Abstract
Center 1 used the National Committee for Clinical Laboratory Standards M27-A2 method and antibiotic medium 3 (AM3) test to determine amphotericin B resistance in 5 of 30 Candida isolates. These isolates were tested at center 2 by AM3 test and flow cytometry (FC). The agreements (C1-C2) were 90% for AM3 test and FC and 73% for the AM3 tests.
Amphotericin B is widely used for the treatment of fungal infections even though there are no reliable breakpoints for correlation of in vitro susceptibility data with clinical outcome (13). A few cases of treatment failures with amphotericin B have also been reported (3, 10). The laboratory detection of amphotericin B-resistant strains remains problematic, notably with the widely used National Committee for Clinical Laboratory Standards (NCCLS) M27-A2 procedure (12, 13). Alternatives, such as antibiotic medium 3 (AM3) with the M27-A2 method, Etest with a predefined gradient of drug concentration, Iso-Sensitest broth, and flow cytometry (FC) have been used to discriminate between amphotericin B-susceptible and -resistant strains (2, 4, 5, 7, 9, 11, 14). Etest with AM3 agar did not yield good results with susceptible and resistant strains, but Etest with RPMI provided reliable discrimination (9). Similarly, Iso-Sensitest broth reportedly allowed a better discrimination of amphotericin B resistance than did AM3 independently of the inoculum size (2).
We compared the FC test developed at center 2 (C2; Mycology Laboratory, Wadsworth Center, Albany, N.Y.) (11) with the AM3 test previously used at center 1 (C1; University of Texas Medical School, Houston) (12, 14) to determine amphotericin B susceptibilities of 30 isolates comprising Candida albicans (5), Candida glabrata (4), Candida krusei (5), Candida lusitaniae (5), Candida parapsilosis (5), and Candida tropicalis (6). This set included five isolates labeled as amphotericin B resistant based on the susceptibility results, animal studies, and/or clinical outcome (1, 6, 7, 12, 14). The cultures were stored in sterile water at 4°C and passaged twice on Sabouraud dextrose agar at 35°C before the assays. Amphotericin B (Sigma Biochemical Company, St. Louis, Mo.) stock solutions were prepared in dimethyl sulfoxide (Amresco, Solon, Ohio) at concentrations of 6,400 μg/ml and were stored at −70°C.
The broth microdilution test was performed at C2 according to the M27-A2 protocol with AM3 (lot 120795JB; Difco Laboratories, Detroit, Mich.) (8). The amphotericin B concentration ranged from 0.03 to 16 μg/ml. The AM3 used at C1 was from a different lot, and the test was read at 24 h at C1 and at 48 h at C2. The endpoints of the test(s) were considered to be the lowest drug concentration with an optically clear well. All samples were tested twice.
The FC assay has been described previously (11). Briefly, serial twofold dilutions of amphotericin B ranging from 0.03 to 16 μg/ml were prepared with RPMI 1640. Yeast suspensions in 0.85% sterile saline were adjusted spectrophotometrically to match an 0.5 McFarland density. One-half milliliter of the yeast suspension was added to 0.5 ml of amphotericin B dilutions and incubated at 35°C for 2 h. The growth control tube contained no drug. After incubation, 200 μl of the suspension was mixed with 200 μl of 25 mM sodium deoxycholate (Sigma Biochemical Company) and 10 μl of propidium iodide (1 μg/ml; Molecular Probes Inc., Eugene, Oreg.). Each sample was analyzed with a FACScan flow cytometer (Becton Dickinson, Lincoln Park, N.J.) as previously described (11). MIC was defined as the lowest concentration of drug at which an increase of 50% in mean channel fluorescence (MCF) was seen, compared to the MCF of growth control. C. parapsilosis ATCC 22019 and C. krusei ATCC 6258 were included as controls. All samples were tested twice, except for the isolates in Table 2, which were tested on three different occasions. Percent agreement was determined as the percentage of isolates with MICs within ±1 dilution of the MIC at C1 for each Candida species.
