Abstract
Background
Advances in therapies for rheumatoid arthritis (RA), particularly biologics, have transformed the treatment paradigm for RA. However, the associated costs of these therapies result in a significant economic burden on the healthcare system. As a chronic disease requiring lifelong treatment, most health plans now position RA drugs as a high-priority therapeutic category.
Objective
To identify provider and payer practices and perceptions regarding coverage of RA biologics in the current marketplace, as well as emerging trends in reimbursement practices.
Method
In November 2011, Reimbursement Intelligence, a healthcare research company, collected and analyzed quantitative and qualitative data via parallel-structure online surveys of 100 rheumatologists and 50 health plan payers (medical and pharmacy directors) who represent more than 80 million covered lives. The surveys included approximately 150 questions, and the surveys were designed to force a response for each question.
Results
Payers reported using tier placement, prior authorization, and contracting in determining coverage strategies for RA biologics. Among providers, experience with older RA agents remains the key driver for the choice of a biologic agent. A majority of payers and providers (68% and 54%, respectively) reported that they did not anticipate a change in the way their plans would manage biologics over the next 2 to 4 years. Payers’ responses indicated uncertainty about how therapeutic positioning of newer, small-molecule drugs at price parity to biologics would affect the current reimbursement landscape. Survey responses show that approval of an indication for early treatment of RA is not likely to change the prescribing and reimbursement landscape for RA biologics. This survey further shows that payers and providers are generally aligned in terms of perceptions of current and future treatments for RA.
Conclusion
Advances in RA therapies allow patients increasing options for effective disease management. However, the high cost of biologic therapies and the need for lifelong treatment raise economic concerns. Payer satisfaction with current therapies and uncertainty about added value of new therapies will create challenges for new medications coming to market.
Rheumatoid arthritis (RA) is a chronic systemic autoimmune disorder and the most common form of inflammatory arthritis.1 RA affects 1% of the population, most often adults aged 40 to 70 years.2 Recent epidemiologic data indicate that the incidence of RA in women has risen in the past 10 years.3
Because RA affects many individuals who are of working age and remains a major cause of disability, the economic burden of RA adds a significant cost not only to patients and their families, but also to society as a whole.1,4 In addition, reduced quality of life, loss of work productivity, and substantial healthcare utilization are factors that must be considered in RA management.4,5
Because complications of RA may begin to develop within months of disease onset, early and aggressive treatment is considered clinically necessary to manage immediate symptoms of pain associated with inflammation, but also to slow disease progression to prevent long-term disability.1,6,7 Historically, estimates of work disability rates for RA have been high, with higher rates associated with longer disease duration; work disability estimates have been shown to reach 30% within 2 to 3 years of diagnosis.4,5 Recent estimates suggest that RA-related work disability rates remain high, although potentially lower than in earlier estimates.8 This 2008 longitudinal analysis showed estimates of 23% work disability at 1 to 3 years of disease onset and of 35% within 10 years.8
KEY POINTS
-
▸
Advances in RA medications, particularly biologics, have transformed the treatment paradigm for RA; however, the associated costs of these therapies result in a significant economic burden on the healthcare system.
-
▸
With a chronic disease requiring lifelong treatment, most health plans are positioning RA drugs as a high-priority therapeutic category.
-
▸
This survey of 100 rheumatologists and 50 payers representing >80 million lives revealed that provider experience and satisfaction with older RA agents remains the underlying driver for choice of biologics.
-
▸
Payers and providers alike reported that they did not anticipate a change in the way their plans would manage biologics over the next 2 to 4 years.
-
▸
Payers were uncertain about the therapeutic positioning for newer, small-molecule drugs at price parity to biologics.
-
▸
Survey responses also suggest that an indication for a biologic to treat early RA will likely not change current prescribing and reimbursement patterns.
