Abstract
Bordetella hinzii was isolated in four biliary specimens collected over 6 months from a liver transplant recipient with cholangitis. The isolates were resistant to most β-lactam antibiotics and fluoroquinolones. Molecular typing was performed by pulsed-field gel electrophoresis. These data add cholangitis to the spectrum of disease manifestations caused by B. hinzii.
CASE REPORT
A 29-year-old male patient was first diagnosed with primary sclerosing cholangitis in April 1994. Several interventional dilatations of biliary tract stenoses were carried out until end-stage disease with liver cirrhosis stage child B (indicative of clinical signs of beginning decompensation of the hepatic function) was diagnosed in January 1999. Elective orthotopic liver transplantation with choledochojejunostomy was performed in June 1999. Primary-organ nonfunction due to severe preservation damage made high-urgency retransplantation necessary after 3 days. Immunosuppressive therapy was performed with cyclosporine, methylprednisolone, and azathioprine. Acute cellular rejection occurred in November 1999 and was treated with high-dose steroids. Ciprofloxacin was administered as a long-term prophylaxis of cholangitis. However, biliary sepsis occurred in January 2000, and Enterococcus faecalis and viridans streptococci were grown in two sets of blood cultures. The patient was successfully treated with imipenem-cilastatin, and the antibiotic prophylaxis was continued with amoxicillin-clavulanate.
In the follow-up period, elevated plasma bilirubin levels occurred repeatedly. In March 2000, fibrotic stenosis of the choledochojejunostomy with marked dilatation of intrahepatic bile ducts was diagnosed by magnetic resonance cholangiography, and bilateral percutaneous transhepatic choledochus drains (PTCD) were implanted, which led to marked improvement of the cholestasis. At 9 months posttransplantation, azathioprine was discontinued, and antibiotic prophylaxis was changed to ciprofloxacin. Repeated bouts of cholangitis developed subsequently. In June 2000, coeliacomesenteriography revealed a markedly reduced blood flow in the common hepatic artery accompanied by considerable rarefaction of the biliary ducts as a consequence of chronic liver ischemia. The patient was registered for elective retransplantation, and immunosuppressive therapy was changed to tacrolimus and methylprednisolone because of unstable cyclosporine levels due to insufficient bile secretion. Chemoprophylaxis with ciprofloxacin as well as daily rinsing of the drain with saline was continued. However, several bouts of cholangitis with C-reactive protein levels up to 60 mg/liter followed. In October 2000, bile secretions were subjected to microbiological analysis, which revealed rich growth of a gram-negative, nonfermenting rod, together with viridans streptococci and an Enterococcus sp. Consecutive cultures performed in January, February, and March 2001 revealed the same gram-negative, nonfermenting rod in all specimens, along with enterococci or streptococci. The bacilli were primarily identified as Bordetella-like organisms and tested for antimicrobial susceptibility by the agar diffusion technique. Two isolates were conserved and subjected to further analysis (see below). The patient was treated with piperacillin-tazobactam, and the PTCD were flushed daily with gentamicin. On 27 March 2001, a third liver transplantation was performed after allocation of a compatible liver graft. Postoperative computed tomography revealed segmentally impaired arterial liver perfusion characteristic of severe preservation damage. A continued increase of hepatic transaminases and subsequent complete breakdown of hepatic protein synthesis led to a fourth transplantation on 2 April 2001. After a short improvement, the clinical situation deteriorated rapidly. A progressive pneumonia occurred, and Aspergillus fumigatus was isolated repeatedly in tracheal secretions and in a nasal swab. Simultaneously, Candida albicans was isolated in one pair of blood cultures and in a pharyngeal swab. Despite antimicrobial therapy with amphotericin B, flucytosine, vancomycin, and meropenem, multiple organs failed, and the patient died on 9 April 2001. Extended aspergillus pneumonia with several metastatic foci in the kidneys, heart, peritoneum, and cerebrum was found on autopsy.
The Bordetella-like organisms isolated from the patient grew primarily on Columbia and MacConkey agar as round, convex, glistening, yellow-to-grayish colonies. One isolate (BL3210) expressed an extremely mucoid phenotype, which was still present after 10 passages on blood agar. The isolates were motile, exhibited oxidase but no catalase activity, and produced alkaline products in the slants and butts of the triple sugar iron reaction tubes. Biochemical identification (ID) by means of API 20NE (BioMérieux, Marcy l'Etoile, France) revealed the profile 0000067, which is consistent with Bordetella avium (95.6%, good ID) and has previously been reported for Bordetella hinzii (2, 7). Further ID was performed by means of the automated ID system Phoenix (Becton Dickinson, Heidelberg, Germany). No ID or susceptibility results were obtained for the isolate BL3210 at the first try, probably because of the mucoid phenotype. Repeat analysis after multiple passages revealed Achromobacter sp. with a confidence level of 90%. Tentative identification was performed by partial sequencing of the 16S rRNA gene (834 bp) using broad-range eubacterial primers (3), which revealed 100% similarity to a published sequence for B. hinzii (6). Final identification was obtained by comparison of the whole-cell protein profile of BL3210 with those of B. hinzii reference cultures. In a previous study, the correlation between whole-cell protein pattern similarity and level of DNA-DNA hybridization within this genus was demonstrated (12).
