Abstract
Background
Nausea and vomiting are serious side effects of cancer chemotherapy that can cause significant negative impacts on patients’ quality of life and on their ability to tolerate and comply with therapy. Despite advances in the prevention and management of chemotherapy-induced nausea and vomiting (CINV), these side effects remain among the most distressing for patients.
Objective
To discuss CINV and the current pharmacologic approaches to its management.
Discussion
This article outlines the mechanism of CINV followed by a review of current approaches to pharmacologic therapy and current practice guidelines from national cancer organizations. This information will help providers and payers understand the optimal management of patients with CINV including practical considerations and value-based decision-making that considers cost issues.
Conclusion
Numerous preventive and treatment options are available to manage CINV Addressing antiemetic regimens requires ongoing patient evaluation to determine the best approach for each individual patient.
Nausea and vomiting are 2 serious and related side effects of cancer chemotherapy. These adverse effects can cause significant negative impacts on patients’ quality of life and on their ability to comply with therapy. Also, nausea and vomiting can result in anorexia, decreased performance status, metabolic imbalance, wound dehiscence, esophageal tears, and nutritional deficiency.1,2 Despite advances in the prevention and management of chemotherapy-induced nausea and vomiting (CINV), these side effects remain among the most distressing for patients. The use of emerging antiemetic medications has reduced the incidence of vomiting substantially, but evaluations show that approximately 30% to 60% of patients still experience either acute or delayed nausea after chemotherapy.3 Serial evaluations throughout the 1980s and into the 2000s show that, although vomiting has fallen further down on the list of side effects that patients perceive as being their most severe, nausea remains either the first or second most severe side effect of chemotherapy.4–8
Risk factors for CINV can be divided into patient-specific and treatment-specific risk factors. Female sex and history of motion or morning sickness are clear risk factors for nausea and vomiting.5,6 Younger age has also been correlated with increased risk, although this may be explained by the more aggressive chemotherapy regimens that tend to be administered to younger patients who have more aggressive diseases.5–7 Finally, alcohol intake tends to be inversely correlated with the risk of developing CINV. Many factors contribute to the treatment-specific risk, including (1) the emetogenicity of the agents being used, (2) the dose and schedule of each agent, and (3) in the case of radiation-induced or postoperative nausea, the site of radiation or surgery.
“Emetogenicity” refers to an agent's tendency to cause nausea and/or vomiting. Initially described in 1997, the emetogenicity scale, also known as the Hesketh scale, divided chemotherapy agents and doses into 5 levels, based on their likelihood to cause CINV.9 Since then, the American Society of Clinical Oncology (ASCO) and the National Comprehensive Cancer Network (NCCN) have modified this scale to be divided into the following 4 categories10,11:
Highly emetogenic: medications or doses that cause CINV in >90% of patients
Moderately emetogenic: medications that induce CINV in 30% to 90% of patients
Low emetogenic: medications that are associated with CINV rates of 10% to 30%
Minimally emetogenic: medications that cause CINV in <10% of patients.
“CINV” is a broad term used to describe the many types of nausea and vomiting that can occur in patients with cancer. The major subtypes of nausea and vomiting associated with chemotherapy are12–16:
Acute: onset of nausea and vomiting within minutes to hours after administration of chemotherapy and resolving within 24 hours
Delayed: occurs 24 hours or later after administration of chemotherapy
Anticipatory: occurs before chemotherapy administration; thought to be an indicator of previous poor control of nausea and vomiting
Breakthrough/refractory: nausea and vomiting that occur despite appropriate prophylaxis; requires the use of rescue medications.
Because there are so many independent and variable risk factors that can influence the risk for CINV in any particular patient, it becomes paramount for providers to individualize the approach to the prevention and treatment of CINV in every patient case.
Pathophysiology of Nausea and Vomiting
The vomiting response is controlled centrally by the emetic center, which lies in the reticular formation of the brain stem. The emetic center receives input from 3 sources: the periphery, the cortex, and the chemoreceptor trigger zone. Peripheral pathways are mediated mainly by serotonin (5-hydroxytryptamine3 [5-HT3]) and neurokinin (NK). The cortical pathway, which is responsible for anticipatory emesis, is mediated by dopamine and histamine. The chemoreceptor trigger zone, which is a collection of neurons at the base of the brain and is exposed to the body's general circulation, mediates signals through all of the above chemokines. Once the emetic center has been triggered, signals are then sent to the salivatory, vasomotor, respiratory, and cranial centers to activate the organs involved with the vomiting reflex, namely the abdominal muscles, diaphragm, stomach, and esophagus.17
Pharmacologic Treatment Options for CINV
Available Agents
Before the 1980s, CINV was primarily managed with dopamine receptor antagonists. Today, we have a multitude of options available, targeting the various pathways of the process, to use in the prevention and management of CINV.
5-HT3 receptor antagonists. Ondansetron was the first US Food and Drug Administration (FDA)-approved 5-HT3 antagonist in 1991. Early trials showed that ondansetron was an effective antiemetic for patients receiving cisplatin-based regimens, and they subsequently showed it to be superior to metoclopramide in patients receiving cisplatin and noncisplatin regimens.18–22 Currently, four 5-HT3 receptor antagonists are available in the United States—ondansetron, granisetron, dolasetron, and palonosetron. Palonosetron, the newest agent, was approved in 2003. These agents are believed to prevent CINV by antagonizing 5-HT3 receptors either peripherally on vagal nerve terminals and/or centrally in the chemoreceptor trigger zone.23–27 Since their introduction, 5-HT3 receptor antagonists have become part of the cornerstone for CINV prevention, thanks to their effectiveness and tolerable side-effect profile. The most common adverse effects reported (in their respective package insert) with these agents are headache and constipation. Transient elevation of liver function enzymes and QTc prolongation have also been noted.
