Abstract
Background
Oxycodone controlled release (CR) and oxymorphone extended release (ER) are frequently prescribed long-acting opioids, which are approved for twice-daily dosing. The US Food and Drug Administration approved a reformulated crush-resistant version of oxycodone CR in April 2010.
Objective
To compare the daily average consumption (DACON) for oxycodone CR and for oxymorphone ER before and after the introduction of the reformulated, crush-resistant version of oxycodone CR.
Methods
This was a retrospective claims database analysis using pharmacy claims from the MarketScan database for the period from January 2010 through March 2011. The interrupted time series analysis was used to evaluate the impact of the introduction of reformulated oxycodone CR on the DACON of the 2 drugs—oxycodone CR and oxymorphone ER. The source of the databases included private-sector health data from more than 150 medium and large employers. All prescription claims containing oxycodone CR and oxymorphone ER dispensed to members from January 1, 2010, to March 31, 2011, were included in the analysis. Prescription claims containing duplicate National Drug Codes, missing member identification, invalid quantities or inaccurate days supply of either drug, and DACON values of <1 and >500 were removed.
Results
The database yielded 483,063 prescription claims for oxycodone CR and oxymorphone ER from January 1, 2010, to March 31, 2011. The final sample consisted of 411,404 oxycodone CR prescriptions (traditional and reformulated) dispensed to 85,150 members and 62,656 oxymorphone ER prescriptions dispensed to 11,931 members. Before the introduction of reformulated oxycodone CR, DACON values for the highest strength available for each of the 2 drugs were 0.51 tablets higher for oxycodone CR than for oxymorphone ER, with mean DACON values of 3.5 for oxycodone CR and 3.0 for oxymorphone ER (P <.001). The differences of mean DACON between the 2 drugs for all lower strengths were 0.46 tablets, with mean DACON values of 2.7 for oxycodone CR and 2.3 for oxymorphone ER (P <.001). After the introduction of the new formulation, the difference in mean DACON between the 2 drugs was slightly lower: 0.45 tablets for the highest-strength and 0.40 tablets for the lower-strength pairs. Regression analyses showed that the immediate and overall impact of the reformulation of oxycodone CR on the DACON of oxycodone CR was minimal, whereas no changes were seen in the DACON of oxymorphone ER. The estimated DACON for oxycodone CR decreased by 0.1 tablets, or 3.7% (P <.001), 6 months after the new formulation was introduced.
Conclusion
The mean DACON was 0.4 tablets per day higher for oxycodone CR compared with oxymorphone ER for all dosage strengths for the entire study period. After the introduction of the reformulated oxycodone CR, the DACON for this drug was slightly mitigated; however, there was a minimal impact on the mean differences between oxycodone CR and oxymorphone ER.
Chronic pain is experienced by more than one third of the US population.1 Regardless of disease etiology or individual characteristics, chronic pain is debilitating and has a profound impact on emotional and physical functioning.2,3 For chronic pain, which is characterized as pain lasting 3 or more months,4 opioid analgesics are considered part of a multifaceted strategy to manage moderate-to-severe pain for a number of conditions involving the musculoskeletal system (eg, osteoarthritis, low back pain), neurologic system (eg, diabetic neuropathy, spinal cord pain), and for pain associated with neoplastic disease.5–7
Two long-acting opioid analgesics, oxycodone controlled release (CR; OxyContin) and oxymorphone extended release (ER; Opana), are often prescribed to reduce the intensity of moderate-to-severe noncancer pain.8 However, the usefulness of long-acting opioids in chronic noncancer pain has been scrutinized, because of their adverse effects (eg, nausea and constipation) and the association with illicit drug behaviors, ranging from drug abuse, illegal distribution, and drug product tampering.9,10 In addition, a prospective study confirmed that some patients with chronic pain may require more frequent dosing of sustained-release opioids beyond the doses recommended by the manufacturer.11 Despite concerns over long-term analgesic use, multiple expert panels have concluded that long-term opioid therapy can be effective for carefully selected and monitored patients with chronic noncancer pain.12
To encourage proper use, new drug formulations are designed to improve pharmacotherapy either by reducing the dosing frequency or by hindering potential misuse and abuse. In August 2010, the US Food and Drug Administration (FDA) approved a newly formulated crush-resistant version of oxycodone CR. The recommended dosing regimen for oxycodone CR, oxymorphone ER, and the reformulated oxycodone CR is 2 tablets daily. Besides the need for product formulations that will provide more consistent and sustained levels of analgesia throughout the recommended dosing interval, the crush-resistant form of oxycodone may present an obstacle to some forms of misuse.
