Abstract
Background
Rare diseases are of increasing concern to private and public healthcare insurance plans. Largely neglected by manufacturers before the 1983 passing of the Orphan Drug Act (ODA), orphan drugs have become a commercialization target of steadily increasing importance to the healthcare industry. The ODA mandates the coverage of rare diseases, which are defined in research communities as diseases that are so infrequent that there is no reasonable expectation of a drugmaker recovering the cost of developing that drug.
Objectives
To determine the views of leading commercial US payers regarding providing access to and coverage for orphan drugs; to assess whether and to what degree cost-effectiveness analysis (CEA) is viewed by payers as relevant to rare disease coverage.
Methods
The study sample was identified through a call for action sent by America's Health Insurance Plans to its members, resulting in 4 interviews conducted and 3 completed surveys from a total of 7 companies. These 7 US health insurance companies represent approximately 75% of the US private insurance market by revenue and include approximately 157 million covered lives (using self-reported data from insurance companies). Representatives of 3 companies responded to the survey, and representatives of 4 companies were interviewed via the phone. The interviews were conducted with subject matter experts at each company and included 2 senior vice presidents of a pharmacy program, 1 chief medical director, and 1 head of pharmacoeconomics. The surveys were completed by 1 vice president of clinical pharmacy strategy, 1 chief pharmacy director, and 1 medical director.
Results
Based on the responses in this study, approximately 67% of US private insurance companies are concerned about orphan drugs, but only approximately 17% have developed meaningful strategies for addressing the cost of orphan drugs. Of the companies who do have such a strategy, 100% are unsure how to determine the best economic assessment tools to control orphan drug costs, and two thirds are relying on prior authorization as a means to control costs. More than 80% of the companies are not using cost-effectiveness methodologies with regard to rare diseases, generally because of a lack of the availability of medicines to facilitate such comparisons. CEA is used by less than 20% of our study sample of payers in dealing with orphan drug policies.
Conclusions
Evaluating cost-effectiveness is a valuable strategy for payers seeking to facilitate appropriate access and coverage decision-making related to orphan drugs, but it is not well understood or adapted by private insurance companies. Health economists, along with providers and payers, must work together to design rational methodologies to evaluate the value of orphan drugs, perhaps by adopting cost-effectiveness methodologies to consider a compound's total research and development and commercialization demands relative to its cost-effectiveness.
Rare diseases have recently been identified as a major source of concern for health insurance companies, with some states seeking to shift a portion of the fiscal burden of orphan drugs to patients, much to patients’ concern.1 Rare diseases and orphan drugs, which have also been referred to as “orphan medicine,” “high-cost drugs,” and “rare medicine,” are subjects of increasing and intense study in pharmacoeconomics and cost-effectiveness analysis (CEA).2–4 By current estimates, between 25 million and 30 million Americans (8%-10% of the US population) have 1 of the more than 6800 diseases deemed rare, because they affect less than 200,000 people, which is the threshold used to define a “rare disease.”5
In some diseases, defined by the UK's National Institute for Clinical Excellence as “ultra-orphan diseases,” only a few hundred patients are identified as having that disease.6 More than 350 orphan drugs have been approved by the US Food and Drug Administration (FDA) since the Orphan Drug Act (ODA) was introduced in 1983 (as opposed to <10 approvals in the 1970s).3 Today, many new orphan drugs are in the pharmaceutical pipeline, which is good news for patients and for their physicians, with 193 new orphan drug designation reviews performed by the FDA in the first 6 months of 2013 alone.6,7
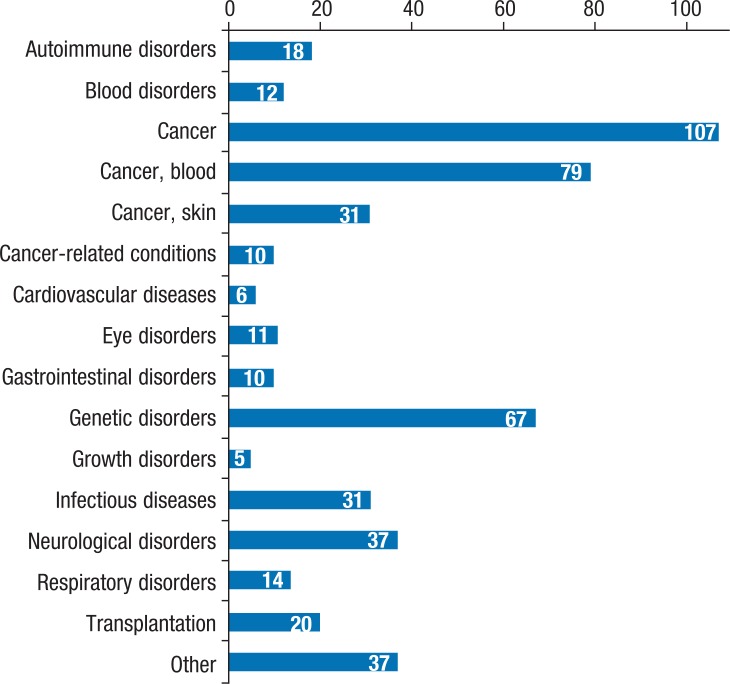
Diseases with the highest number of orphan drug designations include AIDS (57), melanoma (51), cystic fibrosis (46), acute myeloid leukemia (34), ovarian cancer (34), and pancreatic cancer (33).8 An overview of the orphan drugs that are currently in development is shown in Figure 1.
