Key Points
Iron status, erythropoietic drive, inflammation, and malaria season combine to control dynamic fluctuations of hepcidin in African children.
At the end of the malaria season, hepcidin is low and ID is more prevalent, so iron therapy may be beneficial at this time.
Abstract
Hepcidin is the master regulatory hormone that governs iron homeostasis and has a role in innate immunity. Although hepcidin has been studied extensively in model systems, there is less information on hepcidin regulation in global health contexts where iron deficiency (ID), anemia, and high infectious burdens (including malaria) all coexist but fluctuate over time. We evaluated iron status, hepcidin levels, and determinants of hepcidin in 2 populations of rural children aged ≤8 years, in the Gambia and Kenya (total n = 848), at the start and end of a malaria season. Regression analyses and structural equation modeling demonstrated, for both populations, similar combinatorial effects of upregulating stimuli (iron stores and to a lesser extent inflammation) and downregulating stimuli (erythropoietic drive) on hepcidin levels. However, malaria season was also a significant factor and was associated with an altered balance of these opposing factors. Consistent with these changes, hepcidin levels were reduced whereas the prevalence of ID was increased at the end of the malaria season. More prevalent ID and lower hepcidin likely reflect an enhanced requirement for iron and an ability to efficiently absorb it at the end of the malaria season. These results, therefore, have implications for ID and malaria control programs.
Introduction
Malaria, iron deficiency (ID), anemia, and high infectious burden are common in young African children and often coexist. Malaria is estimated to cause 271 million episodes of febrile illness each year,1 and iron deficiency anemia (IDA) is the most common cause of years lived with disability in sub-Saharan Africa.2 ID is known to have a serious impact on child development.3 However, there is concern that iron supplementation may increase the risk of malaria4 and other infectious diseases5 in young children. It is hypothesized that malaria itself might influence iron homeostasis and increase risk of ID.6 Identifying the determinants of iron absorption in the context of children living in sub-Saharan Africa could contribute to the development of strategies to restore healthy iron status.
Hepcidin, an antimicrobial peptide, is the master regulator of iron homeostasis. Hepcidin blocks intestinal iron absorption and iron recycling from macrophages by internalizing and degrading ferroportin, the only known iron exporter.7 Hepcidin integrates signals from diverse physiological processes: it can be induced by infection, inflammation, and iron loading and suppressed by ID, hypoxia, and expanded erythropoiesis.8 Persistently raised hepcidin, due to rare hepatic adenomas9 and mutations in TMPRSS6,10 leads to severe iron-refractory IDA.8 Studies demonstrate that Plasmodium falciparum malaria induces hepcidin production in vitro,11 and in vivo in uncomplicated infection,12-14 in acute illness,15,16 in experimental infections,17 and in asymptomatic infection.18 We previously demonstrated that hepcidin is the key molecular determinant of erythrocytic iron utilization in young anemic African children,19 and studies show that dietary iron absorption is markedly impaired in asymptomatic20 and acute21 malarial infection.
In 2 populations of rural African children, in the Gambia and Kenya, we investigated the relationships of hepcidin concentration with multiple coexisting factors, which individually are known to regulate hepcidin in controlled experimental settings. We further considered for the first time the effect of the malaria season on hepcidin levels and used a structural equation model (SEM) to evaluate the strength of different stimuli in determining hepcidin levels within these populations. Our findings contextualize the current understanding of iron-regulatory biology within a global health setting by demonstrating how hepcidin balances signals indicating the need for iron against those indicating the potential risk of iron acquisition. Our results have implications for public health strategies to combat IDA and ID.
Methods
Study sites and design
West Kiang, the Gambia, 2001: A cohort of 780 rural community-based children aged 2-6 years was recruited for 2 cross-sectional surveys, at the start (July 2001) and end of the malaria season (December 2001/January 2002) as described previously.22,23 Plasma samples were available from both time points for 603 children. At each survey, children had a clinical examination, anthropometric measurements, and a blood sample. Children with pyrexia (temperature >37.5°C) had a malaria blood film and appropriate clinical treatment; a blood sample was then taken 2 weeks later after recovery from illness. All children received a 3-day course of mebendazole at the first survey for possible hookworm infection.
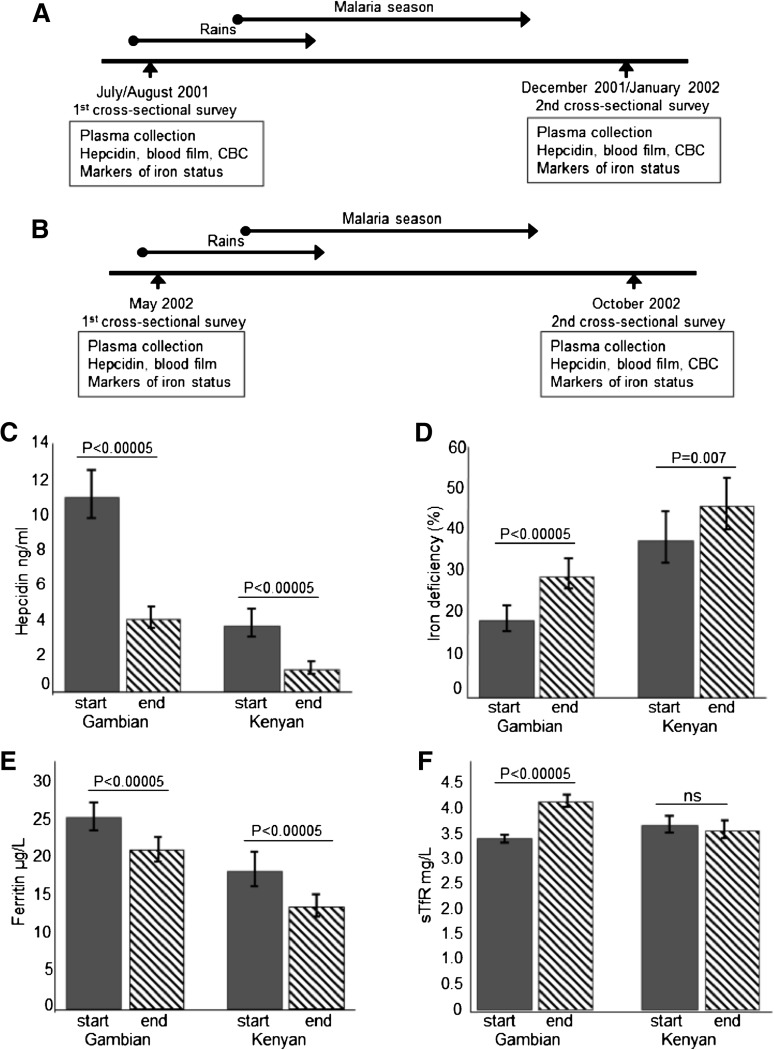
Ngerenya, Kilifi District, Kenya, 2002: This study of 245 children was nested within an ongoing, rolling cohort study evaluating malaria immunity and was used as a replication study for the Gambian study.24 Two cross-sectional surveys were conducted in a cohort of rural community-based children aged 8 months to 8 years at the start (May 2002) and end of the malaria season (October 2002). At each survey, children were clinically assessed, and a blood sample was collected. Weights and heights were available for the May 2002 survey, and weights were available for the October 2002 survey. Complete blood counts were available for a subset of 82 children at the October 2002 survey. Weekly axillary temperatures were measured, and children with an axillary temperature >37.5°C or history of fever during the preceding 48 hours had a blood film and treatment of malaria if the film was positive, as previously described.24 Figure 1A-B shows the study design.