In making pharmaceutical policy decisions, commercial insurers may need to consider utilization patterns in addition to cost. Daily average consumption (DACON) is a readily available measure that is often used to assess drug utilization. DACON has been used in previous studies to examine medication use in patients with arthritis, diabetes, and hypertension.13–16 With chronic pain, the utilization of opioid analgesics may be of concern to pharmacy benefit managers and third-party payers who design and manage prescription drug benefit plans. To build on previous research,17 we wanted to know if differences in the DACON for oxycodone CR compared with oxymorphone ER persist in recent data that include utilization of a reformulated version of oxycodone CR.
KEY POINTS
-
▸
Chronic pain is experienced by at least 38% of the US population.
-
▸
The prevalence of long-term opioid use for noncancer pain in the United States has increased in the past decade.
-
▸
This study compared the overall mean daily average consumption (DACON) for 2 opioids—oxycodone controlled release (CR) and oxymorphone extended release (ER)—after the introduction of the crush-resistant formulation of oxycodone CR.
-
▸
Results showed that the overall DACON for oxycodone CR was higher by 0.4 tablets per day for all dosage strengths than for oxymorphone ER, with means of 2.9 and 2.5, respectively.
-
▸
The difference in mean DACON between oxycodone CR and oxymorphone ER was 0.45 tablets per day for the highest-strength pairs and 0.40 tablets per day for the lower-strength pairs after the introduction of reformulated oxycodone CR.
-
▸
After the introduction of the new formulation, the DACON for oxycodone CR was slightly mitigated; however, there was a minimal impact on the mean DACON differences of the 2 drugs.
-
▸
Because changes in opioid utilization may reflect inappropriate drug use, it is essential to follow the guidelines for opioid use to ensure that these drugs are used judiciously in the management of chronic noncancer pain.
Opportunities to investigate changing patterns of opioid use will allow healthcare benefit managers to monitor opioid use with the goal of maintaining tablet utilization within the expected range of 2 tablets daily. Therefore, the purpose of this study was to evaluate the DACON of oxycodone CR and of oxymorphone ER before and after the introduction of the reformulated oxycodone CR. This study provides some insight into the utilization patterns of 2 branded opioid products used in pain management.
Methods
This was a retrospective, interrupted time series analysis of observational data to assess the DACON of oxycodone CR, oxymorphone ER, and reformulated oxycodone CR over time using the prescription claimslevel analysis. Pharmacy claims for the 2 drugs from the MarketScan Commerical database (Thomson Reuters, Ann Arbor, MI) were used to evaluate average monthly and weekly DACON values of oxymorphone ER and oxycodone CR. The database contains individual-level healthcare claims, including enrollment, medical, and prescription (outpatient) records from all providers of care. For the most recent 3 years, data were collected from more than 150 large employers (>200 carriers) and more than 20 regional health plans that provide healthcare coverage for more than 30 million lives annually.
The covered lives include active employees, early retirees, COBRA continuees, and their dependents who are insured by large employers and health plans. Insurance coverage was provided under a variety of fee-for-service, preferred provider organizations, and capitated and partially captitated health plans. Data were deidentified and used in accordance with the Health Insurance Portability and Accountability Act (HIPAA). Approval by the institutional review board was not required.
Calculation of Opioid Consumption
We evaluated DACON over time for oxycodone CR and oxymorphone ER (traditional and reformulated) from January 2010 to March 2011. All prescription claims containing oxycodone CR and oxymorphone ER dispensed to members aged 18 to 64 years from January 1, 2010, to March 31, 2011, were included in the calculation of DACON. Prescription claims containing duplicate National Drug Codes, missing member identification, invalid quantities or inaccurate days supply for either drug, and DACON of <1 and >500 tablets were removed.
The DACON for each prescription was calculated by dividing the total tablets dispensed by days supplied, as reflected by these data fields in each submitted prescription claim. Overall monthly and weekly DACON was calculated for all doses combined, the highest-strength-dosages (80-mg oxycodone CR and 40-mg oxymorphone ER), and for all lower-strength dosages, respectively (ie, oxycodone CR 10, 20, 30, 40, and 60 mg, and oxymorphone ER 5, 7.5, 10, 20, and 30 mg).
Statistical Analysis
The measurement of DACON over time was interrupted by the introduction of reformulated oxycodone CR. Because the outcome was analyzed with respect to time intervals (ie, weekly), the error terms may be correlated (ie, not independent), thereby violating one of the classic regression assumptions. Therefore, the interrupted time series analysis was used to estimate changes in levels and weekly trends for the mean DACON of oxymorphone ER and oxycodone CR (traditional and reformulated) before and after the introduction of reformulated oxycodone CR. The model is also often referred to as the segmented regression model, which is used to evaluate longitudinal effects (ie, change in intercept and slopes) of interventions, while relaxing the assumption that observations are independent.