Figure 1. Orphan Drugs in Development.
Reprinted from PhRMA. Orphan drugs in development for rare diseases. Report. February 2011. www.phrma.org/sites/default/files/pdf/rarediseases2011.pdf. Accessed November 11, 2012.
In 1983, the founding of the National Organization for Rare Disorders led to the passing of the ODA by President Ronald Reagan, which provided significant incentives for manufacturers to develop drugs for orphan diseases.4 Incentives included a longer period of marketing exclusivity for these drugs to recoup development targeted at orphan diseases.4
KEY POINTS
-
▸
Rare diseases were largely ignored by the pharmaceutical industry before the 1983 passing of the Orphan Drug Act.
-
▸
Rare diseases and the drugs developed to treat them are costly for patients, providers, and payers, and their costs continue to rise.
-
▸
This study is based on responses from 7 large health insurance companies representing approximately 75% of the US private insurance market.
-
▸
The analysis shows that few health insurance companies have a meaningful strategy to address the rising costs of orphan drugs.
-
▸
Payers are unsure how to assess the cost of these drugs and rely on prior authorization as the cost-containment strategy, similar to specialty drugs.
-
▸
Cost-effectiveness is a valuable strategy to guide access and coverage decision-making for orphan drugs but is used by less than 20% of the study payers for dealing with orphan drug policies.
-
▸
Cost-effectiveness methodologies should be adopted to consider an orphan drug's commercial demands relative to its cost and clinical effectiveness.
-
▸
Healthcare stakeholders, including economists, providers, and payers must work together to design rational methodologies for considering the value of orphan drugs.
The FDA has designated at least 2313 medicines as orphan drugs since 1983.3 Although this is good news for countless patients, the issue of orphan drugs is of growing concern for payers, who are faced with covering the high costs of drugs for rare diseases.9 Much of the orphan drug research has occurred through research partnerships with academic institutions.10
US and Global Payer Policies for Orphan Drugs
Health insurance companies, whether public or private, are facing a pivotal point with regard to rare diseases, leading to a paradigm shift on how to best determine the comparative cost and effectiveness of orphan drugs to treat these conditions. For example, the cost of imiglucerase (Cerezyme), a treatment for Gaucher disease, can cost up to $300,000 annually, according to an interview with a pharmaceutical executive.
In the European Union (EU), for example, alipogene tiparvovec (Glybera), a new breed of gene therapy drug for a rare metabolic disease, is a 1-time injection that could cost in the range of $1.6 million and has recently been approved by EU authorities.11 However, the approval of this drug by the FDA is not yet pending, because of multiple approval failures in other countries over the past 20 years.11 Payers in the United States and around the world are questioning these potentially exorbitant annual outlays,11 turning to cost-effectiveness studies to provide insight and a firmer rationale for coverage decision-making.
To drive greater insight into the effectiveness of orphan drugs versus their high cost, there is a need to adopt CEA approaches that go beyond the elementary budgetary impact models that have historically been used to assess the economic dimension of more prevalent medical therapies. Nevertheless, when applied to rare diseases, CEA has been seen by some individuals to limit appropriate access to care and to lead to care rationing; there is also a concern that CEA will be used by payers for cost-containment.12
This is even more challenging because high-quality evidence about the clinical (and certainly economic) added value of orphan drugs is almost never available at the time of orphan drug marketing authorization by the FDA, as a result of the low number of relevant patients.9 Even after market launch of the drug, the challenges to gather the cost-effectiveness data needed to conduct meaningful CEA in rare diseases can often be daunting. The FDA does not take into consideration the payer's perspective, but simply ensures that the drug is safe and is clinically better than doing nothing.