Figure 1.
Study design and hepcidin and iron status at the start and end of the malaria season. (A) Study design at the Gambian site. (B) Study design at the Kenyan site. (C) Plasma hepcidin levels at the start and end of the season. (D) Proportion of iron-deficient children at the start and end of the season. (E) Ferritin levels at the start and end of the season. (F) Soluble transferrin receptor (sTfR) levels at the start and end of the season. CBC indicates complete blood count. Geometric means are presented for continuous variables. The same individual study participants contributed data at the start and end of the season. All analyses were adjusted for age.
Malaria transmission
In the Gambia, malaria transmission is seasonal with the majority of cases occurring from August to November following the rains in July-September, with very little malaria transmission between December and July.25 We therefore defined the malaria season at the Gambian site as the period of peak malaria transmission between August and December. In Kenyan children in the north of Kilifi District, we defined the malaria season as the time of peak malaria transmission between May and October following the long rains from April to July. There is also a second smaller peak in malaria transmission after the short rains in November and lower levels of transmission at other times of the year.24,26 The median number of infective bites per person per year at the time of the studies was 11 in the Gambia (although this varied by geographic location)27 and 10 at the Kenyan study site.24,28
Ethics
Ethical permission was granted for the Gambia 2001 study by the Gambian Government/Medical Research Council (MRC) Ethical Review Committee and for the Kenya 2002 study by the National Ethical Review committee of the Kenya Medical Research Institute (KEMRI), Nairobi. Individual written informed consent was obtained from the parents of all study participants. The study was conducted in accordance with the Declaration of Helsinki.
Laboratory procedures
Plasma ferritin (IMx ferritin assay, Microparticle Enzyme Immunoassay; Abbott Laboratories), sTfR (enzyme-linked immunosorbent assay; R&D systems), hemoglobin (Hb), and mean corpuscular volume (MCV) (Medonic CA 530 Hemoglobinometer) were measured. Markers of inflammation were α-1-antichymotrypsin (ACT) (Immunoturbidimetry, Cobas Mira Plus Bioanalyser; Roche) in Gambian children or C-reactive protein (CRP) (Hitachi Corp.) in Kenyan children as previously described.22,23,29 Thick and thin blood films were stained with Giemsa and examined for P. falciparum parasites according to standard methods. Children were typed for Hb type (HbAA/HbAS) and glucose 6 phosphate dehydrogenase deficiency (G6PDd) type A- as previously described.22,23,29
Plasma hepcidin
Plasma hepcidin was quantified by competitive enzyme-linked immunosorbent assay (Hepcidin-25 [human] EIA Kit; Bachem).19 Standards and samples were analyzed in duplicate or triplicate; a 9-point serial twofold dilution standard curve was used. Samples giving readings outside the standard curve linear region were repeated at appropriate dilutions. Readings with a coefficient of variation (CV) >10% were repeated. The lower limit of detection (LOD) was estimated as 0.08 ng/mL based on the hepcidin value corresponding to 3 standard deviations below the mean no hepcidin blank optical density at 450 nm; undiluted samples giving readings <LOD were reported as LOD/2 = 0.04 ng/mL.
Definitions
Inflammation was defined as ACT >0.6 g/L30 or CRP >5 mg/L31; ID, as ferritin <12 µg/L or <30 µg/L in the presence of inflammation32; and IDA, as ID and Hb <11 g/dL if aged 6 months to 4 years or Hb <11.5 g/dL if aged 5 to 12 years,33 according to standard World Health Organization classifications. Body mass index (BMI) was calculated as weight (kg) per height (m).2
Statistical analyses
Statistical analyses were performed with STATA 12.0 (StataCorp., College Station, TX). Biological data that were not normally distributed were log-transformed, and geometric means were calculated from original untransformed values. Categorical data were expressed in numbers and corresponding percentages. Least squares linear regression analyses were used to evaluate the associations between log-transformed hepcidin concentrations (or other dependent variables) and independent variables in unadjusted and age-adjusted models. The regression coefficients (β) express the change in log-transformed plasma hepcidin associated with a 1-unit change in the independent variable. Odds ratios for risk of ID were derived by logistic regression models. We accounted for potential clustering of observations within individual study children in all of our regression models by use of the Huber-White robust sandwich estimator, which inflates confidence intervals (CIs) and adjusts P values upwards compared with a nonclustered model.34 The likelihood-ratio test was used to assess for interactions between variables. Regression analyses were stratified by site. Final multivariable models included independent variables that were significant (P ≤ .05) in univariable and age-adjusted analyses.
An SEM was used (STATA 12.0, StataCorp.) to test hypothetical explanatory models for hepcidin concentration suggested by the results of regression models. An SEM allows for the simultaneous estimation of correlations and thus testing of hypothesized causal relationships between variables with a test of fit for the overall model as well as individual associations. On the basis of a hypothesized framework for how the different variables may be interrelated, and from the results of univariable and multiple regression analyses, the model was built and iteratively tested. Models were grouped by study site, and then a further model was tested for the Gambian site grouped by season. Pathways that were not statistically significant were not shown unless deemed to be physiologically relevant. Nonnormally distributed variables were log-transformed, and missing values were assumed to be missing at random. Potential clustering of observations within individual study children was accounted for by use of the Huber-White robust sandwich estimator. The model fit was tested by using the comparative fit index (CFI), which compares the model with a baseline model that assumes no relationship among the observed indicator variables, and the root mean squared error of approximation (RMSEA), which considers how much error there is for each degree of freedom within the model. A cutoff of >0.95 for the CFI and a value of <0.05 for the RMSEA indicate a good fit.
Results
Characteristics of the study populations
Plasma samples at 2 time points (before and after the season of peak malaria transmission) were available from a total of 603 Gambian children and 245 Kenyan children. Table 1 summarizes the characteristics of the study populations. Median age was 46.8 months (interquartile range [IQR] 35.6, 59.4) in Gambians and 48.7 months (IQR 25.7, 67.8) in Kenyans (P = .78 for difference between sites), and BMI was higher in Kenyan children compared with Gambian children at the start of the season (Table 1). ID was common, affecting 18.6% of Gambian and 38.2% of Kenyan children at the start of the malaria season (Table 1). Kenyan children had lower hepcidin and ferritin levels compared with Gambian children at each time point (P < .0005 for both analyses, Table 1). Iron-deficient children had significantly lower hepcidin levels compared with non-iron-deficient children (2.05 ng/mL vs 16.43 ng/mL and 1.19 ng/mL vs 7.45 ng/mL in Gambian and Kenyan children, respectively, at the start of the season). Supplemental Table 1 (available on the Blood Web site) summarizes the characteristics of iron-deficient children.