KEY POINTS
-
▸
Risk factors for CINV can be either patient-specific or treatment-specific.
-
▸
Treatment-specific risks include emetogenicity of the agents used, the dose and schedule of each agent, and, if applicable, the radiation or surgery site.
-
▸
Agents available to treat CINV include 5-HT3 receptor antagonists, NK1 receptor antagonists, and corticosteroids, as well as dopamine receptor antagonists, benzodiazepines, olanzapine, and cannabinoids.
-
▸
Guidelines from the NCCN and ASCO can help providers personalize the antiemetic regimens for their patients, but these are only starting points; a “value judgment” must also be made.
-
▸
The preferred status for palonosetron was derived from data in 3 of 4 trials showing significant benefits in the delayed setting; these trials had significant flaws in design, leading us to question this “preferred” status and suggest that all 5-HT3 receptor antagonists are equal if used at equivalent doses and schedules.
-
▸
Providers must consider clinical, logistic, safety, and cost factors when treating CINV, and antiemetic regimens for a particular patient must be evaluated and then reevaluated at every treatment cycle.
NK1 receptor antagonists. NK1 receptor antagonists inhibit substance P in peripheral and central emetic pathways. Aprepitant was the first drug in this class to be approved by the FDA in 2003. Aprepitant was approved at doses of 125 mg orally on day 1 and 80 mg orally on days 2 and 3 for the prevention of nausea and vomiting in patients receiving highly emetogenic or moderately emetogenic single-day chemotherapy.28 Aprepitant was approved after 2 trials showed that the combination of aprepitant, ondansetron, and dexamethasone decreased emesis or decreased the use of rescue medications for patients receiving highly emetogenic chemotherapy during the acute and delayed phases.29,30 The most common adverse effects include fatigue, headache, anorexia, diarrhea, hiccups, and increased transaminases.
Aprepitant is primarily metabolized by cytochrome (CY)P-450 3A4 with minor metabolism by CYP-1A2 and CYP-2C9.28,31 Aprepitant has been shown to be an inhibitor of CYP-3A4 and an inducer of CYP-2A9. Coadministration with corticosteroids such as dexamethasone, a CYP-3A4 substrate, causes an increase in plasma concentrations of dexamethasone. Therefore, when aprepitant is given with dexamethasone for CINV prevention, the dexamethasone dose should be reduced. Because aprepitant is a weak inducer of CYP-2C9, the metabolism of warfarin can be affected. A decrease of international normalized ratio has been noted with this combination, and patients should be monitored, although no empiric dose adjustments for warfarin are recommended.32
Fosaprepitant, which was approved in 2008, is a water-soluble prodrug of aprepitant that is administered intravenously before chemotherapy.33 Fosaprepitant is used as an intravenous (IV) 150-mg dose on day 1 only. The one-time 150-mg IV dose has been shown to be noninferior to the 3-day oral aprepitant regimen.34
Corticosteroids. Corticosteroids were first shown to be efficacious for CINV in the 1980s, and they are now considered a mainstay of antiemetic regimens for the prevention of acute and delayed emesis.10,11,35 Although not approved by the FDA for CINV, corticosteroids have been found to be beneficial when used alone for the prevention of nausea and vomiting in patients receiving low emetogenic chemotherapy and to improve efficacy when combined with 5-HT3 receptor antagonists in patients receiving moderately or highly emetogenic chemotherapies.36–39 Dexamethasone is the recommended corticosteroid according to current guidelines, although no studies have been performed comparing available corticosteroids.10,11
The mechanism of action of corticosteroids as antiemetic agents has not been elucidated, but it may be related to activity in the peripheral nervous system or in the central nervous system (CNS), and possibly by antagonizing serotonin receptors.40–43 Tolerability to corticosteroids can be a concern, because when used for the prevention of delayed nausea and vomiting, common adverse effects have included insomnia, epigastric discomfort, agitation, weight gain, and hyperglycemia.44
Additional Options
Dopamine receptor antagonists. Before the approval of the 5-HT3 antagonists, dopamine receptor antagonists were the primary antiemetics used for CINV. With the current availability of more effective preventive agents, dopamine receptor antagonists are mostly used in the management of breakthrough or refractory emesis. The dopamine antagonists are divided into phenothiazines (eg, prochlorperazine), butyrophenones (eg, haloperidol, droperidol), and substituted benzamides (eg, metoclopramide). These agents antagonize the dopamine (D2) receptor in the chemoreceptor trigger zone.45,46 Metoclopramide antagonizes dopamine, but at high doses it also has activity against the 5-HT3 receptor.47,48 Common side effects of dopamine receptor antagonists, which include extrapyramidal symptoms, dystonia, and drowsiness, make them more suitable for breakthrough nausea rather than for primary prophylaxis.
Benzodiazepines. These agents are anxiolytics that are used in patients receiving chemotherapy. Benzodiazepines are appropriate adjunct therapies to decrease treatment-related anxiety, and they are the preferred agents to treat and prevent anticipatory nausea and vomiting.49–51 Lorazepam and alprazolam are the primary agents used in this class, with sedation being the most common adverse effect, based on our clinical practice experience.