The segmented regression model we developed was derived as follows:
Yt = β0 + β1 * timet + β2* interventiont + β3* timet * interventiont + ∊t
Yt = average DACON in week t
Timet = a continuous variable for time in weeks at time t from the first week of January 2010
Interventiont = a dummy variable taking the values of 0 in the preintroduction period (before August or week 31) and 1 after introduction
β0 estimates the baseline level of outcomes
β1 estimates for the baseline slope of the outcomes to control for secular trends before the introduction
β2 estimates the intercept change immediately after the introduction
β3 estimates the change in slope of mean DACON after the introduction.
Autocorrelation in error terms of consecutive observations often exist when time is a predictor in the time series regression analysis. To assess the regression models for serial correlation of the time series data, the Durbin-Watson, alternative Durbin-Watson, Breusch-Godfrey LM, and Bartlett's statistic white noise tests were used.18,19 The Breusch-Pagan test was used to assess heteroscedasticity or the nonconstant variance assumption.19
Serial correlations were adjusted using one of the autoregressive, integrated, moving-average (ARIMA) models. These models are built by finding the best possible weighted average for a single time series, taking into account past observations (autoregressive terms) and past error terms (moving average terms).20 For this study, interrupted time series analysis allowed researchers to control for previous trends in the assessment of DACON and to study the dynamics of change in response to the introduction of reformulated oxycodone CR.21
To ensure a sufficient number of observations for the segmented regression model, we utilized weekly DACON data of 64 weekly intervals in this study: 31 weeks before the intervention and 33 after the intervention, from January 2010 to March 2011. The number of time points in this study exceeds the range of 50 time points suggested in similar segmented regression analyses and achieves an acceptable level of variability of the estimate at each time point.22 Before analysis, outliers were removed using the standard deviation method, such that DACON values whose deviation exceeds 3 standard deviations of the mean were excluded from the analysis.23
A sensitivity analysis was conducted to examine model stability. The sensitivity analysis used a similar approach to account for the brief transition period after the introduction of reformulated oxycodone CR by excluding the outcome values that occurred during the potential intervention periods of August 2010 and September 2010. Descriptive statistics and overall mean comparison were evaluated using student t-tests. Statistical significance was established for P <.05. SAS version 9.1 (SAS Institute, Inc, Cary, NC) and Stata version 11.2 (StataCorp, College Station, TX) were used for data analysis.
Results
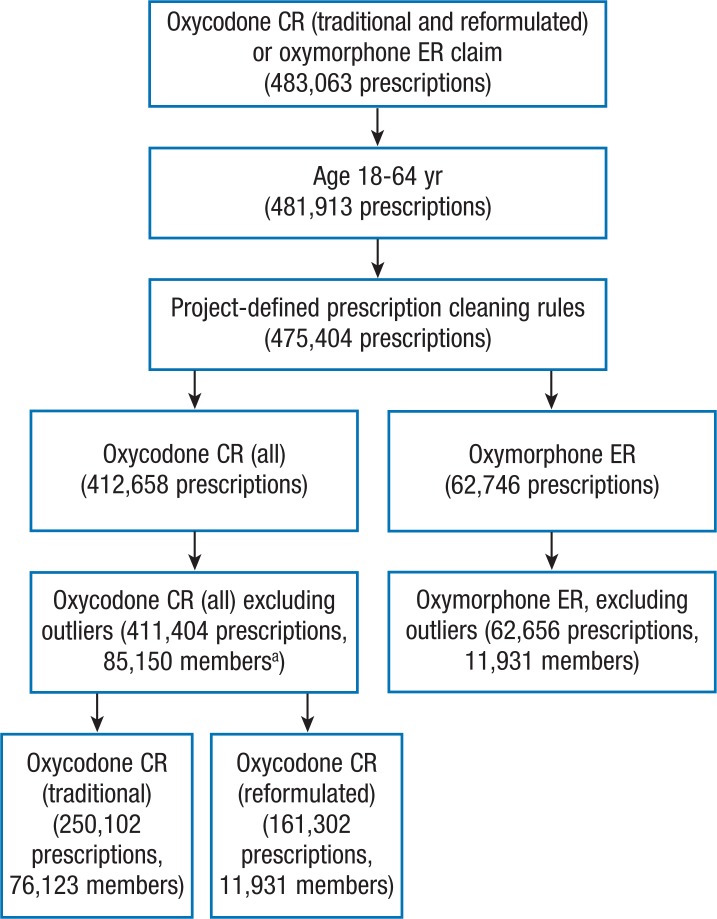
The MarketScan commercial database yielded 483,063 prescription claims for oxycodone CR and oxymorphone ER from January 1, 2010, to March 31, 2011 (Figure 1). After applying the exclusion criteria for age and high DACON outliers, the final sample consisted of 411,404 oxycodone CR prescriptions (traditional and reformulated) dispensed to 85,150 members and 62,656 oxymorphone ER prescriptions dispensed to 11,931 members. Of the total members, 51% were women with the mean age of 48.5 years.
Figure 1. Sample Selection Process, January 1, 2010-March 31, 2011.
aThe number of members for the traditional and reformulated oxycodone CR do not add up, because members could have had multiple prescriptions across the time horizon and across treatment group.