Table 1.
Characteristics of study populations by site
| Characteristic | Season time point | The Gambia | Kenya | P |
|---|---|---|---|---|
| Number | 603 | 245 | — | |
| Median age, mo (IQR) | 46.8 (35.6, 59.4) | 48.7 (25.7, 67.8) | .78 | |
| Median BMI (IQR) | 14.5 (13.8, 15.4) | 15.3 (14.5, 16.2) | <.0005 | |
| Male gender, no. | 318 (52.7%) | 136 (55.5%) | .47 | |
| Minority ethnic group,* no. | 61 (10.1%) | 38 (15.5%) | — | |
| HbAS, no. | 78 (13.4%) | 29 (11.8%) | .54 | |
| G6PDd A- type, no.† | 23 (4.9%) | 72 (34.4%) | <.0005 | |
| Hepcidin, ng/mL | Start | 11.2 (9.9, 12.7) | 3.8 (3.1, 4.7) | <.0005 |
| End | 4.2 (3.6, 4.8)‡ | 1.3 (1.0, 1.7)‡ | <.0005 | |
| ACT, g/L or CRP, mg/L | Start | 0.45 (0.44, 0.46) | 0.78 (0.64, 0.95) | — |
| End | 0.39 (0.39, 0.40)‡ | 0.87 (0.73, 1.05) | — | |
| Ferritin, µg/L | Start | 25.5 (23.7, 27.4) | 18.4 (16.2, 20.9) | <.0005 |
| End | 21.1 (19.5, 22.8)‡ | 13.6 (12.3, 15.2)‡ | <.0005 | |
| sTfR, mg/L | Start | 3.4 (3.3, 3.5) | 3.7 (3.5, 3.9) | .002 |
| End | 4.1 (4.0, 4.3)‡ | 3.6 (3.4, 3.8) | <.0005 | |
| Hb concentration, g/dL | Start | 10.6 (10.5, 10.7) | n/a | — |
| End | 9.9 (9.8, 10.1)‡ | 10.3 (10.0, 10.6) | .13 | |
| MCV, fL | Start | 75.4 (74.6, 76.2) | n/a | — |
| End | 74.7 (73.9, 75.5)‡ | 71.6 (69.8, 73.5) | <.0005 | |
| ID, no. (%) | Start | 112/601 (18.6%) | 91/238 (38.2%) | <.0005 |
| End | 176/597 (29.5%)‡ | 114/245 (46.5%)§ | <.0005 | |
| IDA, no. (%) | Start | 83/595 (14.0%) | n/a | — |
| End | 150/595 (25.2%)‡ | 45/82 (54.9%) | <.0005 | |
| P. falciparum parasitemia, no. (%) | Start | 62/601 (10.3%) | 41/243 (16.9%) | .04 |
| End | 156/602 (25.9%)‡ | 20/242 (8.3%)‡ | <.0005 |
All subjects had hepcidin measurements at 2 time points with no missing data. Other measurements may have been missing data from one or other time point. Only 82 Kenyan children had complete blood count data. Geometric mean values are presented for continuous variables, and categorical variables are represented by proportions (%). All analyses, except for that of age, were adjusted for age. ID was defined as ferritin <12 µg/L or ferritin <30 µg/L with inflammation (ACT >0.6 g/L or CRP >10 mg/L). IDA was defined as ID with Hb <11 g/dL if aged 6 mo to 4 y or Hb <11.5 g/dL if aged 5-12 y.
n/a, not available.
Ethnic groups in the Gambia were Mandinka and Fulani (minority group) and in Kenya were Giriama and Chonyi (minority group).
G6PDd A- heterozygotes and homozygotes combined.
P < .0005 for difference between start and end of season.
P = .007 for difference between start and end of season.
Season predicts hepcidin levels and risk of ID
We evaluated the effect of the malaria season on hepcidin levels and on the prevalence of ID. In both of the populations, the prevalence of ID increased significantly between the start and end of the season (from 18.6% to 29.5% in Gambians and from 38.2% to 46.5% in Kenyans; Table 1; Figure 1). Geometric mean hepcidin levels fell significantly across the season in Gambians (from 11.2 ng/mL to 4.2 ng/mL; P < .0005) and in Kenyans (from 3.8 ng/mL to 1.3 ng/mL; P < .0005). Similarly, ferritin levels fell across the season in both populations (Table 1; Figure 1). Increased ID following the season was further reflected in an increased prevalence of IDA, reduced Hb and MCV levels, and increased sTfR levels in Gambians (Table 1). In Kenyan children, sTfR levels were not higher at the end of the season. Because malaria parasitemia causes raised sTfR levels,35-37 Kenyan children potentially experienced competing effects of increased ID increasing sTfR levels and decreased malaria parasitemia decreasing sTfR levels at the end of the season, resulting in no significant difference in levels between the start and end of the season. The prevalence of malaria parasitemia was higher at the end of the season in Gambian children despite reduced inflammation, which may indicate chronic parasitemia (Table 1). BMI improved significantly over the season in Gambians (P < .0005) likely because of the September harvest; these data were not available for Kenyan children at the end of the season. Odds ratios for risk of ID are shown in supplemental Table 2. Risk of ID was 2.4 times higher at the end of the season in Gambians (95% CI, 1.9, 3.1; P < .0005) and 1.5 times higher at the end of the season in Kenyans (95% CI, 1.1, 1.9; P = .007).
Regulatory signals influencing hepcidin
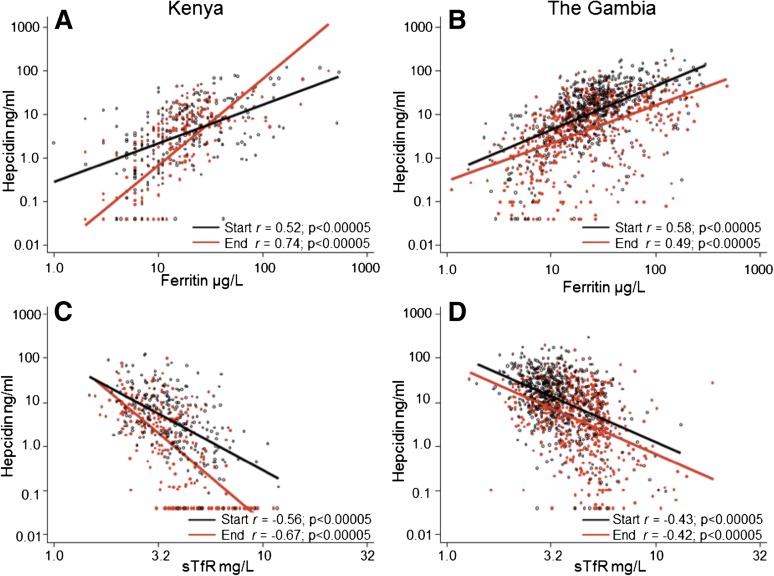
We then evaluated all relevant variables that might predict plasma hepcidin levels because hepcidin regulation integrates different signaling pathways. In univariable and age-adjusted linear regression models, age, season, log-ferritin levels, log-sTfR levels, Hb, MCV, log-ACT, and log-CRP were associated with log-hepcidin levels; the strongest correlations were seen with log-ferritin and log-sTfR (Table 2; Figure 2). We found significant interactions between season and log-ferritin (χ2 46.8, P < .0005) and season and log-sTfR (χ2 20.1, P < .0005) in Kenyan children, so that at the end of the malaria season a smaller unit change in ferritin or sTfR resulted in a significantly larger unit change in hepcidin compared with the start of the season (compare slopes of red and black lines in Figure 2A,C). Thus, at the end of the season the strength of signaling from iron stores and erythropoiesis stimuli had increased in Kenyan children. These interactions were not observed in Gambian children who were less iron deficient (compare slopes of red and black lines in Figure 2B,D).