TABLE 2.
Test results for isolates that appeared resistant or susceptible in one or more tests, at C1, and at C2 by the AM3 test (C2-AM3) and by FC (C2-FC)
| Sp. | Identification no. | Putative statusa | MIC (μg/ml)
|
||
|---|---|---|---|---|---|
| C1b | C2-AM3b,c | C2-FCc | |||
| C. lusitaniae | CL524 | S | 0.12 | 0.03 | 0.25 |
| 5W31 | R | 2.0 | 0.06 | 2.0 | |
| CL2887 | R | 2.0 | 0.12 | 4.0 | |
| Y533 | R | 1.0 | 0.5 | 2.0 | |
| Y534 | R | 1.0 | 0.5 | 2.0 | |
| C. tropicalis | MY1012 | R | 4.0 | 0.12 | 4.0 |
| C. krusei | 505.01 | Not known | 2.0 | 0.5 | 2.0 |
Putative status assigned on the basis of isolate's source from references 1, 7, 12, and 14. S, susceptible; R, resistant.
NCCLS (M27-A2) procedure with AM3.
The modal values of triplicate experiments for the FC method and duplicate experiments for the AM3 test are represented.
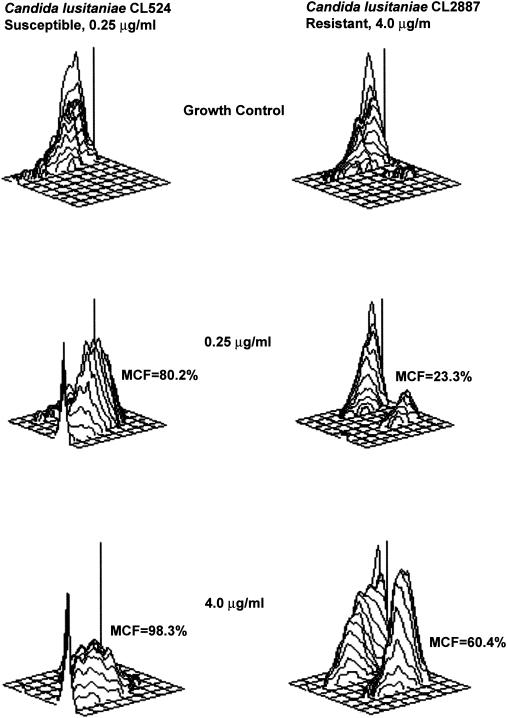
Comparisons of MICs obtained by the AM3 and FC methods are summarized in Table 1. The C1 AM3 and C2 FC tests showed excellent agreements to within 1 dilution, except for C. parapsilosis. One hundred percent agreements to within 1 dilution were obtained for AM3 tests between C1 and C2 for C. albicans and C. parapsilosis, but lower agreements were seen for the other four Candida species. The AM3 and FC tests performed at C2 showed only 60 to 80% agreements to within 1 dilution. At C2, the FC test accurately identified these five amphotericin B-resistant isolates, while the AM3 test failed to identify any of these amphotericin B-resistant isolates (Table 2). The putative susceptible-resistant status for one C. krusei isolate was not known, although the MIC obtained at C1 and at C2 by the FC method was 2.0 μg/ml, and the interpretation was “probable resistance.” Representative three-dimensional plots for two isolates of C. lusitaniae that were considered susceptible and resistant to amphotericin B are shown in Fig. 1.
TABLE 1.