Clinical studies have shown better clinical outcomes when aggressive treatment is initiated early, including treatment with a wide range of disease-modifying antirheumatic drugs (DMARDs) and non-DMARD combination therapies.7–9 A recent joint collaboration of the American College of Rheumatology (ACR) and the European League Against Rheumatism has led to the development of an updated classification system of RA, to shift the focus from late-stage disease features—such as structural changes and joint damage that can be determined from various imaging techniques—to early-stage disease features that are associated with persistent disease.6 Given the advances in treatment for RA, including nonbiologic and biologic options, along with the associated improved outcomes, this classification system update to include early-disease features marked a major shift in the RA disease construct.6
The ACR guidelines outline clinical treatment pathways by first defining disease duration and activity.7 Disease duration is divided into 3 major categories: <6 months (equivalent to early disease), 6 to 24 months (equivalent to intermediate disease duration), and >24 months (equivalent to longer disease duration).7 Disease activity measurements are often qualitative in early-stage disease, and measures are subject to clinical judgment.7
Pharmacotherapy for RA often includes a non-steroidal antiinflammatory drug, selected use of glucocorticoids, and initiation of a DMARD early in the disease course.1,7 Biologic therapies may be added when adequate disease control has not been met by previously initiated drug therapies, which may occur within the first year of diagnosis.1,7 With regard to biologic therapies, the ACR further subdivides “early disease” by disease duration of <3 months or 3 to 6 months, to accommodate the needs for early advancement of the patient to biologic therapies when disease activity is high.7
Despite positive clinical outcomes from treatment advances, healthcare costs associated with the treatment of a prevalent and lifelong disease such as RA are a considerable issue for health plans. The ACR estimates that per-patient treatment with biologic therapies is typically in excess of $12,000 annually.10 The Agency for Healthcare Research and Quality estimates the annual costs for RA medications from as low as a few hundred dollars for oral, nonbiologic DMARDs to a high of more than $16,000 for injectable biologic DMARDs.11 As new therapeutic options for RA become available, provider practices and payer strategies to support evidence-based care within the confines of cost management demand close examination.
This study was conducted to identify provider and payer practices and perceptions regarding therapeutic options and reimbursement for RA. To this end, Reimbursement Intelligence, a healthcare research company, conducted parallel online surveys with health plan payers and rheumatologists. Payers were asked to also consider market trends and potential for formulary coverage of RA therapies currently in development.
Methods
Online parallel-structure surveys were conducted in November 2011 and were completed by 2 groups: 100 rheumatologists and 50 payers identified as advisors to Pharmacy & Therapeutics Committees who are formulary decision makers for RA coverage. The payer group survey respondents included 50 pharmacy and medical directors from national and regional health plans who had held their positions for more than 2 years. The payer group of health plans represented 80 million covered lives.
The distribution of plan types among payer respondents included Medicare Part D, commercial plans, Medicare Advantage, freestanding prescription drug plans, Managed Medicaid, and dual-eligible populations. More than two thirds (69%) of payers represented commercial plans with 3- or 4-tier formularies.
The rheumatologist group represented providers from large and small group practices, and ones with and without in-office infusion capabilities. Rheumatologists were screened as to whether their practice offered in-office biologic infusions, the practice volume of in-office infusions weekly, and the number of rheumatologists in the practice. The sample was weighted toward rheumatology and multispecialty group practices seeing more than 80 patients with RA monthly.
The parallel-structure payer and rheumatologist surveys were comprised of approximately 150 questions, and the survey instrument required answers to all questions. Survey questions included specific probes about 8 biologic therapies currently indicated for RA (Table 1); existing medications that may receive an RA indication; and new, small-molecule oral agents still in development. All respondents received an honorarium for their participation.
Table 1.
Biologic Medications Indicated for Rheumatoid Arthritis
| Brand name | Generic name |
|---|---|
| Actemra | Tocilizumab |
| Cimzia | Certolizumab |
| Enbrel | Etanercept |
| Humira | Adalimumab |
| Orencia | Abatacept |
| Simponi | Golimumab |
| Rituxan | Rituximab |
| Remicade | Infliximab |
Results
Tier Placement
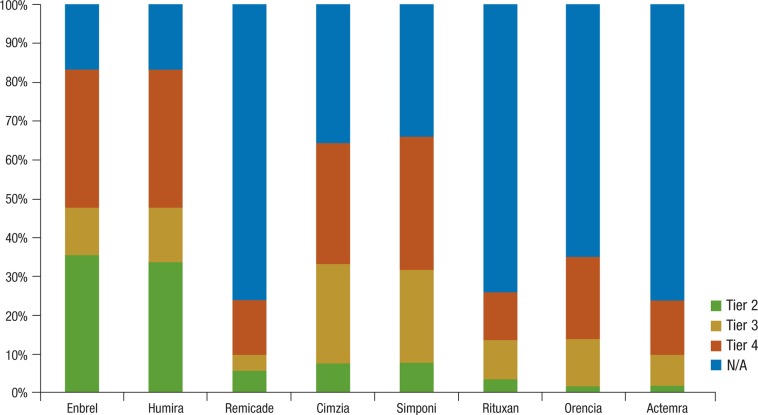
Tiered cost-sharing is a common strategy for therapies covered under a pharmacy benefit. Payers reported that none of the current 8 biologic medications (Table 1, page 85) covered under the pharmacy benefit is placed on tier 1. Tier 2 status was given most frequently to etanercept (Enbrel; 36%) and adalimumab (Humira; 34%), whereas the remaining products were distributed across tiers 3 and 4 (Figure 1, page 85).