Table 2.
Results of linear regression analyses for serum hepcidin concentration by site adjusted for age
| Variable | Gambian children | Kenyan children | ||||
|---|---|---|---|---|---|---|
| β (95% CI)* | P* | R2, % | β (95% CI)* | P* | R2, % | |
| Age, y | 0.07 (0.03, 0.11) | .001 | 1.2 | −0.07 (–0.02, −0.12) | .003 | 2.7 |
| BMI, kg/m2 | −0.01 (–0.06, 0.03) | .51 | 3.3 | 0.02 (–0.05, 0.09) | .51 | 3.3 |
| Gender, female | −0.03 (–0.12, 0.07) | .59 | 1.2 | −0.05 (–0.24, 0.14) | .61 | 2.7 |
| Ethnicity, minority group | −0.04 (–0.22, 0.13) | .63 | 1.2 | 0.26 (–0.03, 0.55) | .08 | 3.6 |
| Hb type, HbAS | −0.04 (–0.19, 0.11) | .62 | 1.1 | −0.09 (–0.36, 0.17) | .49 | 2.8 |
| G6PDd, A- type | 0.11 (–0.12, 0.34) | .33 | 1.2 | −0.15 (–0.36, 0.06) | .16 | 3.2 |
| Iron status | ||||||
| Log-ferritin, µg/L | 0.98 (0.88, 1.08) | <.0005 | 28.7 | 1.43 (1.26, 1.60) | <.0005 | 43.8 |
| Log-sTfR, mg/L | −2.26 (–2.57, −1.96) | <.0005 | 22.3 | −3.26 (–3.60, −2.93) | <.0005 | 37.3 |
| Hematologic status | ||||||
| Hb, g/dL | 0.18 (0.15, 0.21) | <.0005 | 14.3 | 0.37 (0.24, 0.50)† | <.0005 | 28.1 |
| MCV, fL | 0.03 (0.03, 0.04) | <.0005 | 10.5 | 0.07 (0.05, 0.10)† | <.0005 | 36.4 |
| Inflammation | ||||||
| Log-ACT, g/L or log-CRP, mg/L | 1.86 (1.49, 2.22) | <.0005 | 8.4 | 0.65 (0.46, 0.84) | <.0005 | 14.4 |
| Malaria | ||||||
| Season, end of malaria season | −0.45 (–0.40, −0.51) | <.0005 | 10.2 | −0.46 (–0.35, −0.56) | <.0005 | 9.1 |
| P. falciparum parasitemia | −0.15 (–0.27, −0.03) | .01 | 1.8 | 0.50 (0.28, 0.71) | <.0005 | 5.9 |
The dependent variable hepcidin was log-transformed before inclusion in the linear regressions models, and all analyses (except for age) were adjusted for age. Ninety-five percent CIs and P values were adjusted to take account of potential within-subject clustering of events using the sandwich estimator.34
These data were available for only 82 children at the end of the season and should be interpreted with caution. The dependent variable for these models was log-transformed hepcidin at the end of the malaria season (because Hb and MCV were not available at the start of the season).
Figure 2.
Log-hepcidin correlates with log-ferritin and log-sTfR levels at the start and end of the malaria season. (A) Correlation between log-hepcidin and log-ferritin levels in Kenyan children. (B) Correlation between log-hepcidin and log-ferritin levels in Gambian children. (C) Correlation between log-hepcidin and log-sTfR levels in Kenyan children. (D) Correlation between log-hepcidin and log-sTfR levels in Gambian children. Red filled circles indicate values at the end of the malaria season; black unfilled circles, values at the start of the malaria season. The red line is the line of best fit at the end of the malaria season, and the black line is the line of best fit at the start of the malaria season. Pearson’s correlation coefficients and significance values are presented.
To identify independent predictors for hepcidin, we constructed site-specific multivariable models in which log-ferritin, log-sTfR, Hb, CRP, and season remained significantly associated with log-hepcidin (Table 3). In the Kenyan model, the interaction between season and log-ferritin in predicting log-hepcidin levels retained significance. These models explained 49% and 62% of total plasma hepcidin variance in Gambian and Kenyan children, respectively.
Table 3.
Results of multivariable regression models for serum hepcidin levels stratified by site
| Variable | Gambian children* | Kenyan children† | ||
|---|---|---|---|---|
| β (95% CI) | P | β (95% CI) | P | |
| Age in years | −0.05 (–0.02, −0.08) | .004 | −0.06 (–0.03, −0.09) | <.0005 |
| Log-ferritin, µg/L | 0.94 (0.84, 1.03) | <.0005 | 0.42 (0.21, 0.63) | <.0005 |
| Interaction ferritin × season | No interaction | 0.92 (0.65, 1.19) | <.0005 | |
| Log-sTfR, mg/L | −1.19 (–0.88, −1.51) | <.0005 | −2.14 (–1.76, −2.52) | <.0005 |
| Hb, g/dL | 0.10 (0.07, 0.13) | <.0005 | n/a | |
| MCV, fL | –0.006 (–0.01, 0.002) | 0.15 | n/a | |
| Log-ACT, g/L or log-CRP, mg/L | 0.16 (–0.21, 0.53) | 0.40 | 0.18 (0.09, 0.28) | <.0005 |
| Season, end of malaria season | −0.15 (–0.09, −0.21) | <.0005 | −1.48 (–1.83, −1.14) | <.0005 |
| P. falciparum parasitemia | −0.09 (–0.001, −0.19) | .05 | 0.05 (–0.12, 0.22) | .53 |
Terms in italics were not significant and were not included in the final model. Ninety-five percent CIs and P values were adjusted to take account of potential within-subject clustering of events using the sandwich estimator.34
R2 is 0.49 indicating that the model explains 49% of total plasma hepcidin variance.
R2 is 0.62 indicating that the model explains 62% of total plasma hepcidin variance.