Comparison of amphotericin B MICs for Candida spp. by the NCCLS M27-A2-AM3 and FC methods
| Sp. (no. of isolates) | MIC (μg/ml)
|
% Agreement to within 1 dilution | MIC (μg/ml)
|
% Agreement to within 1 dilution | MIC (μg/ml)
|
% Agreement to within 1 dilution | |||
|---|---|---|---|---|---|---|---|---|---|
| C1-AM3 | C2-AM3a | C1-AM3 | C2-FCa | C2-AM3a | C2-FCa | ||||
| C. albicans (5) | 0.12-0.5 | 0.12-0.25 | 100 | 0.12-0.5 | 0.25-1 | 100 | 0.12-0.25 | 0.25-1 | 80 |
| C. glabrata (4) | 0.12-1 | <0.03-0.5 | 50 | 0.12-1 | 0.25-1 | 100 | <0.03-0.5 | 0.25-1 | 75 |
| C. krusei (5) | 0.25-2 | 0.12-0.25 | 60 | 0.25-2 | 0.5-2 | 100 | 0.12-0.25 | 0.5-2 | 80 |
| C. lusitaniae (5) | 0.12-2 | 0.03-0.5 | 60 | 0.12-2 | 0.25-4 | 80 | 0.03-0.5 | 0.25-4 | 60 |
| C. parapsilosis (5) | 0.12-0.25 | 0.12-0.5 | 100 | 0.12-0.25 | 0.25-1 | 60 | 0.12-0.5 | 0.25-1 | 60 |
| C. tropicalis (6) | 0.25-2 | 0.06-0.12 | 66.6 | 0.25-2 | 0.25-4 | 100 | 0.06-0.12 | 0.25-4 | 66.6 |
| Overall | 73 | 90 | 70 | ||||||
A number of tests were done in duplicate.
FIG. 1.
The three-dimensional plots illustrating the increase in MCF of amphotericin B-susceptible and -resistant isolates. At 0.25 μg/ml, the MCF increased to 80.2% in CL524 and 23.3% in CL2887 compared to growth control. At 4.0 μg/ml, the MCF increased to 60.4% for CL2887, while it reached 98.3% for CL524. Therefore, the MIC for a susceptible isolate (CL524) was 0.25 μg/ml, and for a resistant isolate (CL2887) it was 4.0 μg/ml.
The present FC study has independently confirmed the amphotericin B susceptibility and resistance pattern in a set of Candida isolates intensively studied on many occasions at C1. Other studies have reported that the use of AM3 instead of RPMI 1640 in the NCCLS broth dilution protocol (M27-A) allowed ready discrimination between amphotericin B-susceptible and -resistant isolates (5, 12). However, none of the amphotericin B-resistant isolates were correctly identified when AM3 was used at C2. It is quite likely that lot-to-lot variability of AM3 caused this discrepancy, which has also been reported by other investigators (6). It is reasonable to suggest that the FC method is a useful alternative to the AM3 test for determination of amphotericin B resistance, due to multiparametric analysis of individual cells, definition of the cell characteristics with greater precision, and rapid turnaround time (11). In our hands, RPMI was adequate for the FC method, because AM3 fluoresces at an overlapping wavelength with the fluorochrome used for cell analysis. A previous FC study also reported successful identification of a series of C. lusitaniae isolates that were putatively susceptible and resistant to amphotericin B by using DiOC5 as the fluorochrome and RPMI medium (4). Another study reported FC testing, as an alternative to the NCCLS method, for assessment of various Candida spp. for amphotericin B susceptibility, but it did not include any amphotericin B-resistant isolates (9). Thus, the identification of amphotericin B-resistant isolates can be rapidly and accurately performed by the FC method independently of the choice of culture medium and fluorochrome used for cell analyses.
Acknowledgments
We thank Andrea Doney of the Mycology Laboratory, Wadsworth Center, for technical assistance. We thank Robert Dilwith of the Immunology Core, Wadsworth Center, for his skillful operation of the flow cytometer.