CR indicates controlled release; ER, extended release.
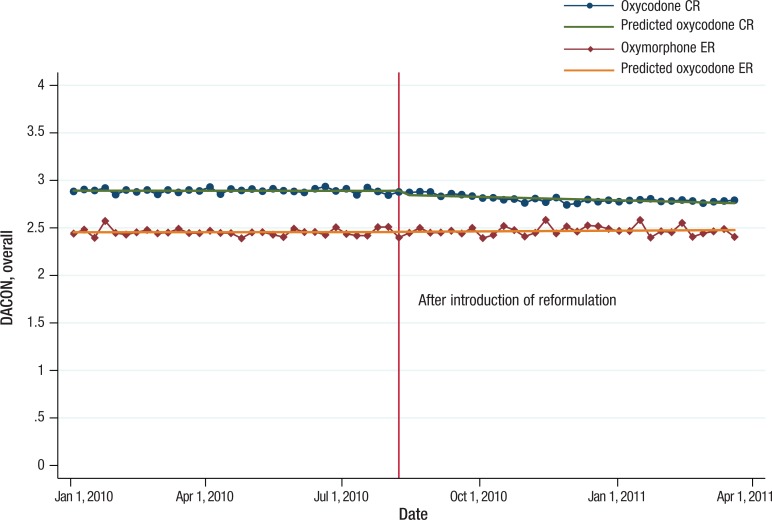
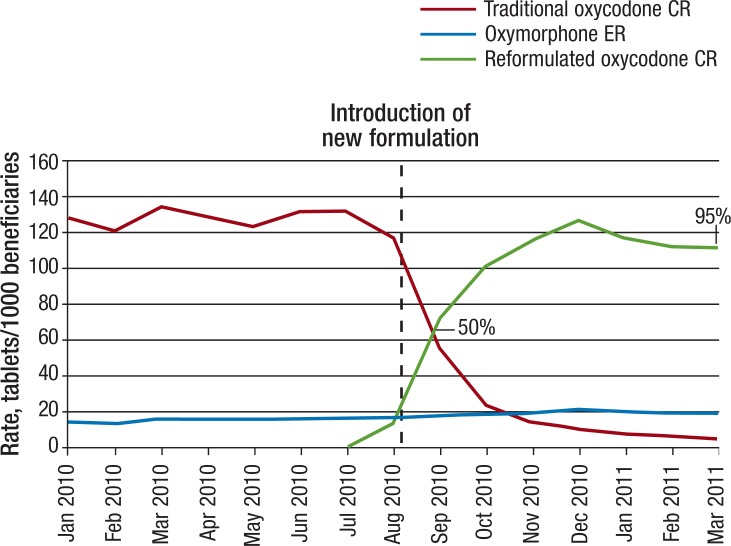
Over the 15-month observation period, the overall mean DACON values for all dosage strengths were approximately 0.4 tablets per day higher for oxycodone CR than for oxymorphone ER, with means of 2.9 for oxycodone CR and 2.5 for oxymorphone ER (Figure 2). Reformulated oxycodone CR accounted for approximately 50% of oxycodone CR tablets dispensed in September 2010, 1 month after its introduction. The proportion of reformulated oxycodone CR relative to all oxycodone CR tablets steadily increased from 50% to 95% by the end of the study period (Figure 3).
Figure 2. Actual and Predicted Trend Values of Overall DACON for Oxycodone CR and Oxymorphone ER from the Interrupted Time Series Models, January 2010-March 2011.
CR indicates controlled release; DACON, daily average consumption; ER, extended release.
Figure 3. Tablets per 1000 Beneficiaries of Oxycodone CR and Oxymorphone ER, January 2010-March 2011.
CR indicates controlled release; ER, extended release.
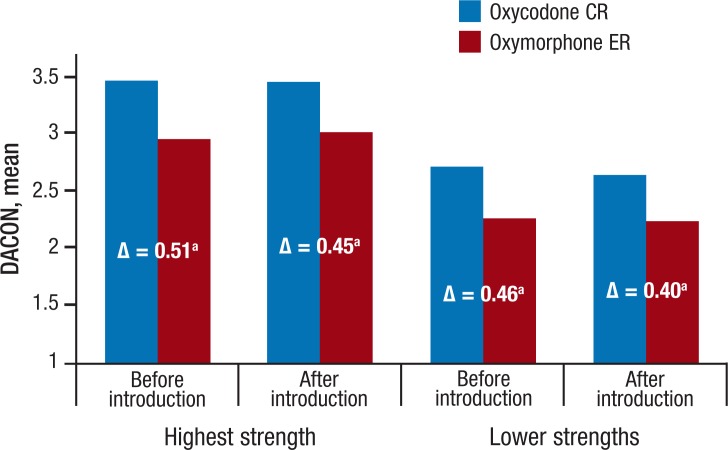
Overall mean DACON values for the 2 drugs over the observation period were relatively stable, ranging from 2.8 to 2.9 tablets per day for oxycodone CR and 2.4 to 2.5 tablets per day for oxymorphone ER, respectively (Figure 2). During the observation period before the introduction of the new formulation, DACON values for the highest strength were 0.51 tablets higher for oxycodone CR than for oxymorphone ER, with a mean DACON of 3.5 for oxycodone CR and 3.0 for oxymorphone ER (P <.001; Figure 4). After the introduction of the new formulation, the difference in mean DACON values between reformulated oxycodone CR and oxymorphone ER decreased slightly to 0.45 tablets per day for the highest-strength pairs (ie, mean DACON, 3.5 for oxycodone CR vs 3.0 for oxymorphone ER; P <.001).