Olanzapine. This atypical antipsychotic has antagonist activity at adrenergic receptors, muscarinic receptors, and multiple dopamine (D1–4) and serotonin receptors (5-HT2A, 5-HT2C, 5-HT3, 5-HT6).52,53 Several trials have shown that olanzapine safely and effectively prevents acute, delayed, and refractory CINV when combined with other antiemetics in patients receiving moderately and highly emetogenic chemotherapy.54–56 Adverse effects such as sedation, weight gain, orthostatic hypotension, hyperglycemia, and a black box warning for increased mortality in elderly patients with dementia-related psychosis limit its use.57
Cannabinoids. Dronabinol and nabilone are 2 cannabinoids that are currently approved by the FDA for CINV in patients who have not adequately responded to conventional antiemetics. Cannabinoids are thought to prevent nausea and vomiting by antagonizing cannabinoid receptor CB1 in the CNS and possibly CB2 receptors as well.58 Cannabinoids have been shown to be as effective as or slightly more effective than dopamine receptor antagonists.59,60 Only 1 trial has directly compared a cannabinoid with standard treatment.61 Ondansetron with dexamethasone plus dronabinol was found to be equally efficacious to ondansetron, dexamethasone, and placebo.61 This lack of added benefit has limited the use of cannabinoids in the preventive setting. In addition, vertigo, euphoria, and somnolence are adverse effects that limit the use of cannabinoids.
Current Practice Guidelines
Practice guidelines from the NCCN and ASCO are available to help providers determine optimal prophylaxis and the treatment of CINV.10,11 The NCCN Antiemesis Guideline™, a consensus-based guideline that incorporates evidence and expert opinion to make recommendations, is revised annually.11 ASCO guidelines are purely evidence-based guidelines and are updated periodically; the last update was in 2011.10 Table 1 summarizes specific recommendations for antiemesis from the NCCN and from ASCO. For CINV, both guidelines outline primary prophylaxis based on the emetogenicity of the patient's chemotherapy: high, moderate, low, and minimal.
Table 1.
Recommended Antiemetic Regimens for CINV Prophylaxis
| Emetic risk | Treatment for acute phasea | Treatment for delayed phase |
|---|---|---|
| High | 3-drug combination treatment with an NK1 receptor antagonist, a 5-HT3 receptor antagonist, and dexamethasone | NK1 receptor antagonist if oral route was used; dexamethasone |
| Moderate | 2-drug combination treatment with a 5-HT3 receptor antagonist and dexamethasone | Dexamethasone |
| Low | Dexamethasone | |
| Minimal | No routine prophylaxis recommended |
All patients should have “as-needed” rescue medication available, which can include prochlorperazine, promethazine, or lorazepam, regardless of emetic risk level.
5-HT indicates serotonin; CINV, chemotherapy-induced nausea and vomiting; NK, neurokinin.
For patients receiving highly emetogenic chemotherapy, both guidelines recommend a 3-drug combination that includes a 5-HT3 receptor antagonist, an NK1 receptor antagonist, and dexamethasone to prevent CINV. The NCCN specifies that the preferred 5-HT3 receptor antagonist for highly emetogenic chemotherapy is palonosetron,11 whereas ASCO does not list a preferred 5-HT3 receptor antagonist.
For patients receiving moderately emetogenic chemotherapy, the NCCN and ASCO recommend a 2-drug combination of a 5-HT3 receptor antagonist, preferably palonosetron, with dexamethasone. Dexamethasone is recommended by both organizations for the prevention of CINV in patients with low or minimal emetogenic potential. The NCCN also lists metoclopramide or prochlorperazine as possible alternatives. For patients receiving minimal-risk chemotherapy, no medications are recommended primarily as prophylaxis.
For anticipatory nausea and vomiting (ANV), ASCO and the NCCN recommend that prevention with optimal primary prophylaxis is the best approach.10,11 Both organizations state that behavioral therapy, including desensitization, is recommended for treatment of ANV. The NCCN guidelines recommend the use of benzodiazepines to treat ANV.
For radiation-induced nausea and vomiting, 5-HT3 receptor antagonists are the preferred class of antiemetic. The NCCN divides types of radiation into high risk (eg, total body irradiation), moderate risk (eg, radiation to upper abdomen), and combined radiation with chemotherapy.11 For moderate- and high-risk radiation, granisetron or ondansetron before each radiation treatment, with or without dexamethasone, is recommended. Prophylaxis of nausea and vomiting with combination chemotherapy and radiation is determined by the emetogenic potential of the chemotherapy.
ASCO categorizes emetogenic risk of radiation as high (eg, total body irradiation), moderate (eg, upper abdomen), low (eg, head and neck), minimal (eg, breast), and combination of radiation and chemotherapy.10 For moderate- and high-risk radiation, a 5-HT3 receptor antagonist before each radiation treatment, along with dexamethasone during fractions 1 to 5, are recommended. Granisetron and ondansetron are preferred 5-HT3 receptor antagonists in this setting, but dolasetron can be considered. Palonosetron is listed as an option, although there are no trials to indicate appropriate dosing frequency. Patients receiving radiation with a low risk for nausea and vomiting can be offered a 5-HT3 receptor antagonist or a dopamine receptor antagonist, such as metoclopramide or prochlorperazine, as a rescue treatment. Prophylaxis for nausea and vomiting for patients receiving a combination of chemotherapy and radiation is determined by the chemotherapy regimen, unless the radiation causes a higher risk.
Practical Considerations
Even with the current published guidelines, there are unique challenges for clinicians who manage patients with CINV or patients at risk of developing CINV. In this article, we focus on the following 3 practical challenges.
1. Are all 5-HT3 receptor antagonists created equal? Currently, there are four 5-HT3 antagonists available in the US market—dolasetron, granisetron, ondansetron, and palonosetron. Studies with these agents show relatively similar rates of success in the prevention of CINV in patients receiving cisplatin-based chemotherapy regimens. In addition, studies have established acceptable oral: IV conversions that result in similar levels of emesis control. Equivalent doses and pharmacokinetic properties of the agents are listed in Table 2 and Table 3. When used in equivalent doses, ondansetron, granisetron, and dolasetron are considered similar for the prevention of nausea and vomiting.62,63 The pharmacokinetics of ondansetron, granisetron, and dolasetron are slightly different, but not enough to result in any clinically significant differences.
