.Fox J, Weisberg S. 2011. An R companion to applied regression. Thousand Oaks, CA: Sage. [Google Scholar]
- 32.Augspurger CK. 1986. Morphology and dispersal potential of wind-dispersed diaspores of neotropical trees. Am. J. Bot. 73, 353–363. ( 10.2307/2444078) [DOI] [Google Scholar]
- 33.Benkman CW. 1995. Wind dispersal capacity of pine seeds and the evolution of different seed dispersal modes in pines. Oikos 73, 221–224. ( 10.2307/3545911) [DOI] [Google Scholar]
- 34.Levey DJ. 1987. Seed size and fruit-handling techniques of avian frugivores. Am. Nat. 129, 471–485. ( 10.1086/284652) [DOI] [Google Scholar]
- 35.Wheelwright NT. 1985. Fruit size, gape width, and the diets of fruit-eating birds. Ecology 66, 808–818. ( 10.2307/1940542) [DOI] [Google Scholar]
- 36.Henery ML, Westoby M. 2001. Seed mass and seed nutrient content as predictors of seed output variation between species. Oikos 92, 479–490. ( 10.1034/j.1600-0706.2001.920309.x) [DOI] [Google Scholar]
- 37.Niklas K. 1994. Plant allometry: the scaling of form and process. Chicago, IL: The University of Chicago Press. [Google Scholar]
- 38.Nathan R. 2006. Long-distance dispersal of plants. Science 313, 786–788. ( 10.1126/science.1124975) [DOI] [PubMed] [Google Scholar]
- 39.Burns KC. 2013. What causes size coupling in fruit–frugivore interaction webs? Ecology 94, 295–300. ( 10.1890/12-1161.1) [DOI] [PubMed] [Google Scholar]
- 40.Clegg SM, Owens IPF. 2002. The ‘island rule’ in birds: medium body size and its ecological explanation. Proc. R. Soc. Lond. B 269, 1359–1365. ( 10.1098/rspb.2002.2024) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lomolino MV. 2005. Body size evolution in insular vertebrates: generality of the island rule. J. Biogeogr. 32, 1683–1699. ( 10.1111/j.1365-2699.2005.01314.x) [DOI] [Google Scholar]
- 42.Aubret F. 2012. Body-size evolution on islands: are adult size variations in tiger snakes a nonadaptive consequence of selection on birth size? Am. Nat. 179, 756–767. ( 10.1086/665653) [DOI] [PubMed] [Google Scholar]
- 43.Moles AT, Falster DS, Leishman MR, Westoby M. 2004. Small-seeded species produce more seeds per square metre of canopy per year, but not per individual per lifetime. J. Ecol. 92, 384–396. ( 10.1111/j.0022-0477.2004.00880.x) [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
For data accessibility, see the electronic supplementary material, S2.