Antimicrobial susceptibility testing was performed primarily by the disk diffusion method. All four Bordetella isolates from the patient revealed identical results. Isolate BL3210 was further analyzed by means of the E-Test (AB BIODISK, Solna, Sweden) and broth microdilution technique (Phoenix; Becton Dickinson). The susceptibility results obtained by different methods were concordant and revealed high resistance to many antimicrobial agents, including most β-lactam antibiotics and fluoroquinolones (Table 1). These results are consistent with previously published data (4, 7), except for the marked resistance to ciprofloxacin, with a MIC of >32 mg/liter, which has not been reported before. Since ciprofloxacin was used as a long-term prophylaxis in this patient, resistance may have been induced under its prophylactic application.
TABLE 1.
Antimicrobial susceptibility results and MICs for B. hinzii isolate BL3210
| Antibiotic | Susceptibility testing result (MIC[s]) by means ofa:
|
||
|---|---|---|---|
| Disk diffusion | E-Test | Broth microdilution | |
| Ampicillinb | R | I (12) | R (16) |
| Amoxicillin-clavulanateb | R | R (>32, 2) | |
| Piperacillin-tazobactam | S | S (2, 4) | |
| Cefuroximeb | R | R (>16) | |
| Ceftriaxone | R | I or R (>16) | |
| Cefotaxime | R | R (>32) | I or R (>16) |
| Ceftazidime | S | S (4) | S (8) |
| Imipenem | S | S (1) | S (2) |
| Meropenem | S | S (≤0.25) | |
| Tetracycline | S | S (0.38) | S (1) |
| Trimethoprim-sulfamethoxazole | S | S (≤0.4, 7.6) | |
| Ciprofloxacin | R | R (>32) | R (>4) |
| Levofloxacin | R | R (>4) | |
| Gentamicin | S | S (4) | S (4) |
| Amikacin | S | S (8) | |
MICs are in milligrams per liter. R, resistant; S, sensitive; I, intermediate. Two MICs are given for drug combinations, and the values are for the individual drugs.
Results were interpreted according to NCCLS breakpoints for Enterobacteriaceae.
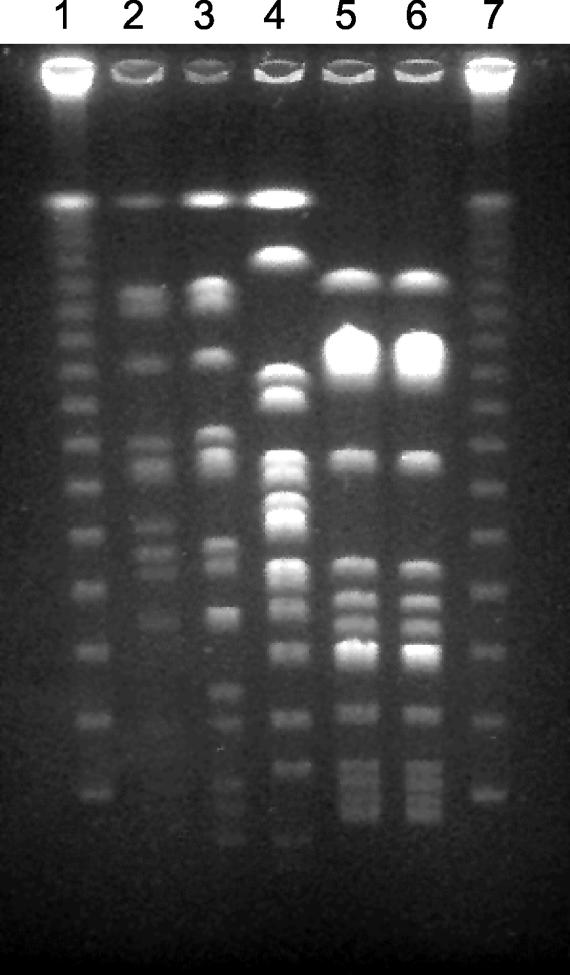
Molecular characterization of the two preserved B. hinzii isolates (BL3210 and BL6043) was performed by means of pulsed-field gel electrophoresis (PFGE) of genomic macrorestriction fragments as described elsewhere (9). BcuI (Fermentas, St. Leon-Rot, Germany) was used as a restriction enzyme, and electrophoresis was performed at 13°C for 23 h with a voltage of 6 V/cm and pulse times of 5 to 60 s. Isolate BL3210 had to be subcultured repeatedly (10 times) on blood agar before sufficient amounts of bacterial DNA could be obtained for the analysis. Three epidemiologically unrelated isolates from human sources were also analyzed in parallel. B. hinzii LMG 1872 was isolated from a sputum sample in France. B. hinzii LMG 3470 was a subculture of the latter isolate sent to the Laboratory of Microbiology Bacteria Collection at the University of Ghent (LMG) 13 years after the initial isolation. B. hinzii LMG 15873 was grown from the sputum of a patient with cystic fibrosis in Switzerland (4). Restriction with BcuI resulted in approximately 14 bands (Fig. 1). The B. hinzii isolates from the patient revealed similar but not identical banding patterns, which differed in two bands and suggested a single genetic modification (11). This event might have occurred naturally during the course of the chronic infection, or alternatively, it might have been induced by the in vitro passages. Variations of two or three bands have been observed among strains of some species when they are cultured repeatedly over time or isolated multiple times from the same patient (10, 11; M. Arvand, unpublished data).