Table 2.
Dosing Ranges for Antiemetics Used for Primary Prophylaxis of CINV
| Antiemetic | Dose | Antiemetic | Dose |
|---|---|---|---|
| NK1 receptor antagonists | Granisetron | 2 mg oral or 1 mg oral twice daily; 1 mg IV or 0.01 mg/kg IV | |
| Fosaprepitant | 150 mg IV | ||
| Aprepitant | 125 mg oral on day 1 and 80 mg oral on days 2 and 3 | Dolasetron | 100 mg oral |
| Palonosetron | 0.25 mg IV | ||
| 5-HT3 receptor antagonists | Corticosteroid | ||
| Ondansetron | 16–24 mg oral; 8 mg IV | Dexamethasone | 8–20 mg oral IV |
5-HT indicates serotonin; CINV, chemotherapy-induced nausea and vomiting; IV, intravenous; NK, neurokinin.
Table 3.
Pharmacokinetic Properties of 5-HT3 Receptor Antagonists
| Agent | Ondansetron | Granisetron | Dolasetron | Palonosetron |
|---|---|---|---|---|
| Half-life (hrs) | 3–4 | 7–9 | 7–8 | 40 |
| Oral bioavailability, % | 56 | 60 | 75 | N/A |
| Renal elimination, % | 5 | 12 | 67 | 42 |
| Hepatic metabolism | CYP-3A, CYP-1A, CYP-2D6, CYP-2E1 | CYP-3A | CYP-3A, CYP-2D6 | CYP-3A4, CYP-2D6, CYP-1A2 |
5-HT indicates serotonin; CYP, cytochrome P; N/A, not applicable.
Palonosetron differs from the other 5-HT3 antagonists by having increased binding affinity to the 5-HT3 receptor, higher potency, and a longer half-life.64,65 The half-life of palonosetron is approximately 40 hours compared with the significantly lower half-lives of ondansetron, granisetron, and dolasetron.64 This results in altered dosing recommendations for palonosetron, which is dosed once per cycle rather than on a daily basis.
As discussed earlier, the NCCN and ASCO guidelines have stated a preference for palonosetron for the prevention of CINV. These recommendations are based on data from 4 trials.66–69 In 3 of these trials, palonosetron was compared with various other 5-HT3 receptor antagonists to determine noninferiority.67–69 Only 1 trial was designed to detect superiority in the comparison.66 All 4 trials demonstrated similar success rates in preventing acute CINV between palonosetron and the comparator. The preferred status for palonosetron was derived from the data showing significant benefits for palonosetron in the delayed setting in 3 of the 4 trials, including the trial sized to measure superiority.
All of these trials, however, had significant flaws in their design. Most notable, all of the trials compared a single dose of palonosetron to a single dose of the comparator 5-HT3 receptor antagonist. Given palonosetron's extended half-life of 40 hours, compared with the half-lives of between 3 and 8 hours for other 5-HT3 receptor antagonists, comparisons at any time after 24 hours are pharmacokinetically irrelevant. In addition, only 1 of the trials mandated the use of corticosteroids, which are the backbone of any combination antiemetic regimen.69 Given these significant flaws in design, we call into question the “preferred” status of palonosetron and instead endorse the idea that all 5-HT3 receptor antagonists are indeed equal if used at equivalent doses and schedules.
2. Multiday chemotherapy. Most data on the use of 5-HT3 receptor antagonists, especially NK1 receptor antagonists, are in the setting of single-day chemotherapy. However, numerous malignancies are treated with multiple sequential days of chemotherapy, often with various agents being given on different days. Current guidelines recommend using the appropriate level of prophylaxis, according to the emetogenicity of the regimen, on each day of the regimen, and continuing delayed prophylaxis for 2 to 3 days after the completion of chemotherapy.10,11 In patients receiving moderately emetogenic regimens, this is a relatively straightforward approach. In patients receiving highly emetogenic regimens, however, it becomes more difficult.
The timing of the NK1 receptor antagonist dose or of the frequency of palonosetron dosing in these regimens is poorly defined. Einhorn and colleagues evaluated the use of every 2-day palonosetron in patients receiving the highly emetogenic chemotherapeutic combination of bleomycin, etoposide, and cisplatin for testicular cancer and showed favorable results.70 Another study evaluated the use of aprepitant in combination with granisetron and dexamethasone, in patients receiving multiday highly emetogenic and moderately emetogenic chemotherapy. Aprepitant was administered as 125 mg, followed by 80 mg daily for the remainder of chemotherapy days, and continued for 2 more days, all along with dexamethasone. This study demonstrated a complete remission rate of almost 58% in highly emetogenic and 73% in moderately emetogenic regimens.71
3. Breakthrough/refractory nausea and vomiting. Breakthrough/refractory nausea and vomiting are challenging to treat. In particular, refractory nausea and vomiting may cause significant morbidity, including weight loss, metabolic imbalances, and nutritional deficiency, and may result in the inability of patients to remain on their therapy schedule. The use of antidopaminergic and anticholinergic agents is very appropriate in this setting. More important, however, is the need to continually reassess the patient's response to therapy with each cycle. In some cases, severe or refractory nausea could be predicted, given a patient's history of poor tolerance of therapy, and adjustments could have been made to decrease the risk of nausea and vomiting. Also, regular reevaluation of risk factors can help to identify patients who may have an increased risk of breakthrough nausea and vomiting.