SEMs
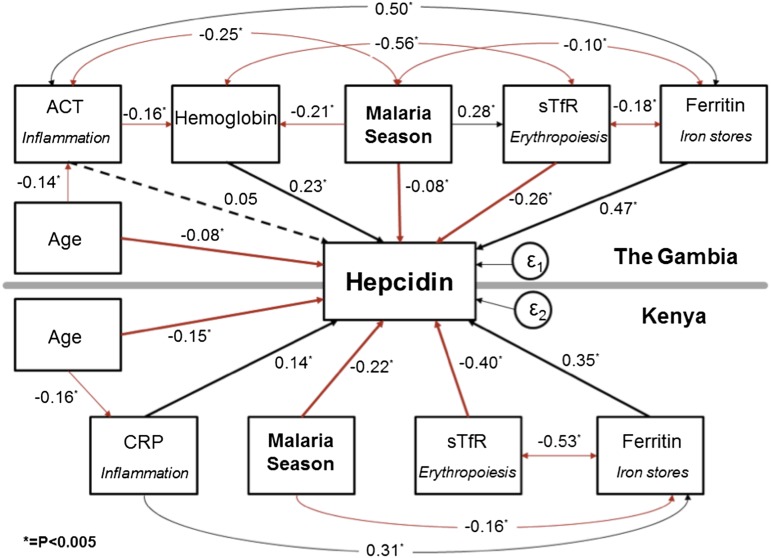
To further explore the relative strengths and interactions of signaling pathways that determine plasma hepcidin, we created a SEM grouped by study site (Figure 3). This SEM allowed a holistic assessment of both the complex interactions of hepcidin with the variables controlling its expression and the associations that exist between those different variables. The SEM also gave standardized coefficients enabling the comparison of strength of stimuli within and between study sites. In both populations, the transition from start to end of the malaria season was a highly significant negative predictor of log-hepcidin and was negatively correlated with log-ferritin (P < .0005). The iron stores signal represented by log-ferritin and the erythropoiesis signal represented by log-sTfR were the predominant predictors of log-hepcidin with the largest standardized coefficients in both populations. In Kenyan children, erythropoietic drive represented by log-sTfR was a particularly strong signal (β −0.40, P < .0005), and log-ferritin was strongly negatively associated with log-sTfR (β −0.53, P < .0005) suggesting that (lack of) iron stores were limiting the rate of erythropoiesis, consistent with IDA. Hb levels were also strongly associated with log-hepcidin (β 0.23, P < .0005) in Gambian children. The inflammation signal, represented by log-ACT and log-CRP, was a relatively weak predictor of log-hepcidin compared with the other signaling pathways in these well community-based children (Figure 3). Supplemental Figure 1 illustrates how the balance of hepcidin regulatory signals changes from the start to the end of the season as signaling from inflammation wanes and signaling from erythropoiesis increases in Gambian children. The CFI and the RMSEA for both models were 1.0 and < 0.005, respectively, indicating a good fit.
Figure 3.
SEM of variables associated with hepcidin. A single SEM was created, grouped by site. The sizes of the associations are indicated by the standardized regression coefficients. *P < .005. A positive association is indicated by a black line, an inverse association by a red line, and a nonsignificant association by a dotted line. In Gambian children, age was additionally associated with log-ferritin levels (β 0.24; P < .0005), log-sTfR (β −0.17; P < .0005), and Hb levels (β 0.25; P < .0005); data not shown for simplicity. The R2 was 0.49 for the Gambian group and 0.58 for the Kenyan group. The standard error (ε) was 0.02 for the Gambian group and 0.02 for the Kenyan group. The overall CFI was 1.0, and the RMSEA was <0.005 indicating a good fit.
Discussion
In this study, we demonstrate that at the population level multiple factors independently associate with hepcidin levels in African children, notably time relative to the malaria season. Furthermore, we define the associations between prevalence of ID and changes in hepcidin and other parameters, giving insight into the competing regulatory drivers of iron status in a global health context.
ID affects ∼30% of the world’s population, and young children are at particular risk.38 Because hepcidin controls iron homeostasis, it is important to understand how hepcidin, and the factors that regulate it, relate to ID in developing-world populations. We explored the relationships of hepcidin with parameters that represent measures of these signals. In age-adjusted, multivariable, and structural equation models, we found that iron stores represented by ferritin and erythropoietic drive represented by sTfR levels39 were the predominant regulatory signals associated with hepcidin levels in our populations. The strongly positive association seen between log-ferritin and log-hepcidin levels is in agreement with large studies in Italian40 and Dutch adults,41 and our finding of markedly low hepcidin levels in ID children agrees with previous studies in African refugee children42 and Korean children.43 Normal Korean children without ID had a mean serum hepcidin level of 16.71 ng/mL (± 14.74), similar to the level of 16.43 ng/mL seen in Gambian children at the start of the malaria season.43 In keeping with studies in Tanzanian15 and Indonesian children,18 sTfR levels were strongly negatively associated with hepcidin levels. Hb levels were strongly positively associated with hepcidin levels in both populations as previously found in African refugee children.42
Our findings further suggest that there may be nonlinear effects involving a threshold of ID below which downregulation of hepcidin takes precedence over other stimuli. This threshold appears to be reached in Kenyan children at the end of the malaria season when iron stores (ferritin) and erythropoiesis (sTfR) become significantly more powerful determinants of plasma hepcidin compared with the start of the season. However, in Gambian children who are less iron deficient, season does not significantly alter the strength of the iron stores and erythropoiesis signals on hepcidin (Figure 2). In an SEM, erythropoietic drive (sTfR) was a strong negative regulator of hepcidin and was strongly associated with plasma ferritin in Kenyan children, suggesting that ID erythropoiesis is an important regulatory signal in this population (Figure 3).
Inflammation, as represented by ACT and CRP, was significantly associated with hepcidin in age-adjusted analyses, but only weakly associated in multivariable analyses and in an SEM. This study consisted of well children living in rural communities, and thus the inflammatory signal may be weaker than in febrile malaria patients.13,15,16 A study in African refugee patients showed no association between inflammatory cytokines and hepcidin levels.42 Moreover, studies suggest that the erythroid demand for iron may be a more powerful regulator of hepcidin expression than inflammation.44-46 In our model, age was negatively associated with hepcidin levels, likely attributable to younger children being more inflamed. Gender, BMI, and carriage of HbAS or G6PDd A- did not influence hepcidin levels.
One striking aspect of our findings, best illustrated in the SEM, was the similarity of the balance of factors that regulate hepcidin in children in the Gambia and Kenya, despite these 2 communities living in different ecosystems on opposite sides of the African continent. In particular, the strong effect of malaria season on both prevalence of ID and on hepcidin was apparent in both settings. In both populations, the prevalence of ID increased and plasma hepcidin levels dropped significantly between the start and end of the malaria season. The effect of season on plasma hepcidin levels was robust to adjustment for other variables in multivariable and structural equation models. Risk of ID varied by site as well as by season. Despite better access to health care, children living on the coast of Kenya were considerably more iron deficient than children living in the Gambia. This could relate to dietary differences between the populations or may also relate to differences in malaria transmission, with Kenyan children experiencing higher levels of malaria transmission outside the main malaria season.