FIG. 1.
PFGE analysis of B. hinzii isolates after restriction with BcuI. Lane 1, lambda ladder PFGE marker; lanes 2 and 3, B. hinzii isolates BL3210 and BL6043 from the patient, respectively; lane 4, B. hinzii LMG 15873 from a Swiss patient with cystic fibrosis; lanes 5 and 6, B. hinzii LMG 1872 and LMG 3470 obtained from a French patient but submitted to the LMG in 1966 and 1979, respectively; lane 7, lambda ladder.
Discussion.
Biochemical ID of the gram-negative rods by means of the API or Phoenix system did not reveal the correct ID, since B. hinzii is not included in the databases of those ID systems. Careful evaluation of the ID results, especially those obtained by automated systems, in light of the clinical findings and the patient's history, was necessary to conduct further investigation and to avoid misdiagnosis. Thus, this case report emphasizes the pivotal role of clinically interested microbiologists in the rapidly changing field of diagnostic microbiology.
Since the description of B. hinzii in 1994, to our knowledge, five reports on the isolation of this unusual organism from human clinical sources have been published. B. hinzii was isolated from respiratory tract specimens from patients with evidence of a lower respiratory tract infection (4, 5). Two strains were isolated from blood cultures, one strain was isolated from a febrile human immunodeficiency virus-infected patient with catheter-associated septicemia (2), and one was isolated from a patient with cholestasis and fatal septicemia (7). Finally, one additional sputum isolate has been described, but there was no clinical information available (12). In addition, one of us (P.V.) has received several putative B. hinzii isolates for ID confirmation. Isolates confirmed as B. hinzii were obtained from the sputa of cystic fibrosis patients, a bronchial aspirate of a farmer, and a gastric tubage (P. Vandamme, unpublished data). In the present study, the patient had a history of multiple liver transplantations, was treated with PTCD, and presented with recurrent bouts of cholangitis. B. hinzii was isolated repeatedly from different biliary specimens collected over a period of 6 months. Although the interpretation of laboratory and histological findings is difficult for patients who have undergone liver transplantation because of primary sclerosing cholangitis (6), we assume that B. hinzii was a causative agent of the chronic infection of biliary ducts in this patient. The patient neither had close contact to birds or other animals nor presented with signs of a respiratory tract infection. Therefore, we hypothesize that he was colonized by B. hinzii in the gastrointestinal tract and developed an ascending infection of the biliary ducts. A similar route of infection was also possible in the case reported by Kattar et al., in which the patient developed fatal bacteremia after endoscopic retrograde cholangiopancreatography (7). Since a gastrointestinal colonization usually implies an oral transmission route for the pathogen, we assume that B. hinzii may have been acquired per os, e.g., by ingestion of contaminated poultry products.
Molecular characterization of the B. hinzii isolates was performed by means of PFGE. This technique has a high discriminatory power and excellent reproducibility and has been used in epidemiological studies of different bacterial species, including Bordetella holmesii (1, 8-10). The two preserved isolates from the patient displayed similar banding patterns, suggesting that they represent one strain. Likewise, the two subcultures of a single French isolate that were sent to the Belgian Coordinated Collection of Microorganisms-LMG research group 13 years apart revealed identical restriction patterns, confirming that they represent a single strain. The Swiss patient's isolate also revealed a unique pattern. Therefore, despite the limited number of the isolates analyzed in this study, our results suggest that PFGE may be a useful method for further epidemiological studies of B. hinzii isolates. In conclusion, this case report adds chronic cholangitis to the spectrum of B. hinzii-associated human diseases.