Proposal for a Value-Based Decision-Making Algorithm
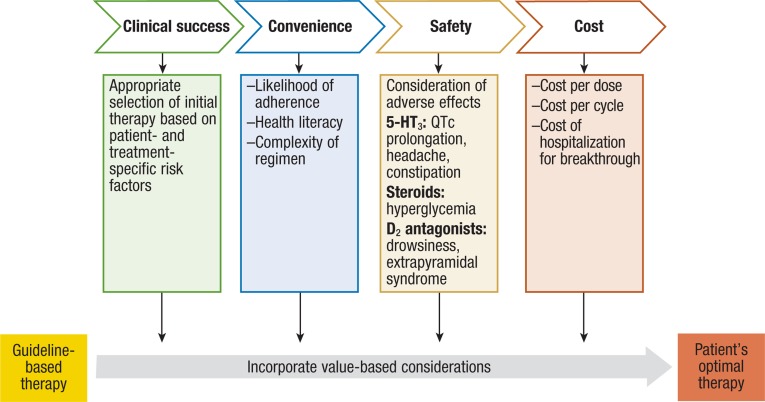
It must be emphasized that the currently available guidelines are not meant to be clear-cut decisions on how to approach every patient. The guidelines are meant to be viewed as a starting point from which to build the case for every individual's antiemetic regimen. Along with these guidelines, a “value judgment” must be made to determine the most optimal regimen for any patient.
Value, as it relates to CINV, may be determined based on a number of factors. The most relevant factors include (1) clinical factors (ie, freedom from nausea and decreased use of rescue medications), (2) logistic factors (ie, convenience of a regimen and likelihood of adherence), (3) safety factors (ie, consideration of potential adverse effects of agents in particular scenarios), and (4) cost factors (ie, affordability, coverage, and reimbursement). In many cases, treatment decisions must be made considering whether the patient will be able to afford or comply with the regimen that would technically be “ideal.” For example, clinicians may consider using IV NK1 receptor antagonists and/or IV palonosetron in a patient who either cannot afford to pay for continued oral NK1 receptor antagonist or 5-HT3 receptor antagonist therapy, or in a patient who will be unlikely to remember to take doses as scheduled for the days after chemotherapy.
A proposed algorithm for decision-making, including considerations regarding the abovementioned value considerations, is outlined in the Figure.
Figure. Proposed Value-Based Decision Algorithm for CINV.
5-HT indicates serotonin; CINV, chemotherapy-induced nausea and vomiting.
Conclusions
Antiemetic regimens for a particular patient must be evaluated and then reevaluated at every treatment cycle. At every step of a patient's care, clinicians must incorporate clinical decision-making with value-based considerations to determine each patient's individual, most optimal approach to treatment. With such an approach, we hope to continue to make progress in the prevention and management of this troublesome and problematic adverse effect of chemotherapy, thereby helping to improve the therapy experience and quality of life for our patients.
Author Disclosure Statement
Dr Rao and Dr Faso have reported no conflicts of interest.
Contributor Information
Kamakshi V. Rao, Oncology/BMT clinical pharmacist practitioner, University of North Carolina Hospitals and Clinics, Chapel Hill, NC.
Aimee Faso, Hematology/oncology clinical pharmacist practitioner, University of North Carolina Hospitals and Clinics, Chapel Hill, NC.
References
- 1.Laszlo J. Antiemetics and cancer chemotherapy. Baltimore, MD: Williams & Wilkins; 1983 [Google Scholar]
- 2.Fernández-Ortega P, Caloto MT, Chirveches E, et al. Chemotherapy-induced nausea and vomiting in clinical practice: impact on patients’ quality of life. Support Care Cancer. [Epub ahead of print Mar 31 2012]. [DOI] [PubMed]
- 3.Cohen L, de Moor CA, Eisenberg P, et al. Chemotherapy-induced nausea and vomiting: incidence and impact on patient quality of life at community oncology settings. Support Care Cancer. 2007; 15: 497–503 [DOI] [PubMed] [Google Scholar]
- 4.Coates A, Abraham S, Kaye SB, et al. On the receiving end—patient perception of the side-effects of cancer chemotherapy. Eur J Cancer Clin Oncol. 1983; 19: 203–208 [DOI] [PubMed] [Google Scholar]
- 5.de Boer-Dennert M, de Wit R, Schmitz PI, et al. Patient perceptions of the side-effects of chemotherapy: the influence of 5HT3 antagonists. Br J Cancer. 1997; 76: 1055–1061 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Griffin AM, Butow PN, Coates AS, et al. On the receiving end. V: Patient perceptions of the side effects of cancer chemotherapy in 1993. Ann Oncol. 1996; 7: 189–195 [DOI] [PubMed] [Google Scholar]
- 7.Hofman M, Morrow GR, Roscoe JA, et al. Cancer patients’ expectations of experiencing treatment-related side effects: a University of Rochester Cancer Center—Community Clinical Oncology Program study of 938 patients from community practices. Cancer. 2004; 101: 851–857 [DOI] [PubMed] [Google Scholar]
- 8.Lindley C, McCune JS, Thomason TE, et al. Perception of chemotherapy side effects cancer versus noncancer patients. Cancer Pract. 1999; 7: 59–65 [DOI] [PubMed] [Google Scholar]
- 9.Hesketh PJ, Kris MG, Grunberg SM, et al. Proposal for classifying the acute emetogenicity of cancer chemotherapy. J Clin Oncol. 1997; 15: 103–109 [DOI] [PubMed] [Google Scholar]
- 10.Basch E, Prestrud AA, Hesketh PJ, et al. Antiemetics: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2011; 29: 4189–4198 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelines in Oncology. Antiemesis. Version 1.2012. www.nccn.org/professionals/physician_gls/pdf/antiemesis.pdf Accessed June 20, 2012.