The possible causes, and consequences, of the strong effect of season on prevalence of ID and hepcidin concentrations require further consideration. There are a number of possible explanations for an increased prevalence of ID at the end of the malaria season. Nutritional deficiency may result in ID, and children in both populations have diets low in heme and total iron and high in phytates.47 However, BMI increased significantly across the malaria season in Gambian children, probably because of the September harvest, despite an increased prevalence of ID. This suggests that although nutrition almost certainly contributes to ID in general, the changes we observed in ID prevalence over the malaria season are unlikely to be explained by poor nutrition. Another potential explanation is hookworm infestation. However, hookworm prevalence is very low in both populations, and the Gambian children were treated with an antihelminthic at the time of the first survey.
A third explanation is that iron absorption is impaired during the malaria season. Malaria raises hepcidin levels in febrile and asymptomatic infection,12-18 and raised plasma hepcidin impairs iron absorption.7,19 In agreement with de Mast et al,18 we found that asymptomatic malaria parasitemia was associated with increased hepcidin levels in Kenyan children (age-adjusted β 0.50, 0.28, 0.71; P < .0005). This positive association was not found in Gambian children, which might be accounted for by the different study design because Gambian children were excluded if they had a temperature ≥37.5°C. However, not only malaria but other common infections and inflammatory conditions such as gastroenteritis also peak during the wet season.25 Therefore, it is likely that during the malaria season hepcidin levels are relatively raised in both populations because of a generally increased burden of inflammatory and infectious events including malaria.
Our recent data demonstrate that hepcidin is the major determinant of utilization of oral iron in African children.19 Thus, a hepcidin-mediated block in iron absorption over a prolonged time period, such as the malaria season, may lead to ID. This might also explain the differences in ID by site that we observed; children in the Gambia have a period of time free of malaria transmission during which iron may be absorbed and stores replenished, whereas children living on the coast of Kenya experience perennial malaria transmission (and have more prevalent ID). If infections and inflammation cause ID via effects on increasing hepcidin, it follows that measures that reduce the burden of infection may of themselves also reduce the burden of ID. Further work is required to examine this idea, but if true, it could be a significant consideration for calculating the benefit of anti-infective programs.
In both populations studied, plasma hepcidin was lower at the end of the malaria season. We hypothesize that the accumulated ID and IDA act to downregulate hepcidin expression in order to facilitate release of iron from stores as hepcidin stimulatory signals from inflammation during the season are waning. Therefore, the balance of hepcidin upregulatory signals (inflammation plus iron stores) and downregulatory signals (ID and erythropoietic drive) shifts from the beginning to the end of the season. An SEM by season for the Gambian site is consistent with this hypothesis (supplemental Figure 1) because a weak inflammatory signal is significantly positively associated with hepcidin at the beginning of the season, but not at the end, and the coefficient of association of sTfR with hepcidin is larger at the end of the season.
The combination of increased prevalence of ID (and IDA) with lower hepcidin at the end of the malaria season needs to be placed within a more general context. Evidence from several studies strongly suggests that iron supplements can increase risk of morbidity from malaria and other infections in children.4,5,48,49 There is therefore a dilemma of how best to combat ID and IDA while avoiding harmful effects of universal iron supplementation, and many iron supplementation programs have become stalled as a result of this difficulty. Our data suggest one possible way forward: although ID and IDA coexist geographically with malaria, they do not precisely coincide temporally; prevalence of ID/IDA is higher at the end of the malaria season, but from this time, malaria transmission is in decline (to negligible levels in the Gambia) and the overall burden of infection is lower. Furthermore, at the end of the malaria season, hepcidin is relatively decreased. Together, this suggests that iron supplementation may be of most benefit well after the end of the season when requirement for iron and the ability to absorb it are elevated and the risk of iron exacerbating infections is diminished. In addition, it may be of benefit to give iron and antimalarials together to further reduce any potential risk of reactivating malarial infection, particularly in those areas (such as Kenya) where malaria transmission decreases postseason but is still present throughout the year. Based on our previous studies correlating iron utilization against hepcidin levels in Gambian children,19 we estimate that iron would be used approximately twice as effectively at the end of the malaria season. Targeting programs of iron supplementation in this way could therefore allow lower and safer doses of iron to be employed.
In conclusion, analysis of hepcidin and parameters known to regulate hepcidin in 848 African children has revealed the combinatorial and dynamic nature of iron homeostasis in a population where ID is a significant cause of morbidity. The data show how different physiological inputs contribute to hepcidin levels and how the balance of these inputs changes over time. The finding of increased prevalence of ID coupled with low hepcidin, which will facilitate iron absorption at the end of the malaria season, in both East and West Africa may have important implications for strategies aimed at restoring healthy iron status in children.
Acknowledgments
The authors thank the children who took part in the study and their parents; Baba Jobarteh, Sosseh Sanyang, Kabiru Ceesay, Musa Colley, Khalilu Sanneh, and Ibrima Camera for their assistance in the field and laboratory in the Gambia; Karen Chamberlain, Hanneke Mfuni, Steve Austin, and Lorna Cox of the Nutritional Biomarker Analysis laboratory at MRC Human Nutrition Research, Cambridge, for performing assays and retrieving samples; and the staff of the human genetics laboratory at the KEMRI-Wellcome Trust Programme, including Alex Macharia, Emily Nyatichi, Herbert Opi, Metrine Tendwa, Johnstone Makale, Adan Mohamed, Gideon Nyutu, and Ruth Mwarabu for their help with sample processing and database support. This paper is published with the permission of the director of KEMRI.
This work was supported by a Career Support Award from Oxford University Clinical Academic Graduate School and by funding from the Academy of Medical Sciences, the Wellcome Trust, the British Heart Foundation, and Arthritis Research UK. The research was jointly funded by the UK MRC (grant MC-A760-5QX00) and the UK Department for International Development (DFID) under the MRC/DFID Concordat agreement, and by a Bill and Melinda Gates Foundation Global Health Grant (Hepcidin and Iron in Global Health, HIGH Partnership, OPP1055865). During this work, H.D. was funded by a Beit Memorial Fellowship for Medical Research, an MRC New Investigator award, and NIHR Oxford Biomedical Research Centre. T.N.W. is funded by a fellowship from the Wellcome Trust (091758) and by funds from the European Union FP7 EVIMalR Consortium.
Footnotes
The online version of this article contains a data supplement.
There is an Inside Blood Commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: S.H.A. conducted the Gambian field study, analyzed data, obtained funding, and wrote the manuscript; A.E.A. performed experiments, contributed to data analysis, and wrote the manuscript; S.K. and S.U. performed experiments and contributed to data analysis; T.W.M. conducted the Kenyan field study and contributed to the write-up; P.A.B. provided statistical assistance and contributed to data analysis and the write-up; T.N.W. obtained funding and contributed to data analysis and the write-up; A.M.P. conceived the study, obtained funding, and contributed to data analysis and the write-up; and H.D. performed experiments, obtained funding, interpreted data, and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Sarah H. Atkinson, Kenya Medical Research Institute/Wellcome Trust Research Programme, Centre of Geographic Medicine Research-Coast, Kilifi District Hospital, Kilifi, Kenya; e-mail: satkinson@kemri-wellcome.org; and Hal Drakesmith, MRC Human Immunology Unit, Weatherall Institute of Molecular Medicine, University of Oxford, Oxford, United Kingdom; e-mail: alexander.drakesmith@ndm.ox.ac.uk.