Acknowledgments
We thank Hannah Schubert, Hygiene-Institut, University of Heidelberg, for help with PFGE analysis; Patrick Finzer, Deutsches Krebsforschungszentrum, Heidelberg, Germany, for providing some of the clinical isolates; and Sabine Albert, Department of Microbiology, University of Frankfurt/Main, for conducting the 16S rRNA-DNA sequence analysis.
REFERENCES
- 1.Arvand, M., A. J. Klose, D. Schwartz-Porsche, H. Hahn, and C. Wendt. 2001. Genetic variability and prevalence of Bartonella henselae in cats in Berlin, Germany, and analysis of its genetic relatedness to a strain from Berlin that is pathogenic for humans. J. Clin. Microbiol. 39:743-746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cookson, B. T., P. Vandamme, L. C. Carlson, A. M. Larson, J. V. Sheffield, K. Kersters, and D. H. Spach. 1994. Bacteremia caused by a novel Bordetella species, “B. hinzii.” J. Clin. Microbiol. 32:2569-2571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Edwards, U., T. Rogall, H. Blocker, M. Emde, and E. C. Bottger. 1989. Isolation and direct complete nucleotide determination of entire genes. Characterization of a gene coding for 16S ribosomal RNA. Nucleic Acids Res. 17:7843-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Funke, G., T. Hess, A. von Graevenitz, and P. Vandamme. 1996. Characteristics of Bordetella hinzii strains isolated from a cystic fibrosis patient over a 3-year period. J. Clin. Microbiol. 34:966-969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gadea, I., M. Cuenca-Estrella, N. Benito, A. Blanco, M. L. Fernandez-Guerrero, P. L. Valero-Guillen, and F. Soriano. 2000. Bordetella hinzii, a “new” opportunistic pathogen to think about. J. Infect. 40:298-299. [DOI] [PubMed] [Google Scholar]
- 6.Jeyarajah, D. R., G. J. Netto, S. P. Lee, G. Testa, O. Abbasoglu, B. S. Husberg, M. F. Levy, R. M. Goldstein, T. A. Gonwa, G. W. Tillery, J. S. Crippin, and G. B. Klintmalm. 1998. Recurrent primary sclerosing cholangitis after orthotopic liver transplantation: is chronic rejection part of the disease process? Transplantation 66:1300-1306. [DOI] [PubMed] [Google Scholar]
- 7.Kattar, M. M., J. F. Chavez, A. P. Limaye, S. L. Rassoulian-Barrett, S. L. Yarfitz, L. C. Carlson, Y. Houze, S. Swanzy, B. L. Wood, and B. T. Cookson. 2000. Application of 16S rRNA gene sequencing to identify Bordetella hinzii as the causative agent of fatal septicemia. J. Clin. Microbiol. 38:789-794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mazengia, E., E. A. Silva, J. A. Peppe, R. Timperi, and H. George. 2000. Recovery of Bordetella holmesii from patients with pertussis-like symptoms: use of pulsed-field gel electrophoresis to characterize circulating strains. J. Clin. Microbiol. 38:2330-2333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pfaller, M. A., C. Wendt, R. J. Hollis, R. P. Wenzel, S. J. Fritschel, J. J. Neubauer, and L. A. Herwaldt. 1996. Comparative evaluation of an automated ribotyping system versus pulsed-field gel electrophoresis for epidemiological typing of clinical isolates of Escherichia coli and Pseudomonas aeruginosa from patients with recurrent gram-negative bacteremia. Diagn. Microbiol. Infect. Dis. 25:1-8. [DOI] [PubMed] [Google Scholar]
- 10.Steinbrueckner, B., F. Ruberg, and M. Kist. 2001. Bacterial genetic fingerprint: a reliable factor in the study of the epidemiology of human Campylobacter enteritis? J. Clin. Microbiol. 39:4155-4159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tenover, F. C., R. D. Arbeit, R. V. Goering, P. A. Mickelsen, B. E. Murray, D. H. Persing, and B. Swaminathan. 1995. Interpreting chromosomal DNA restriction patterns produced by pulsed-field gel electrophoresis: criteria for bacterial strain typing. J. Clin. Microbiol. 33:2233-2239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Vandamme, P., J. Hommez, M. Vancanneyt, M. Monsieurs, B. Hoste, B. Cookson, C. H. Wirsing von König, K. Kersters, and P. J. Blackall. 1995. Bordetella hinzii sp. nov., isolated from poultry and humans. Int. J. Syst. Bacteriol. 45:37-45. [DOI] [PubMed] [Google Scholar]