- 12.Jacobsen PB, Redd WH. The development and management of chemotherapyrelated anticipatory nausea and vomiting. Cancer Invest. 1988; 6: 329–336 [DOI] [PubMed] [Google Scholar]
- 13.Kris MG, Gralla RJ, Clark RA, et al. Consecutive dose-finding trials adding lorazepam to the combination of metoclopramide plus dexamethasone: improved subjective effectiveness over the combination of diphenhydramine plus metoclopramide plus dexamethasone. Cancer Treat Rep. 1985; 69: 1257–1262 [PubMed] [Google Scholar]
- 14.Moher D, Arthur AZ, Pater JL. Anticipatory nausea and/or vomiting. Cancer Treat Rev. 1984; 11: 257–264 [DOI] [PubMed] [Google Scholar]
- 15.Morrow GR. Clinical characteristics associated with the development of anticipatory nausea and vomiting in cancer patients undergoing chemotherapy treatment. J Clin Oncol. 1984; 2: 1170–1176 [DOI] [PubMed] [Google Scholar]
- 16.Roila F, Boschetti E, Tonato M, et al. Predictive factors of delayed emesis in cisplatin-treated patients and antiemetic activity and tolerability of metoclopramide or dexamethasone. A randomized single-blind study. Am J Clin Oncol. 1991; 14: 238–242 [DOI] [PubMed] [Google Scholar]
- 17.Allan SG. Mechanisms and management of chemotherapy-induced nausea and vomiting. Blood Rev. 1987; 1: 50–57 [DOI] [PubMed] [Google Scholar]
- 18.Bonneterre J, Chevallier B, Metz R, et al. A randomized double-blind comparison of ondansetron and metoclopramide in the prophylaxis of emesis induced by cyclophosphamide, fluorouracil, and doxorubicin or epirubicin chemotherapy. J Clin Oncol. 1990; 8: 1063–1069 [DOI] [PubMed] [Google Scholar]
- 19.Grunberg SM, Stevenson LL, Russell CA, McDermed JE. Dose ranging phase I study of the serotonin antagonist GR38032F for prevention of cisplatin-induced nausea and vomiting. J Clin Oncol. 1989; 7: 1137–1141 [DOI] [PubMed] [Google Scholar]
- 20.Hainsworth J, Harvey W, Pendergrass K, et al. A single-blind comparison of intravenous ondansetron, a selective serotonin antagonist, with intravenous metoclopramide in the prevention of nausea and vomiting associated with high-dose cisplatin chemotherapy. J Clin Oncol. 1991; 9: 721–728 [DOI] [PubMed] [Google Scholar]
- 21.Kris MG, Gralla RJ, Clark RA, Tyson LB. Dose-ranging evaluation of the serotonin antagonist GR-C507/75 (GR38032F) when used as an antiemetic in patients receiving anticancer chemotherapy. J Clin Oncol. 1988; 6: 659–662 [DOI] [PubMed] [Google Scholar]
- 22.Marty M, Pouillart P, Scholl S, et al. Comparison of the 5-hydroxytryptamine3 (serotonin) antagonist ondansetron (GR 38032F) with high-dose metoclopramide in the control of cisplatin-induced emesis. N Engl J Med. 1990; 322: 816–821 [DOI] [PubMed] [Google Scholar]
- 23.Endo T, Minami M, Hirafuji M, et al. Neurochemistry and neuropharmacology of emesis—the role of serotonin. Toxicology. 2000; 153: 189–201 [DOI] [PubMed] [Google Scholar]
- 24.Fozard JR. Neuronal 5-HT receptors in the periphery. Neuropharmacology. 1984; 23: 1473–1486 [DOI] [PubMed] [Google Scholar]
- 25.Fukui H, Yamamoto M, Sato S. Vagal afferent fibers and peripheral 5-HT3 receptors mediate cisplatin-induced emesis in dogs. Jpn J Pharmacol. 1992; 59: 221–226 [DOI] [PubMed] [Google Scholar]
- 26.Kilpatrick GJ, Jones BJ, Tyers MB. Binding of the 5-HT3 ligand, [3H]GR65630, to rat area postrema, vagus nerve and the brains of several species. Eur J Pharmacol. 1989; 159: 157–164 [DOI] [PubMed] [Google Scholar]
- 27.Miner WD, Sanger GJ, Turner DH. Evidence that 5-hydroxytryptamine3 receptors mediate cytotoxic drug and radiation-evoked emesis. Br J Cancer. 1987; 56: 159–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Emend (aprepitant) capsules [package insert]. Whitehouse Station, NJ: Merck; 2006. www.merck.com/product/usa/pi_circulars/e/emend/emend_pi.pdf?WT.mc_id=N02N3 Accessed June 21, 2012.
- 29.Hesketh PJ, Grunberg SM, Gralla RJ, et al. The oral neurokinin-1 antagonist aprepitant for the prevention of chemotherapy-induced nausea and vomiting: a multinational, randomized, double-blind, placebo-controlled trial in patients receiving high-dose cisplatin—the Aprepitant Protocol 052 Study Group. J Clin Oncol. 2003; 21: 4112–4119 [DOI] [PubMed] [Google Scholar]
- 30.Schmoll HJ, Aapro MS, Poli-Bigelli S, et al. Comparison of an aprepitant regimen with a multiple-day ondansetron regimen, both with dexamethasone, for antiemetic efficacy in high-dose cisplatin treatment. Ann Oncol. 2006; 17: 1000–1006 [DOI] [PubMed] [Google Scholar]
- 31.Sanchez RI, Wang RW, Newton DJ, et al. Cytochrome P450 3A4 is the major enzyme involved in the metabolism of the substance P receptor antagonist aprepitant. Drug Metab Dispos. 2004; 32: 1287–1292 [DOI] [PubMed] [Google Scholar]
- 32.Depré M, Van Hecken A, Oeyen M, et al. Effect of aprepitant on the pharmacokinetics and pharmacodynamics of warfarin. Eur J Clin Pharmacol. 2005; 61: 341–346 [DOI] [PubMed] [Google Scholar]
- 33.Emend (fosaprepitant dimeglumine) for injection [package insert]. Whitehouse Station, NJ: Merck; 2009. www.merck.com/product/usa/pi_circulars/e/emend_iv/emend_iv_pi.pdf Accessed June 21, 2012.