Figure 4. Mean DACON by Strength Before and After Introduction of New Formulation of Oxycodone CR.
aMean differences by each pair were all significant (P <.001). CR indicates controlled release; DACON, daily average consumption; ER, extended release.
The differences of mean DACON for all lower strengths between oxycodone CR and oxymorphone ER were 0.46 tablets per day before the introduction of the new formulation, with mean DACON values of 2.7 and 2.3 tablets (P <.001) for oxycodone CR and oxymorphone ER, respectively (Figure 4). After the introduction of the new formulation, the difference in mean DACON values between the 2 drugs was 0.40 tablets per day, with a mean DACON of 2.7 for oxycodone CR and 2.3 for oxymorphone ER (P <.001).
Interrupted Time Series Results
Figure 2 shows the actual and predicted values of weekly DACON trends for oxycodone CR and oxymorphone ER over time using the interrupted time series models. Before the introduction of reformulated oxycodone CR, there was no significant week-to-week impact in the mean DACON for oxycodone CR and oxymorphone ER (P = .956 and P = .878, respectively; Table 1). Immediately after the introduction of the new formulation, the estimated mean DACON for oxycodone CR dropped slightly, by 0.05 tablets weekly. Throughout the study period, there was neither immediate change nor any weekly change in oxymorphone ER's DACON as a result of the introduction of oxycodone CR.
Table 1.
Results from the Interrupted Time Series Models: Impact of Introduction of Reformulated Oxycodone CR on Each Medication Strength
| Before introduction | After introduction | ||||||
|---|---|---|---|---|---|---|---|
| Medication/strength | Change in DACON each week before the introduction (β1 baseline slope) | P value | Change in DACON immediately after the introduction (β2 intercept change) | P value | Change in DACON slope after the introduction (β3) | Change in DACON slope after the introduction (1 month) | P value |
| Oxycodone CR | |||||||
| All strengths | 0.00003 | .956 | 0.052 | .034 | −0.003 | −0.012 | <.001 |
| Highest strength | −0.002 | <.001 | 0.0002 | .996 | 0.0013 | −0.005 | .124 |
| Lower strengths | 0.0002 | .658 | 0.055 | <.001 | −0.003 | −0.011 | <.001 |
| Oxymorphone ER | |||||||
| All strengths | −0.0001 | .878 | −0.011 | .828 | 0.001 | −0.002 | .695 |
| Highest strength | −0.0003 | .822 | 0.041 | .518 | 0.0005 | −0.002 | .797 |
| Lower strengths | 0.00003 | .947 | 0.031 | .302 | −0.001 | −0.005 | .105 |
CR indicates controlled release; DACON, daily average consumption; ER, extended release.
In terms of changes in trends for the mean DACON after the introduction of oxycodone CR, the weekly trends for oxycodone CR slightly decreased, by 0.003 tablets weekly (0.01 tablets per month). Because the absolute change in weekly DACON was minimal, we compared the overall change by combining the immediate and trend effects for the estimated DACON 6 months after the introduction of the new formulation, with the outcomes as if that introduction had not occurred.
The models showed that the average DACON for oxycodone CR decreased by 0.11 tablets or 3.7% (P <.001) 6 months after the new formulation was introduced compared with the DACON level before the introduction (Table 2).
Table 2.
Estimated Overall Change of DACON 6 Months after Introduction of Reformulated Oxycodone CR from Interrupted Time Series Models
| Medication strength | Overall change (intercept + slope) 6 months after introduction | P value | |
|---|---|---|---|
| Daily tablets | Change, % | ||
| Oxycodone CR | |||
| All strengths | −0.11 | −3.7 | <.001 |
| Highest strength | 0.07 | 2.1 | .003 |
| Lower strengths | −0.09 | −3.4 | <.001 |
| Oxymorphone ER | |||
| All strengths | 0.02 | 0.7 | .611 |
| Highest strength | 0.07 | 2.3 | .236 |
| Lower strengths | −0.03 | −1.4 | .087 |
CR indicates controlled release; DACON, daily average consumption; ER, extended release.