References
- 1.Hay SI, Okiro EA, Gething PW, et al. Estimating the global clinical burden of Plasmodium falciparum malaria in 2007. PLoS Med. 2010;7(6):e1000290. doi: 10.1371/journal.pmed.1000290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010 [published correction appears in Lancet. 2013;381(9867):628]. Lancet. 2012;380(9859):2163–2196. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Grantham-McGregor S, Ani C. A review of studies on the effect of iron deficiency on cognitive development in children. J Nutr. 2001;131(2, suppl 2):649S–666S. doi: 10.1093/jn/131.2.649S. [DOI] [PubMed] [Google Scholar]
- 4.Sazawal S, Black RE, Ramsan M, et al. Effects of routine prophylactic supplementation with iron and folic acid on admission to hospital and mortality in preschool children in a high malaria transmission setting: community-based, randomised, placebo-controlled trial. Lancet. 2006;367(9505):133–143. doi: 10.1016/S0140-6736(06)67962-2. [DOI] [PubMed] [Google Scholar]
- 5.Soofi S, Cousens S, Iqbal SP, et al. Effect of provision of daily zinc and iron with several micronutrients on growth and morbidity among young children in Pakistan: a cluster-randomised trial. Lancet. 2013;382(9886):29–40. doi: 10.1016/S0140-6736(13)60437-7. [DOI] [PubMed] [Google Scholar]
- 6.Spottiswoode N, Fried M, Drakesmith H, Duffy PE. Implications of malaria on iron deficiency control strategies. Adv Nutr. 2012;3(4):570–578. doi: 10.3945/an.111.001156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nemeth E, Tuttle MS, Powelson J, et al. Hepcidin regulates cellular iron efflux by binding to ferroportin and inducing its internalization. Science. 2004;306(5704):2090–2093. doi: 10.1126/science.1104742. [DOI] [PubMed] [Google Scholar]
- 8.Ganz T. Hepcidin and iron regulation, 10 years later. Blood. 2011;117(17):4425–4433. doi: 10.1182/blood-2011-01-258467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Weinstein DA, Roy CN, Fleming MD, Loda MF, Wolfsdorf JI, Andrews NC. Inappropriate expression of hepcidin is associated with iron refractory anemia: implications for the anemia of chronic disease. Blood. 2002;100(10):3776–3781. doi: 10.1182/blood-2002-04-1260. [DOI] [PubMed] [Google Scholar]
- 10.Finberg KE, Heeney MM, Campagna DR, et al. Mutations in TMPRSS6 cause iron-refractory iron deficiency anemia (IRIDA). Nat Genet. 2008;40(5):569–571. doi: 10.1038/ng.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Armitage AE, Pinches R, Eddowes LA, Newbold CI, Drakesmith H. Plasmodium falciparum infected erythrocytes induce hepcidin (HAMP) mRNA synthesis by peripheral blood mononuclear cells. Br J Haematol. 2009;147(5):769–771. doi: 10.1111/j.1365-2141.2009.07880.x. [DOI] [PubMed] [Google Scholar]
- 12.Howard CT, McKakpo US, Quakyi IA, et al. Relationship of hepcidin with parasitemia and anemia among patients with uncomplicated Plasmodium falciparum malaria in Ghana. Am J Trop Med Hyg. 2007;77(4):623–626. [PubMed] [Google Scholar]
- 13.Burté F, Brown BJ, Orimadegun AE, et al. Circulatory hepcidin is associated with the anti-inflammatory response but not with iron or anemic status in childhood malaria. Blood. 2013;121(15):3016–3022. doi: 10.1182/blood-2012-10-461418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ayoya MA, Stoltzfus RJ, Spiekermann-Brouwer GM, et al. Hepcidin and Plasmodium falciparum malaria in anemic school children in Mali [in French]. Bull Soc Pathol Exot. 2009;102(4):219–220. [PubMed] [Google Scholar]
- 15.de Mast Q, Nadjm B, Reyburn H, et al. Assessment of urinary concentrations of hepcidin provides novel insight into disturbances in iron homeostasis during malarial infection. J Infect Dis. 2009;199(2):253–262. doi: 10.1086/595790. [DOI] [PubMed] [Google Scholar]
- 16.Casals-Pascual C, Huang H, Lakhal-Littleton S, et al. Hepcidin demonstrates a biphasic association with anemia in acute Plasmodium falciparum malaria. Haematologica. 2012;97(11):1695–1698. doi: 10.3324/haematol.2012.065854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Mast Q, van Dongen-Lases EC, Swinkels DW, et al. Mild increases in serum hepcidin and interleukin-6 concentrations impair iron incorporation in haemoglobin during an experimental human malaria infection. Br J Haematol. 2009;145(5):657–664. doi: 10.1111/j.1365-2141.2009.07664.x. [DOI] [PubMed] [Google Scholar]
- 18.de Mast Q, Syafruddin D, Keijmel S, et al. Increased serum hepcidin and alterations in blood iron parameters associated with asymptomatic P. falciparum and P. vivax malaria. Haematologica. 2010;95(7):1068–1074. doi: 10.3324/haematol.2009.019331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Prentice AM, Doherty CP, Abrams SA, et al. Hepcidin is the major predictor of erythrocyte iron incorporation in anemic African children. Blood. 2012;119(8):1922–1928. doi: 10.1182/blood-2011-11-391219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cercamondi CI, Egli IM, Ahouandjinou E, et al. Afebrile Plasmodium falciparum parasitemia decreases absorption of fortification iron but does not affect systemic iron utilization: a double stable-isotope study in young Beninese women. Am J Clin Nutr. 2010;92(6):1385–1392. doi: 10.3945/ajcn.2010.30051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Doherty CP, Cox SE, Fulford AJ, et al. Iron incorporation and post-malaria anaemia. PLoS ONE. 2008;3(5):e2133. doi: 10.1371/journal.pone.0002133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Atkinson SH, Rockett K, Sirugo G, et al. Seasonal childhood anaemia in West Africa is associated with the haptoglobin 2-2 genotype. PLoS Med. 2006;3(5):e172. doi: 10.1371/journal.pmed.0030172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Atkinson SH, Rockett KA, Morgan G, et al. Tumor necrosis factor SNP haplotypes are associated with iron deficiency anemia in West African children. Blood. 2008;112(10):4276–4283. doi: 10.1182/blood-2008-06-162008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mwangi TW, Ross A, Snow RW, Marsh K. Case definitions of clinical malaria under different transmission conditions in Kilifi District, Kenya. J Infect Dis. 2005;191(11):1932–1939. doi: 10.1086/430006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Brewster DR, Greenwood BM. Seasonal variation of paediatric diseases in The Gambia, west Africa. Ann Trop Paediatr. 1993;13(2):133–146. doi: 10.1080/02724936.1993.11747637. [DOI] [PubMed] [Google Scholar]