Figure 1. Payer Formulary Tier Placement for RA Biologics.
N/A indicates not applicable; RA, rheumatoid arthritis.
Prior Authorizations and Step Edits
To target medications to appropriate patients, health plans may require patients to meet predetermined clinical criteria and receive prior authorization before reimbursement is approved. Similarly, health plans may use step edits, or a “fail-first” requirement, where payment for a therapy will be made only after certain therapies have been used first. If the patient does not respond appropriately (ie, considered a “step” or “failure”), then the provider will likely recommend a second-line therapy.12
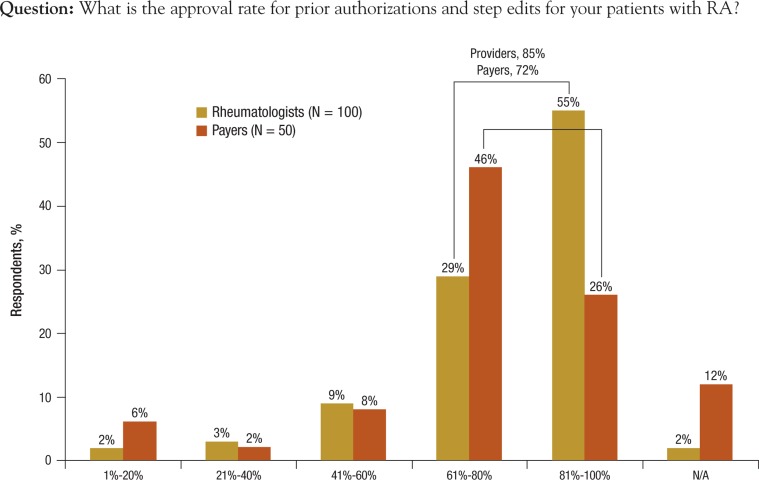
Payers were asked about approval rates when prior authorizations or step edits are required. Survey results show that most health plans use prior authorizations to manage utilization of RA therapies; 80% to 88% reported they require prior authorizations for the 8 biologic options presented in the survey. More than half (55%) of the providers reported approval rates between 81% and 100% of the time, whereas 29% reported approval rates from 61% to 80% of the time (Figure 2).
Figure 2. Approval Rates for Prior Authorization/Step Edits.
N/A indicates not applicable; RA, rheumatoid arthritis.
Distribution Strategy and Use of Specialty Pharmacies
Most (80%) payers in the survey reported they use specialty pharmacies for distribution of biologic therapies for RA. Among those who use specialty pharmacies, 65% reported using closed networks, whereas 83% reported they do not mandate the use of specialty pharmacies for distribution of office-infused biologic agents.
Specialty pharmacy services often offer services beyond product dispensing. Payers reported that the most valuable add-on services are patient education (60%), compliance programs (54%), compliance/adherence data reported back to payers (46%), and reimbursement assistance (28%).
Perceptions of Management Approach
Payer respondents characterized their general approach to RA management in terms of level of stringency of drug approval requirements for providers. Specifically, payers were asked, “How would you characterize your plan's general approach toward the management of rheumatoid arthritis?” Respondents were asked to choose from 5 categories—“open access,” “somewhat regimented,” “regimented approach,” and “other.”
Regimented was defined as “patients must have documented failure on a DMARD and a preferred biologic.” Open access was defined as “physician decides patient therapy and no documentation needed.”
Overall, 40% of payers characterized their plan's approach as regimented. In contrast, only 33% of providers characterized their plans as regimented. A little more than one third (36%) of payers characterized their management approach as somewhat regimented, in which patients must have a documented failure while using a DMARD before a biologic drug will be approved.