When analyzing the data by strength, the average DACON for the 80-mg (highest strength) oxycodone CR 6 months after the introduction increased slightly by 0.07 tablets or 2.1% (P = .003), whereas the DACON for the lower strengths decreased by 0.09 tablets, or 3.4% (P <.001).
Sensitivity analyses were conducted by excluding the outcome values that occurred during the potential intervention period of August and September 2010. The results (not shown) indicate that the introduction of reformulated oxycodone CR had a slight, mitigating effect on DACON; however, there was no significant change in DACON over time. In addition, there was no significant change in DACON for oxymorphone ER associated with the introduction of the newly formulated oxycodone CR.
Discussion
To evaluate the impact on utilization, differences in DACON were assessed between traditional oxycodone CR and oxymorphone ER before and after the introduction of the reformulated oxycodone CR. Results from the interrupted time series analyses and sensitivity analyses revealed that the impact on DACON associated with the introduction of reformulated oxycodone CR was minimal, whereas there was no impact on oxymorphone ER's DACON. The models estimated that the average DACON for oxycodone CR decreased by 0.1 tablets, or 3.7%, 6 months after the new formulation was introduced (P <.001).
In addition, differences in mean DACON between oxycodone CR and oxymorphone ER were quite stable before and after the introduction of reformulated oxycodone CR. Throughout the 15-month study period, the overall DACON was higher for oxycodone CR (traditional or reformulated) compared with oxymorphone ER, by 0.4 to 0.5 tablets per day for all dosage strengths. For a subgroup analysis by strength, the differences of mean DACON between oxycodone CR and oxymorphone ER slightly decreased to 0.45 tablets for the highest strength and 0.4 tablets for lower strengths after the introduction of the new formulation.
These results are consistent with previous research in this area. For example, there is evidence from other studies to support a higher DACON with oxycodone CR compared with oxymorphone ER. Malkin and colleagues found that the DACON for all strengths of oxycodone CR was 3.4 and that higher strengths were associated with a higher DACON value, ranging from 2.9 for the 10-mg tablets to 5.2 for the 80-mg tablets.24
In another study by Berner and colleagues, a retrospective analysis of administrative claims data for commercially insured patients was conducted to compare the DACON of oxycodone CR and oxymorphone ER in patients with low back pain.25 Again, the DACON was higher for the maximum-strength tablets of oxycodone CR 80 mg, which were 3.9 tablets per day and significantly higher than the DACON of 2.9 for an equipotent oxymorphone ER maximum-strength tablet of 40 mg (P <.01).25 The investigators estimated that if oxymorphone ER 40-mg tablets were substituted for oxycodone CR 80-mg tablets in the 688 patients in their analysis of a health plan with 32,325 patients having at least 1 prescription for oxycodone CR or oxymorphone ER, the monthly cost difference would be $217,985 based on the DACON difference, assuming per-tablet wholesale acquisition costs of $10.83 and $10.93, respectively.25
Given the consistent patterns in DACON for these drugs, pharmaceutical policymakers may want to consider these results in related decisions.
Findings from this study provide additional information when DACON is considered for oxycodone CR (traditional and reformulated) across all tablet strengths compared with oxymorphone ER. A notable finding is the continued difference in DACON between oxycodone CR and oxymorphone ER, both before and after introduction of the reformulated oxycodone CR. Considering the potential for dose escalation during long-term opioid use,26 the consistent DACON values observed throughout the 15-month study period suggest that current dosing schedules were not altered in response to the introduction of reformulated oxycodone CR.
Interrupted time series analysis is useful when changes over time are interrupted by events, such as the introduction of reformulated oxycodone CR in this study. However, longitudinal designs can be influenced by events outside the control of researchers. Therefore, a sensitivity analysis was performed to assess model robustness. The concordance of results through the transition period of reformulated oxycodone CR indicates that our findings will provide decision makers with a valid assessment of the DACON for this population.
Although the DACON provides an accurate assessment of drug utilization, with long-term opioid use there may be some members who either fail to achieve adequate analgesic effects despite reaching the maximum tablet strength for frequently prescribed opioids or may experience analgesic tolerance if more frequent dosing is necessary.27
Realizing that it may be difficult to distinguish analgesic tolerance from potential drug abuse, especially if members are requesting traditional oxycodone CR over reformulated oxycodone CR, health professionals should be cognizant of and recognize that changes in utilization may reflect inappropriate drug use. It is, therefore, essential to follow the guidelines for opioid use to ensure that these drugs are used judiciously in the management of chronic noncancer pain, especially for patients who require higher doses of opioids, have issues with drug abuse, or who report numerous comorbid conditions.28,29
Limitations
There are certain caveats associated with the use of claims data. First, it was not possible to examine whether patients were actually using the medications examined in this database. Thus, it was not possible to determine if changes in pain intensity required patients to alter the dose or dosing frequency of opioids for medical emergencies or procedures.