- 26.Hay SI, Snow RW, Rogers DJ. Predicting malaria seasons in Kenya using multitemporal meteorological satellite sensor data. Trans R Soc Trop Med Hyg. 1998;92(1):12–20. doi: 10.1016/s0035-9203(98)90936-1. [DOI] [PubMed] [Google Scholar]
- 27.Hay SI, Rogers DJ, Toomer JF, Snow RW. Annual Plasmodium falciparum entomological inoculation rates (EIR) across Africa: literature survey, Internet access and review. Trans R Soc Trop Med Hyg. 2000;94(2):113–127. doi: 10.1016/s0035-9203(00)90246-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mbogo CN, Snow RW, Khamala CP, et al. Relationships between Plasmodium falciparum transmission by vector populations and the incidence of severe disease at nine sites on the Kenyan coast. Am J Trop Med Hyg. 1995;52(3):201–206. doi: 10.4269/ajtmh.1995.52.201. [DOI] [PubMed] [Google Scholar]
- 29.Nyakeriga AM, Troye-Blomberg M, Dorfman JR, et al. Iron deficiency and malaria among children living on the coast of Kenya. J Infect Dis. 2004;190(3):439–447. doi: 10.1086/422331. [DOI] [PubMed] [Google Scholar]
- 30.Thurnham DI, McCabe GP. Influence of Infection and Inflammation on Biomarkers of Nutritional Status with an Emphasis on Vitamin A and Iron. Geneva, Switzerland: World Health Organization; 2012. [Google Scholar]
- 31.World Health Organization (WHO), Centers for Disease Control and Prevention (CDC) Assessing the Iron Status of Populations: Including Literature Reviews. Report of a Joint World Health Organization/Centers for Disease Control and Prevention Technical Consultation on the Assessment of Iron Status at the Population Level, Geneva, Switzerland, 6–8 April, 2004. 2nd ed. Geneva, Switzerland: WHO/CDC; 2007. [Google Scholar]
- 32.United Nations Children’s Fund, United Nations University, World Health Organization (WHO) Iron Deficiency Anaemia: Assessment, Prevention, and Control. A Guide for Programme Managers. Geneva, Switzerland: WHO; 2001. [Google Scholar]
- 33.World Health Organization (WHO) Haemoglobin Concentrations for the Diagnosis of Anaemia and Assessment of Severity. Geneva, Switzerland: WHO; 2011. [Google Scholar]
- 34.Armitage P, Berry G, Matthews JNS. Using STATA's robust cluster command as appropriate. In: Armitage P, Berry G, Matthews JNS, editors. Statistical Methods in Medical Research. 4th ed. Oxford, United Kingdom: Blackwell Scientific Publications; 2001. pp. 378–454. [Google Scholar]
- 35.Verhoef H, West CE, Ndeto P, Burema J, Beguin Y, Kok FJ. Serum transferrin receptor concentration indicates increased erythropoiesis in Kenyan children with asymptomatic malaria. Am J Clin Nutr. 2001;74(6):767–775. doi: 10.1093/ajcn/74.6.767. [DOI] [PubMed] [Google Scholar]
- 36.Menendez C, Quinto LL, Kahigwa E, et al. Effect of malaria on soluble transferrin receptor levels in Tanzanian infants. Am J Trop Med Hyg. 2001;65(2):138–142. doi: 10.4269/ajtmh.2001.65.138. [DOI] [PubMed] [Google Scholar]
- 37.Mockenhaupt FP, May J, Stark K, Falusi AG, Meyer CG, Bienzle U. Serum transferrin receptor levels are increased in asymptomatic and mild Plasmodium falciparum-infection. Haematologica. 1999;84(10):869–873. [PubMed] [Google Scholar]
- 38.Yadav D, Chandra J. Iron deficiency: beyond anemia. Indian J Pediatr. 2011;78(1):65–72. doi: 10.1007/s12098-010-0129-7. [DOI] [PubMed] [Google Scholar]
- 39.Kemna EH, Kartikasari AE, van Tits LJ, Pickkers P, Tjalsma H, Swinkels DW. Regulation of hepcidin: insights from biochemical analyses on human serum samples. Blood Cells Mol Dis. 2008;40(3):339–346. doi: 10.1016/j.bcmd.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 40.Traglia M, Girelli D, Biino G, et al. Association of HFE and TMPRSS6 genetic variants with iron and erythrocyte parameters is only in part dependent on serum hepcidin concentrations. J Med Genet. 2011;48(9):629–634. doi: 10.1136/jmedgenet-2011-100061. [DOI] [PubMed] [Google Scholar]
- 41.Galesloot TE, Vermeulen SH, Geurts-Moespot AJ, et al. Serum hepcidin: reference ranges and biochemical correlates in the general population. Blood. 2011;117(25):e218–e225. doi: 10.1182/blood-2011-02-337907. [DOI] [PubMed] [Google Scholar]
- 42.Cherian S, Forbes DA, Cook AG, et al. An insight into the relationships between hepcidin, anemia, infections and inflammatory cytokines in pediatric refugees: a cross-sectional study. PLoS ONE. 2008;3(12):e4030. doi: 10.1371/journal.pone.0004030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Choi HS, Song SH, Lee JH, Kim HJ, Yang HR. Serum hepcidin levels and iron parameters in children with iron deficiency. Korean J Hematol. 2012;47(4):286–292. doi: 10.5045/kjh.2012.47.4.286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Theurl I, Aigner E, Theurl M, et al. Regulation of iron homeostasis in anemia of chronic disease and iron deficiency anemia: diagnostic and therapeutic implications. Blood. 2009;113(21):5277–5286. doi: 10.1182/blood-2008-12-195651. [DOI] [PubMed] [Google Scholar]
- 45.Darshan D, Frazer DM, Wilkins SJ, Anderson GJ. Severe iron deficiency blunts the response of the iron regulatory gene Hamp and pro-inflammatory cytokines to lipopolysaccharide. Haematologica. 2010;95(10):1660–1667. doi: 10.3324/haematol.2010.022426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Huang H, Constante M, Layoun A, Santos MM. Contribution of STAT3 and SMAD4 pathways to the regulation of hepcidin by opposing stimuli. Blood. 2009;113(15):3593–3599. doi: 10.1182/blood-2008-08-173641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.McCrae JE, Paul AA. Foods of Rural Gambia. 2nd ed. Cambridge, United Kingdom: Medical Research Council Dunn Nutrition Unit; 1996. [Google Scholar]
- 48.Veenemans J, Milligan P, Prentice AM, et al. Effect of supplementation with zinc and other micronutrients on malaria in Tanzanian children: a randomised trial. PLoS Med. 2011;8(11):e1001125. doi: 10.1371/journal.pmed.1001125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Prentice AM, Verhoef H, Cerami C. Iron fortification and malaria risk in children. JAMA. 2013;310(9):914–915. doi: 10.1001/jama.2013.6771. [DOI] [PMC free article] [PubMed] [Google Scholar]