Conversely, 57% of providers characterized their health plans as somewhat regimented. Of the payers, 10% reported that they offer open access, in which the physician decides on the patient's therapy and no documentation is needed for treatment initiation or changes. More payers (16%) than providers (10%) reported open access in this survey.
Impact of Reimbursement Process on Access to Biologics
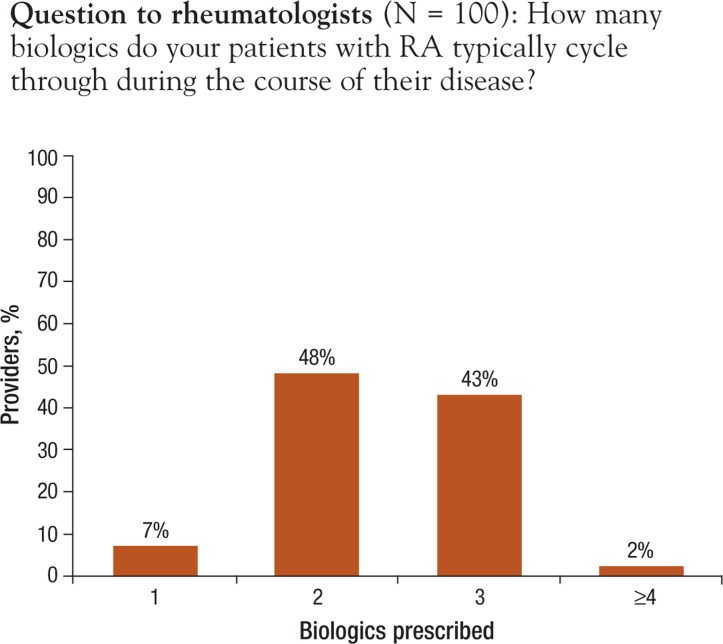
Providers reported that oral methotrexate (Trexall, Rheumatrex) is initiated within the first 3 months of RA diagnosis: 74% reported immediate initiation of the drug; 22% reported initiating methotrexate within 1 to 3 months. In addition, 21% of providers also reported initiating biologics within 1 to 3 months after initiation of methotrexate; 61% reported initiating biologics between 3 to 6 months; and 8% reported initiation of biologics between 6 months and 1 year. With regard to the number of biologics used for each patient, only 7% of providers reported that patients remained using the first biologic throughout the duration of their disease; 48% and 43% of providers indicated that patients typically cycle through 2 or 3 biologics, respectively, in the course of the disease (Figure 3).
Figure 3. Provider-Reported Number of RA Biologics Prescribed per Patient during the Course of Disease.
RA indicates rheumatoid arthritis.
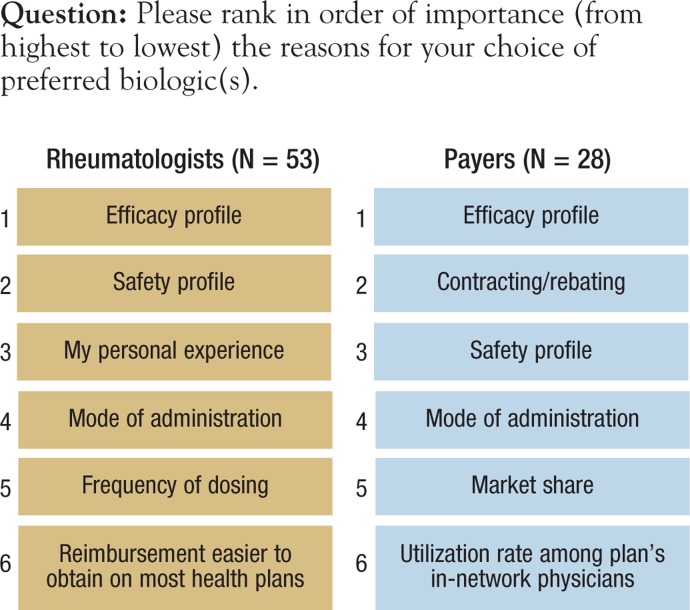
Payers and providers were asked to rank, in order of importance (from highest to lowest), the reasons for choice of preferred biologic (Figure 4). For both respondent groups, efficacy ranked the highest in influence on choice of biologics. Payers ranked etanercept, adalimumab, and infliximab (Remicade)—the 3 most established of the 8 biologics studied—as the most frequent first- and second-line drug choices. Payers reported that contracting/rebating is the second most frequent influence in determining a preferred biologic for patients with commercial coverage. For the Medicare population, payers ranked safety as more important than contracting/rebating.