Second, it was not possible to determine if prescribers changed medications in response to oxycodone CR or to oxymorphone ER failures, or changed the dosing schedule to accommodate other medication use or additional diagnoses. For these reasons, it was not possible to control for patient-initiated self-management of pain.
Third, although the calculations of DACON were statistically robust, as observed from sensitivity analyses, additional changes in utilization could occur from inappropriate use of opioids.
Fourth, a common limitation about the claims database is that there is no diagnosis code entered on prescription claims; this study was based on prescription claims, not patients. Therefore, information related to diagnosis codes is not provided in this study.
Finally, because claims databases do not contain data regarding pain severity, it was not possible to evaluate whether one population suffered from more severe pain. To monitor opioid use, managed care plans often place quantity limits on long-acting opioids. Although the patterns of drug use examined in this study were relatively stable over time, it is important to continue monitoring changes in long-term utilization of these opioids.
Conclusion
In this study, the introduction of a crush-resistant oxycodone CR very slightly lowered the DACON for that drug; however, the change was minimal. Therefore, this research supports the notion that differences in DACON are more likely a result of differences in the oxycodone and oxymorphone molecules and not the effects of oxycodone CR reformulation.
Acknowledgments
The authors would like to thank Robert Garvin, MA, for programming support.
Study Funding
This study was supported by funding from Endo Pharmaceuticals.
Author Disclosure Statement
Drs Puenpatom, Ma, Ben-Joseph, and Summers are employees of and Dr Szeinbach received financial support from Endo Pharmaceuticals.
Contributor Information
R. Amy Puenpatom, Associate Director of Health Outcomes and Pharmacoeconomics, Endo Pharmaceuticals, Chadds Ford, PA.
Sheryl L. Szeinbach, Professor, Division of Pharmacy Practice and Administration, College of Pharmacy, Ohio State University, Columbus.
Larry Ma, Director, Health Outcomes and Pharmacoeconomics at Endo Pharmaceuticals, Chadds Ford, PA.
Rami H. Ben-Joseph, Vice President, Health Outcomes and Pharmacoeconomics at Endo Pharmaceuticals, Chadds Ford, PA.
Kent H. Summers, Senior Director, Health Outcomes and Pharmacoeconomics at Endo Pharmaceuticals, Chadds Ford, PA.
References
- 1.Institute of Medicine. Relieving Pain in America: A Blueprint for Transforming Prevention, Care, Education and Research. Washington, DC: The National Academies Press; 2011. http://books.nap.edu/openbook.php?record_id=13172&page=17 Accessed January 25, 2012. [PubMed] [Google Scholar]
- 2.Turk DC, Wilson HD, Cahana A. Treatment of chronic non-cancer pain. Lancet. 2011; 377: 2226–2235 [DOI] [PubMed] [Google Scholar]
- 3.Annemans L. Pharmacoeconomic impact of adverse events of long-term opioid treatment for the management of persistent pain. Clin Drug Investig. 2011; 31: 73–86 [DOI] [PubMed] [Google Scholar]
- 4.Portenoy RK, Ugarte C, Fuller I, Haas G. Population-based survey of pain in the United States: differences among white, African American, and Hispanic subjects. J Pain. 2004; 5: 317–328 [DOI] [PubMed] [Google Scholar]
- 5.Markenson JA, Croft J, Zhang PG, Richards P. Treatment of persistent pain associated with osteoarthritis with controlled-release oxycodone tablets in a randomized controlled clinical trial. Clin J Pain. 2005; 21: 524–535 [DOI] [PubMed] [Google Scholar]
- 6.Watson CP, Moulin D, Watt-Watson J, et al. Controlled-release oxycodone relieves neuropathic pain: a randomized controlled trial in painful diabetic neuropathy. Pain. 2003; 105: 71–78 [DOI] [PubMed] [Google Scholar]
- 7.Desandre P, Quest TE. Management of cancer-related pain. Hematol Oncol Clin North Am. 2010; 24: 643–658 [DOI] [PubMed] [Google Scholar]
- 8.Chou R, Clark E, Helfand M. Comparative efficacy and safety of long-acting oral opioids for chronic non-cancer pain: a systematic review. J Pain Symptom Manag. 2003; 26: 1026–1048 [DOI] [PubMed] [Google Scholar]
- 9.Leider HL, Dhaliwal J, Davis EJ, et al. Healthcare costs and nonadherence among chronic opioid users. Am J Manag Care. 2011; 17: 32–40 [PubMed] [Google Scholar]