- 34.Grunberg S, Chua D, Maru A, et al. Single-dose fosaprepitant for the prevention of chemotherapy-induced nausea and vomiting associated with cisplatin therapy: randomized, double-blind study protocol—EASE. J Clin Oncol. 2011; 29: 1495–1501 [DOI] [PubMed] [Google Scholar]
- 35.Ioannidis JP, Hesketh PJ, Lau J. Contribution of dexamethasone to control of chemotherapy-induced nausea and vomiting: a meta-analysis of randomized evidence. J Clin Oncol. 2000; 18: 3409–3422 [DOI] [PubMed] [Google Scholar]
- 36.The Italian Group for Antiemetic Research. Dexamethasone, granisetron, or both for the prevention of nausea and vomiting during chemotherapy for cancer. N Engl J Med. 1995; 332: 1–5 [DOI] [PubMed] [Google Scholar]
- 37.Hesketh PJ, Harvey WH, Harker WG, et al. A randomized, double-blind comparison of intravenous ondansetron alone and in combination with intravenous dexamethasone in the prevention of high-dose cisplatin-induced emesis. J Clin Oncol. 1994; 12: 596–600 [DOI] [PubMed] [Google Scholar]
- 38.Latreille J, Stewart D, Laberge F, et al. Dexamethasone improves the efficacy of granisetron in the first 24 h following high-dose cisplatin chemotherapy. Support Care Cancer. 1995; 3: 307–312 [DOI] [PubMed] [Google Scholar]
- 39.Markman M, Sheidler V, Ettinger DS, et al. Antiemetic efficacy of dexamethasone. Randomized, double-blind, crossover study with prochlorperazine in patients receiving cancer chemotherapy. N Engl J Med. 1984; 311: 549–552 [DOI] [PubMed] [Google Scholar]
- 40.Ho CM, Ho ST, Wang JJ, et al. Dexamethasone has a central antiemetic mechanism in decerebrated cats. Anesth Analg. 2004; 99: 734–739 [DOI] [PubMed] [Google Scholar]
- 41.Mantovani G, Maccio A, Esu S, Lai P. Evidence that cisplatin induces serotonin release from human peripheral blood mononuclear cells and that methylprednisolone inhibits this effect. Eur J Cancer. 1996; 32A: 1983–1985 [DOI] [PubMed] [Google Scholar]
- 42.Suzuki T, Sugimoto M, Koyama H, et al. Inhibitory effect of glucocorticoids on human-cloned 5-hydroxytryptamine3A receptor expressed in xenopus oocytes. Anesthesiology. 2004; 101: 660–665 [DOI] [PubMed] [Google Scholar]
- 43.Tanihata S, Oda S, Nakai S, Uchiyama T. Antiemetic effect of dexamethasone on cisplatin-induced early and delayed emesis in the pigeon. Eur J Pharmacol. 2004; 484: 311–321 [DOI] [PubMed] [Google Scholar]
- 44.Vardy J, Chiew KS, Galica J, et al. Side effects associated with the use of dexamethasone for prophylaxis of delayed emesis after moderately emetogenic chemotherapy. Br J Cancer. 2006; 94: 1011–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Edmonds-Seal J, Prys-Roberts C. Pharmacology of drugs used in neuroleptanalgesia. Br J Anaesth. 1970; 42: 207–216 [DOI] [PubMed] [Google Scholar]
- 46.Wyant GM. A comparative study of eleven anti-emetic drugs in dogs. Can Anaesth Soc J. 1962; 9: 399–407 [DOI] [PubMed] [Google Scholar]
- 47.Bianchi C, Beani L, Crema C. Effects of metoclopramide on isolated guinea-pig colon. 2. Interference with ganglionic stimulant drugs. Eur J Pharmacol. 1970; 12: 332–341 [DOI] [PubMed] [Google Scholar]
- 48.Fontaine J, Reuse JJ. Pharmacological analysis of the effects of metoclopramide on the guinea-pig ileum in vitro. Arch Int Pharmacodyn Ther. 1973; 204: 293–305 [PubMed] [Google Scholar]
- 49.Laszlo J, Clark RA, Hanson DC, et al. Lorazepam in cancer patients treated with cisplatin: a drug having antiemetic, amnesic, and anxiolytic effects. J Clin Oncol. 1985; 3: 864–869 [DOI] [PubMed] [Google Scholar]
- 50.Malik IA, Khan WA, Qazilbash M, et al. Clinical efficacy of lorazepam in prophylaxis of anticipatory, acute, and delayed nausea and vomiting induced by high doses of cisplatin. A prospective randomized trial. Am J Clin Oncol. 1995; 18: 170–175 [DOI] [PubMed] [Google Scholar]
- 51.Razavi D, Delvaux N, Farvacques C, et al. Prevention of adjustment disorders and anticipatory nausea secondary to adjuvant chemotherapy: a double-blind, placebo-controlled study assessing the usefulness of alprazolam. J Clin Oncol. 1993; 11: 1384–1390 [DOI] [PubMed] [Google Scholar]
- 52.Bymaster FP, Calligaro DO, Falcone JF, et al. Radioreceptor binding profile of the atypical antipsychotic olanzapine. Neuropsychopharmacology. 1996; 14: 87–96 [DOI] [PubMed] [Google Scholar]
- 53.Bymaster FP, Falcone JF, Bauzon D, et al. Potent antagonism of 5-HT(3) and 5-HT(6) receptors by olanzapine. Eur J Pharmacol. 2001; 430: 341–349 [DOI] [PubMed] [Google Scholar]
- 54.Navari RM, Einhorn LH, Loehrer PJ, Sr, et al. A phase II trial of olanzapine, dexamethasone, and palonosetron for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group study. Support Care Cancer. 2007; 15: 1285–1291 [DOI] [PubMed] [Google Scholar]
- 55.Navari RM, Einhorn LH, Passik SD, et al. A phase II trial of olanzapine for the prevention of chemotherapy-induced nausea and vomiting: a Hoosier Oncology Group study. Support Care Cancer. 2005; 13: 529–534 [DOI] [PubMed] [Google Scholar]
- 56.Passik SD, Navari RM, Jung SH, et al. A phase I trial of olanzapine (Zyprexa) for the prevention of delayed emesis in cancer patients: a Hoosier Oncology Group study. Cancer Invest. 2004; 22: 383–388 [DOI] [PubMed] [Google Scholar]
- 57.Zyprexa Relprevv (olanzapine) [package insert]. Indianapolis, IN: Eli Lilly; 2011.