Figure 4. Ranked Order of Importance for Choosing Preferred Biologics.
Payers and providers were asked about the main reason for low utilization of newer tumor necrosis factor (TNF) inhibitors, such as certolizumab (Cimzia) and golimumab (Simponi), and asked to rank in order from highest to lowest the reason for low utilization. Although contracting/price had some impact on product choice, ranking third, provider comfort with older agents was the first-ranked reason given by both providers and payers. More than three fourths (79%) of providers reported that some documentation of medical need for a biologic is always required. However, providers, like payers, stated that approval is granted more than two thirds of the time (Figure 2).
Only 15% of providers reported that plans require quantitative measurements (ie, x-ray) of active disease to allow the initiation or change of biologics. One third (34%) of providers and 40% of payers reported ACR 20% criteria for improvement (ACR20) achievement as a requirement for approval of a biologic therapy, and 42% of providers and 44% of payers reported that demonstrated safety and tolerability are required to initiate biologic drug therapy.
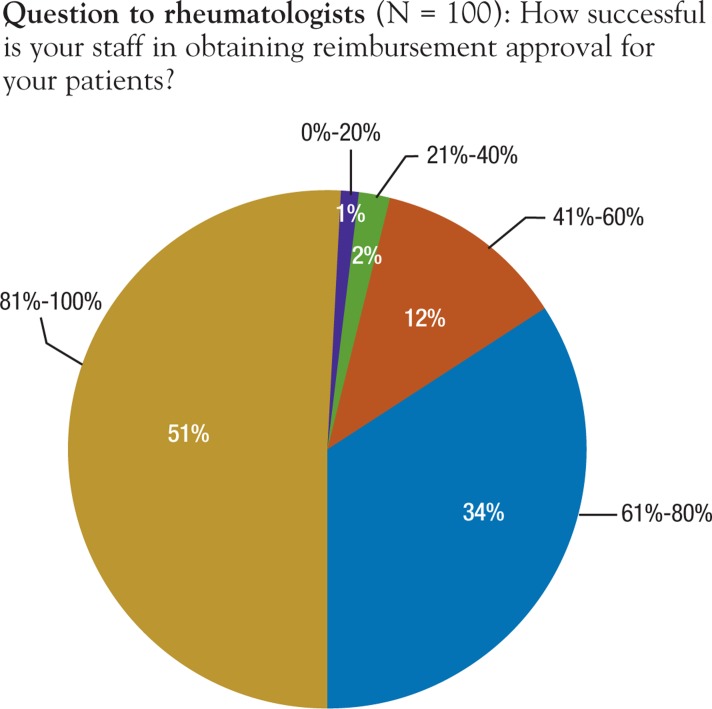
Providers were asked, “Under what circumstances would you be willing to take steps to dispute a payer decision?” More than two thirds (68%) agreed with the statement, “To appeal a denied prior authorization of one autoimmune biologic because payer requires failure of a different autoimmune biologic agent (eg, step edit).” Similarly, 60% chose the statement, “To counter onerous administrative or documentation requirements (eg, to eliminate burdens some request for additional documentation beyond reasonable medical notes),” with 68% choosing the statement, “to secure reimbursement following a claim denial for an on-label diagnosis.” In a follow-up question, providers were asked how many times they would dispute a denial before accepting the denial as a defined payer policy. Nearly one third (32%) of providers reported 1 time, 38% reported 2 times, and 17% reported 3 times. In this survey, providers reported that their support staff was generally successful at overcoming prior authorization and stepedit requirements (Figure 5).
Figure 5. Provider-Reported Staff Success Rate in Obtaining Reimbursement Approval.
Overall, payers and providers were consistent in their responses in regard to changes in the way RA will be managed over the next 2 to 4 years, with 54% of rheumatologists and 68% of payers reporting no change. Payers were asked if changes in reimbursement for office-infused products influenced the treatment selection rheumatologists currently make. More than 6 of 10 (62%) payers said that these changes would not influence treatment selection.