- 10.Boscarino JA, Rukstalis MR, Hoffman SN, et al. Prevalence of prescription opioid-use disorder among chronic pain patients: comparison of the DSM-5 vs. DSM-4 diagnostic criteria. J Addict Dis. 2011; 30: 185–194 [DOI] [PubMed] [Google Scholar]
- 11.Gallagher RM, Welz-Bosna M, Gammaitoni A. Assessment of dosing frequency of sustained-release opioid preparations in patients with chronic nonmalignant pain. Pain Med. 2007; 8: 71–74 [DOI] [PubMed] [Google Scholar]
- 12.Manchikanti L, Ailinani H, Koyyalagunta D, et al. A systematic review of randomized trials of long-term opioid management for chronic non-cancer pain. Pain Physician. 2011; 14: 91–121 [PubMed] [Google Scholar]
- 13.Schnitzer TJ, Kong SX, Mitchell JH, et al. An observational, retrospective, cohort study of dosing patterns for rofecoxib and celecoxib in the treatment of arthritis. Clin Ther. 2003; 25: 3162–3172 [DOI] [PubMed] [Google Scholar]
- 14.McAdam-Marx C, Yu J, Bouchard J, et al. Comparison of daily insulin dose and other antidiabetic medications usage for type 2 diabetes patients treated with an analog basal insulin. Curr Med Res Opin. 2010; 26: 191–201 [DOI] [PubMed] [Google Scholar]
- 15.Borah BJ, Darkow T, Bouchard J, et al. A comparison of insulin use, glycemic control, and health care costs with insulin detemir and insulin glargine in insulinnaïve patients with type 2 diabetes. Clin Ther. 2009; 31: 623–631 [DOI] [PubMed] [Google Scholar]
- 16.Jan SA, Patel JV, Welz J, Ishak P. A retrospective database analysis of prescribing patterns for specific angiotensin receptor blockers. Drug Benefit Trends. 2005; 17: 23–29 [Google Scholar]
- 17.Rubino M, Summers KH, Puenpatom A, Fu C, Ohsfeldt RL, Ben-Joseph RH. A comparison of daily average consumption (DACON) of oxycodone and oxymorphone long-acting oral tablets. J Manag Care Pharm. 2011; 17: 367–376 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brockwell PJ, Davis RA. Time Series: Theory and Methods (Springer Series in Statistics). 2nd edition New York, NY: Springer; 2006 [Google Scholar]
- 19.Baltagi BH. Econometrics (Springer Texts in Business and Economics). 5th ed.New York, NY: Springer; 2011 [Google Scholar]
- 20.Durbin J. Testing for serial correlation in least squares regressions when some of the regressions are lagged dependent variables. Econometrica. 1970; 38: 410–421 [Google Scholar]
- 21.Wagner AK, Soumerai SB, Zhang F, Ross-Degnan D. Segmented regression analysis of interrupted time series studies in medication use research. J Clin Pharm Ther. 2002; 27: 299–309 [DOI] [PubMed] [Google Scholar]
- 22.Shardell M, Harris AD, El-Kamary SS, et al. Statistical analysis and application of quasi experiments to antimicrobial resistance intervention studies. Clin Infect Dis. 2007; 45: 901–907 [DOI] [PubMed] [Google Scholar]
- 23.Maronna RA, Martin DR, Yohai VJ. Robust Statistics: Theory and Methods (Wiley Series in Probability and Statistics). Chichester, England: John Wiley & Sons, Ltd; 2006 [Google Scholar]
- 24.Malkin JD, Ackerman SJ, Schein J, et al. Cost and utilization patterns of fentanyl transdermal system and oxycodone hydrochloride controlled-release in a California Medicaid population. J Manag Care Pharm. 2002; 8: 132–140 [Google Scholar]
- 25.Berner T, Thomson H, Hartry A, et al. A comparison of daily average consumption of oxycodone controlled release (OxyContin CR) and oxymorphone extended release (Opana ER) in patients with low back pain. PT. 2011; 36: 139–144 [PMC free article] [PubMed] [Google Scholar]
- 26.Cifuentes M, Webster B, Genevay S, Pransky G. The course of opioid prescribing for a new episode of disabling low back pain: opioid features and dose escalation. Pain. 2010; 151: 22–29 [DOI] [PubMed] [Google Scholar]
- 27.Graziottin A, Gardner-Nix J, Stumpf M, Berliner MN. Opioids: how to improve compliance and adherence. Pain Pract. 2011; 11: 574–581 [DOI] [PubMed] [Google Scholar]
- 28.Chou R, Ballantyne JC, Fanciullo GJ, et al. Research gaps on use of opioids for chronic noncancer pain: findings from a review of the evidence for an American Pain Society and American Academy of Pain Medicine clinical practice guideline. J Pain. 2009; 10: 147–159 [DOI] [PubMed] [Google Scholar]
- 29.Chapman CR, Lipschitz DL, Angst MS, et al. Opioid pharmacotherapy for chronic non-cancer pain in the United States: a research guideline for developing an evidence-base. J Pain. 2010; 11: 807–829 [DOI] [PubMed] [Google Scholar]