- 58.Martin BR, Wiley JL. Mechanism of action of cannabinoids: how it may lead to treatment of cachexia, emesis, and pain. J Support Oncol. 2004; 2: 305–314; discussion 314–316. [PubMed] [Google Scholar]
- 59.Herman TS, Einhorn LH, Jones SE, et al. Superiority of nabilone over prochlorperazine as an antiemetic in patients receiving cancer chemotherapy. N Engl J Med. 1979; 300: 1295–1297 [DOI] [PubMed] [Google Scholar]
- 60.Steele N, Gralla RJ, Braun DW, Jr, Young CW. Double-blind comparison of the antiemetic effects of nabilone and prochlorperazine on chemotherapy-induced emesis. Cancer Treat Rep. 1980; 64: 219–224 [PubMed] [Google Scholar]
- 61.Meiri E, Jhangiani H, Vredenburgh JJ, et al. Efficacy of dronabinol alone and in combination with ondansetron versus ondansetron alone for delayed chemotherapy-induced nausea and vomiting. Curr Med Res Opin. 2007; 23: 533–543 [DOI] [PubMed] [Google Scholar]
- 62.Billio A, Morello E, Clarke MJ. Serotonin receptor antagonists for highly emetogenic chemotherapy in adults. Cochrane Database Syst Rev. 2010;CD006272. [DOI] [PubMed]
- 63.Jordan K, Hinke A, Grothey A, et al. A meta-analysis comparing the efficacy of four 5-HT3-receptor antagonists for acute chemotherapy-induced emesis. Support Care Cancer. 2007; 15: 1023–1033 [DOI] [PubMed] [Google Scholar]
- 64.Rojas C, Stathis M, Thomas AG, et al. Palonosetron exhibits unique molecular interactions with the 5-HT3 receptor. Anesth Analg. 2008; 107: 469–478 [DOI] [PubMed] [Google Scholar]
- 65.Rojas C, Thomas AG, Alt J, et al. Palonosetron triggers 5-HT(3) receptor internalization and causes prolonged inhibition of receptor function. Eur J Pharmacol. 2010; 626: 193–199 [DOI] [PubMed] [Google Scholar]
- 66.Aapro MS, Grunberg SM, Manikhas GM, et al. A phase III, double-blind, randomized trial of palonosetron compared with ondansetron in preventing chemotherapy-induced nausea and vomiting following highly emetogenic chemotherapy. Ann Oncol. 2006; 17: 1441–1449 [DOI] [PubMed] [Google Scholar]
- 67.Eisenberg P, Figueroa-Vadillo J, Zamora R, et al. Improved prevention of moderately emetogenic chemotherapy-induced nausea and vomiting with palonosetron, a pharmacologically novel 5-HT3 receptor antagonist: results of a phase III, single-dose trial versus dolasetron. Cancer. 2003; 98: 2473–2482 [DOI] [PubMed] [Google Scholar]
- 68.Gralla R, Lichinitser M, Van Der Vegt S, et al. Palonosetron improves prevention of chemotherapy-induced nausea and vomiting following moderately emetogenic chemotherapy: results of a double-blind randomized phase III trial comparing single doses of palonosetron with ondansetron. Ann Oncol. 2003; 14: 1570–1577 [DOI] [PubMed] [Google Scholar]
- 69.Saito M, Aogi K, Sekine I, et al. Palonosetron plus dexamethasone versus granisetron plus dexamethasone for prevention of nausea and vomiting during chemotherapy: a double-blind, double-dummy, randomised, comparative phase III trial. Lancet Oncol. 2009; 10: 115–124 [DOI] [PubMed] [Google Scholar]
- 70.Einhorn LH, Brames MJ, Dreicer R, et al. Palonosetron plus dexamethasone for prevention of chemotherapy-induced nausea and vomiting in patients receiving multiple-day cisplatin chemotherapy for germ cell cancer. Support Care Cancer. 2007; 15: 1293–1300 [DOI] [PubMed] [Google Scholar]
- 71.Jordan K, Kinitz I, Voigt W, et al. Safety and efficacy of a triple antiemetic combination with the NK-1 antagonist aprepitant in highly and moderately emetogenic multiple-day chemotherapy. Eur J Cancer. 2009; 45: 1184–1187 [DOI] [PubMed] [Google Scholar]