Regarding sites of care, 82% of the rheumatologists reported providing in-office infusion, although 62% of these providers also reported using an alternate site of care at least part of the time. When asked does “site of care affect your choice of therapy,” 72% of providers agreed with the statement, “It does not affect my choice of biologic.”
Potential Impact of Expanding Therapeutic Options for RA
Payers and providers were asked to assess the potential impact of several emerging trends in RA therapies, including new, small-molecule oral disease-modifying agents (Table 2, page 88). Respondents were asked, “Once FDA [US Food and Drug Administration] approved, how will they change your treatment strategy? Assume efficacy and safety are similar to current TNF inhibitors. Assume price parity to current biologics.” One third (36%) of providers and one fifth (20%) of payers reported these agents will be used after methotrexate but before TNF inhibitors. One third (33%) of providers and 16% of payers reported these agents will be used after TNF inhibitor failure. Smaller numbers, 15% of providers and 18% of payers, reported these agents will be used after TNF inhibitors fail. Only 11% of providers and 34% of payers say they are not sure.
Table 2.
Disease-Modifying Rheumatoid Arthritis Agents in Development
| Generic name/drug type | Development phase |
|---|---|
| Fostamatinib, oral, spleen tyrosine kinase inhibitor | Phase 2 clinical trial |
| Tofacitinib, oral, Janus kinase inhibitor | NDA submitted to the FDA on December 21, 2011 |
| Tabalumab, IV, human immunoglobulin G4 monoclonal antibody | Phase 3 clinical trial |
FDA indicates US Food and Drug Administration; IV, intravenous; NDA, New Drug Application.
If small-molecule drugs are priced at a 15% to 20% discount to biologics, a little more than half (52%) of payers reported they would position them before TNF inhibitors. However, if small-molecule drugs were priced at a 15% to 20% premium to biologics in product costs, they would position them after TNF inhibitors. If the price of these compounds were discounted relative to TNF inhibitors, an expedited review would be expected by 46% of payer respondents.
Most payers (88%) and more than half (57%) of providers reported they believe small-molecule therapies to be in the same therapeutic class as biologics. More than two thirds (68%) of payers noted that approval of the first small-molecule therapy would likely trigger a class review of the biologics. Regarding compliance potential of orals, which will be dosed 2 or 3 times daily,13,14 58% of payers and 44% of providers reported that oral dosing would improve patient compliance; 25% of providers and 8% of payers reported they believe it will reduce compliance as a result of the 2- or 3-timesdaily dosing regimen, and 29% of providers and 28% of payers reported that compliance will not be an issue.
Consideration of adoption of new therapies at launch was reported by only 27% of providers. However, 46% of providers reported they would require <1 year of experience with a new therapy to regularly prescribe it, with the balance of respondents (27%) reporting a 1- to 3-year time frame to adoption.
In addition, payers and providers were asked if they were aware of any ongoing head-to-head trials in RA; affirmative responses were given by 12% of payers and 26% of providers. Payers and providers were then asked, “How significant would the results of a head-to-head trial be in selecting your preferred biologic, if the trial design is for superiority?” On a scale of 1 to 7, with 1 representing “not significant at all” and 7 representing “most significant,” 40% of both payers and providers selected 6, whereas 34% and 33%, respectively, selected 7.
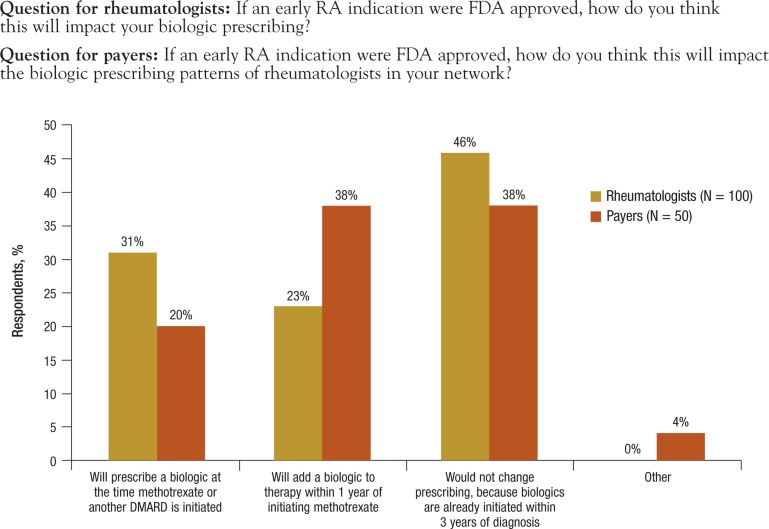
Payers and providers in this survey were asked if there would be an impact on prescribing patterns if an early RA indication was approved by the FDA. Among rheumatologists, 46% reported they would not change prescribing of biologics, with 23% reporting they will add a biologic within 1 year of initiating methotrexate. Nearly one third (31%) of rheumatologists reported they would prescribe a biologic at the time methotrexate or another DMARD is initiated (Figure 6, page 89).