REFERENCES
- 1.Anaissie, E. J., N. C. Karyotakis, R. Hachem, M. C. Dignani, J. H. Rex, and V. Paetznick. 1994. Correlation between in vitro and in vivo activity of antifungal agents against Candida species. J. Infect. Dis. 170:384-389. [DOI] [PubMed] [Google Scholar]
- 2.Cuenca-Estrella, M., T. M. Diaz-Guerra, E. Mellado, and J. L. Rodriguez-Tudela. 2001. Detection of resistance to amphotericin B in Candida isolates by using Iso-Sensitest broth. Antimicrob. Agents Chemother. 45:2070-2074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Drutz, D. J., and R. I. Lehrer. 1978. Development of amphotericin B-resistant Candida tropicalis in a patient with defective leukocyte function. Am. J. Med. Sci. 276:77-92. [PubMed] [Google Scholar]
- 4.Favel, A., F. Peyron, M. De Meo, A. Michel-Nguyen, J. Carriere, C. Chastin, and P. Regli. 1999. Amphotericin B susceptibility testing of Candida lusitaniae isolates by flow cytofluorometry: comparison with the Etest and the NCCLS broth macrodilution method. J. Antimicrob. Chemother. 43:227-232. [DOI] [PubMed] [Google Scholar]
- 5.Law, D., C. B. Moore, and D. W. Denning. 1997. Amphotericin B resistance testing of Candida spp.: a comparison of methods. J. Antimicrob. Chemother. 40:109-112. [DOI] [PubMed] [Google Scholar]
- 6.Lozano-Chiu, M., P. W. Nelson, M. Lancaster, M. A. Pfaller, and J. H. Rex. 1997. Lot-to-lot variability of antibiotic medium 3 used for testing susceptibility of Candida isolates to amphotericin B. J. Clin. Microbiol. 35:270-272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lozano-Chiu, M., M. V. Lancaster, and J. H. Rex. 1998. Evaluation of a colorimetric method for detecting amphotericin B-resistant Candida isolates. Diagn. Microbiol. Infect. Dis. 31:417-424. [DOI] [PubMed] [Google Scholar]
- 8.National Committee for Clinical Laboratory Standards. 2002. Reference method for broth dilution antifungal susceptibility testing of yeasts. M27-A2. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 9.Peyron, F., A. Favel, A. Michel-Nguyen, M. Gilly, P. Regli, and A. Bolmstrom. 2001. Improved detection of amphotericin B-resistant isolates of Candida lusitaniae by Etest. J. Clin. Microbiol. 39:339-342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Powderly, W. G., G. S. Kobayashi, G. P. Herzig, and G. Medoff. 1988. Amphotericin B-resistant yeast infection in severely immunocompromised patients. Am. J. Med. 84:826-832. [DOI] [PubMed] [Google Scholar]
- 11.Ramani, R., and V. Chaturvedi. 2000. Flow cytometry antifungal susceptibility testing of pathogenic yeasts other than Candida albicans and comparison with the NCCLS broth microdilution test. Antimicrob. Agents Chemother. 44:2752-2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rex, J. H., C. R. Cooper, Jr., W. G. Merz, J. N. Galgiani, and E. J. Anaissie. 1995. Detection of amphotericin B-resistant Candida isolates in a broth-based system. Antimicrob. Agents Chemother. 39:906-909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rex, J. H., M. A. Pfaller, T. J. Walsh, V. Chaturvedi, A. Espinel-Ingroff, M. A. Ghannoum, L. L. Gosey, F. C. Odds, M. G. Rinaldi, D. J. Sheehan, and D. W. Warnock. 2001. Antifungal susceptibility testing: practical aspects and current challenges. Clin. Microbiol. Rev. 14:643-658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wanger, A., K. Mills, P. W. Nelson, and J. H. Rex. 1995. Comparison of Etest and National Committee for Clinical Laboratory Standards broth macrodilution method for antifungal susceptibility testing: enhanced ability to detect amphotericin B-resistant Candida isolates. Antimicrob. Agents Chemother. 39:2520-2522. [DOI] [PMC free article] [PubMed] [Google Scholar]