Figure 6. Impact of Early RA Indication on Prescribing Patterns.
DMARD indicates disease-modifying antirheumatic drug; FDA, US Food and Drug Administration; RA, rheumatoid arthritis.
Discussion
Cost Burden
RA represents a significant burden to payers and is positioned as the highest priority therapeutic category for most health plans.15 Specialty pharmacy costs for RA therapies account for more than 25% of total spending for specialty drugs.16 Although nonspecialty drug costs in many categories are expected to grow more slowly over the next 3 years (because of increased availability of generics), costs for specialty therapies are expected to grow between 15% and 17% annually.16
Between 2011 and 2013, the costs for RA biologics are expected to be the single largest contributor to increases in specialty drug spending and are predicted to represent approximately one fifth (21%) of all health plan drug spending by 2014.16
Payers continue to implement and refine a variety of reimbursement strategies to balance quality of care in light of the economic burden of RA biologics, with varying effects.
Payer Reimbursement Management
The high costs associated with RA therapies have led payers to look for strategies to manage immediate costs, while weighing the potential for long-term costs of delayed treatment (eg, disability). Health plans have historically covered biologic therapies with subcutaneous delivery under a pharmacy benefit, because patients can self-administer these therapies.15 Intravenous and infusion therapies have been covered largely under a medical benefit, because drug administration needs to be delivered by a healthcare professional.15
Drugs covered under the medical benefit lack the scrutiny of drug utilization review and coverage management protocols used in pharmacy benefit structures. The entry of new oral and self-injectable products will generate greater scrutiny, which may result in an impact on cost.16 As new biologics enter the market, health plans will need to consider how coverage channels, including rebates or other discounts offered under a pharmacy benefit structure, will impact future costs.
Currently, there is no biologic therapy on the market that is specifically indicated for “early” RA. Because early stages of RA are diagnosed by clinical presentation rather than a definitive test, diagnostic parameters of early RA remain unclear.1
The survey findings highlight emerging trends in RA cost-management strategies, including payers’ efforts to follow evidence-based guidelines for use of RA biologics, and the trend toward shifting a greater proportion of the cost to patients; the resulting framework can inform the coordination of cost and clinical management of RA.
Results from this survey also show that payers and providers were generally aligned in terms of perceptions of current and future treatments for RA. Because provider experience and satisfaction with older RA agents were reported as the underlying driver for using current therapies, uptake of newer agents may also follow a similar pattern. Of note were responses that having an indication for early RA would not influence prescribing patterns, because biologics generally are already being used within 1 year of diagnosis, which is still considered early in the course of the disease.
Limitations
The limitations of this study include those inherent to all surveys. Survey questions must be developed broadly to be appropriate to as many respondents as possible. Surveys in general are inflexible to adaptation to individuals or subsets of respondents, so captured data may not reveal the richer context of the questions posed.
In addition, this survey was administered online and was formatted with forced-answer questions. Although there is a benefit to capturing responses for all questions and all respondents, question saliency may not be uniform for all respondents, thereby impacting the weight of each question in relation to others.
Furthermore, the sample size of the provider group was twice that of the payer group, which could skew intergroup comparisons.
Conclusion
Although the availability of highly effective biologic therapies for RA has greatly improved patient care, the cost of these therapies remains a priority concern for patients, providers, and payers alike. Provider and payer satisfaction with older RA agents, and skepticism about the incremental value of new therapies, will continue to raise the hurdles for new RA therapies coming to market.
Biography

Author Disclosure Statement
Ms Greenapple reported no conflicts of interest.
References
- 1.Rindfleisch JA, Muller D. Diagnosis and management of rheumatoid arthritis. Am Fam Physician. 2005; 72: 1037–1047 [PubMed] [Google Scholar]
- 2.Lee DM, Weinblatt ME. Rheumatoid arthritis. Lancet. 2001; 358: 903–911 [DOI] [PubMed] [Google Scholar]
- 3.Myasoedova E, Crowson CS, Kremers HM, et al. Is the incidence of rheumatoid arthritis rising? Results from Olmsted County, Minnesota, 1955–2007. Arthritis Rheum. 2010; 62: 1576–1582 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokka T. Work disability in early rheumatoid arthritis. Clin Exp Rheumatol. 2003; 21 (5 suppl 31): S71–S74 [PubMed] [Google Scholar]
- 5.Verstappen SM, Bijlsma JW, Verkleij H, et al. Utrecht Rheumatoid Arthritis Cohort Study Group. Overview of work disability in rheumatoid arthritis patients as observed in cross-sectional and longitudinal surveys. Arthritis Rheum. 2004; 51: 488–497 [DOI] [PubMed] [Google Scholar]
- 6.Aletaha D, Neogi T, Silman AJ, et al. 2010 rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann Rheum Dis. 2010; 69: 1580–1588 [DOI] [PubMed] [Google Scholar]
- 7.Saag KG, Teng GG, Patkar NM, et al. American College of Rheumatology 2008 recommendations for the use of nonbiologic and biologic disease-modifying antirheumatic drugs in rheumatoid arthritis. Arthritis Rheum. 2008; 59: 762–784 [DOI] [PubMed] [Google Scholar]
- 8.Allaire S, Wolfe F, Niu J, LaValley MP. Contemporary prevalence and incidence of work disability associated with rheumatoid arthritis in the US. Arthritis Rheum. 2008; 59: 474–480 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Resman-Targoff BH, Cicero MP. Aggressive treatment of early rheumatoid arthritis: recognizing the window of opportunity and treating to target goals. Am J Manag Care. 2010; 16 (9 suppl): S249–S258 [PubMed] [Google Scholar]
- 10.Cush JJ. American College of Rheumatology. Biologic treatments for rheumatoid arthritis. 2010. www.rheumatology.org/practice/clinical/patients/medications/biologics.pdf Accessed January 26, 2012.
- 11.Effective Health Care, Agency for Healthcare Research and Quality. Rheumatoid arthritis medicines: a guide for adults. AHRQ Publication Number 08-EHC004-2A; April 2008. www.effectivehealthcare.ahrq.gov/repFiles/RheumArthritisConsumerGuide_Singlepage.pdf Accessed March 1, 2012.
- 12.Hoadley J, Henry J. Kaiser Foundation. Cost containment strategies for prescription drugs: assessing the evidence in the literature. March 2005. www.kff.org/rxdrugs/upload/Cost-Containment-Strategies-for-Precription-Drugs-Assessing-The-Evidence-in-the-Literature-Report.pdf Accessed March 1, 2012.
- 13.Rigel Pharmaceuticals, Inc. Rigel's R788 significantly improves rheumatoid arthritis in phase 2b clinical trial: expected and manageable safety profile demonstrated in TASKi2. Media release. July 9, 2009. www.drugs.com/clinical_trials/rigels-r788-significantly-improves-rheumatoid-arthritis-phase-2b-clinical-trial-7714.html Accessed March 2, 2012.
- 14.van Vollenhoven RF, Fleischmann RM, Cohen SB, et al. Tofacitinib (CP-690,550), an oral Janus kinase inhibitor, or adalimumab versus placebo in patients with rheumatoid arthritis on background methotrexate: a phase 3 study. Arthritis Rheum. 2011; 63 (suppl 10):408 [Google Scholar]
- 15.EMD Serono. Managed care strategies for management of specialty injectable drugs. EMD Serono Injectables Digest. 4th ed.2008. www.amcp.org/WorkArea/DownloadAsset.aspx?id=12974 Accessed January 26, 2012.
- 16.Medco. 2011 Drug Trend Report: Healthcare 2020. Franklin Lakes, NJ: Medco; 2011. www.drugtrendreport.com/Medco-2011-Drug-Trend-Report-Executive-Summary.pdf Accessed March 1, 2012. [Google Scholar]