This challenge is becoming a growing concern to payers. A 2010 study of orphan drug pricing and payer management strategies emphasizes the nature of the gap between the methods that payers are using and the rising tide of orphan drug use.9 Payers not only expect orphan drug costs to rise, they also see the number of orphan drugs being introduced by large pharmaceutical companies increasing as well.13 Yet no new management cost-effectiveness assessment approaches appear to be in development. Clinical data (ie, FDA-approved indications) remain the primary basis for prior authorization and for continued use of a specific orphan drug.13
The United States is not alone in facing the payer challenge to orphan drugs; other countries with government-regulated payment structures have adopted various stances toward orphan drugs.9 Based on our study, US private insurers are looking to other countries’ national policies on orphan drugs as potential models for new orphan disease policy development, and are closely watching the outcomes from these various approaches.
A summary of how global government agencies differ in their payment policies on orphan drugs is summarized in Table 1,9 which has potential implications to the US healthcare system. As shown in Table 1, the approaches vary from tougher negotiations with drug companies to limits on profitability to cost-effectiveness studies based on the “human value principle,” but no single measure seems to be emerging across the board.9
Table 1.
Orphan Drug Policies by International Payers
| Country: program | Policy |
|---|---|
| Belgium: National Institute for Health and Disability Insurance | Restricts reimbursement of some orphan drugs to prescribers belonging to specialized centers, but no policy or research incentives exist |
| France: National Plan for Rare Diseases | Temporary use authorization; a committee negotiates the price of an orphan drug with the pharmaceutical company, taking into account the improvement in clinical added value of the drug, with prices based on those that serve the same therapeutic purposes in other European countries |
| The Netherlands | Majority of orphan drugs incorporated in List 1B of the Medicine Reimbursement system; price is considered together with reimbursement decisions, and a maximum price is set based on therapeutically equivalent drugs |
| Italy: National Health Service Plan/National Network for Rare Diseases | Price negotiation takes into account the efficacy of the drug and price comparison with other countries |
| Sweden | Reimbursement considers cost-effectiveness, the human value principle (ie, equality of all people), and the need and solidarity principle (ie, products that treat those with the greatest health need take precedence), but not budget impact |
| United Kingdom | Prices are set by the pharmaceutical company but need to meet profit control criteria, as stated in the Pharmaceutical Price Regulation Scheme; reimbursement considers budget impact and cost-effectiveness; the UK's NICE has stated that many orphan drugs had incremental cost-effectiveness ratios at the high end of what the appraisal committee deemed to be cost-effective14; in addition, NICE has defined a category of “ultra-orphan disease” for conditions affecting <1000 patients6 |
NICE indicates National Institute for Health and Care Excellence.
In the United States, no specific coverage of orphan drugs is addressed in the Affordable Care Act (ACA). To some extent, payment policies are still in motion, because this legislation is composed of many provisions that become effective at various times, starting with its signing into law in March 2010. However, 2 elements related to orphan drugs are clear in the ACA:
Medicare does not have special or different coverage rules for orphan drugs: Medicare largely covers drugs, regardless of whether they are “reasonable and necessary,” and based on delivery mechanism
The ACA specifically excludes Medicare and Medicaid from making coverage decisions for any drug (not just orphan products) based solely on its cost-effectiveness.
To the extent that they are discussed, orphan drugs are addressed in the ACA under provisions dealing with biosimilars, annual tax on pharmaceutical sales, and on the 340B drug pricing program. Orphan drugs are not granted special coverage rules by the ACA that would distinguish them from other drugs. However, numerous states are creating laws to limit out-of-pocket (OOP) payments for patients, including yearly caps (eg, in Hawaii, New York, Maine, Connecticut, and Rhode Island).1 The laws being passed are for limitations on OOP payments for plans in the exchange, but most commercial health plans already have an OOP maximum. Pharmaceutical companies are generally supportive of such bills, because high copayments discourage patient adherence.1 Cost is only 1 factor that drives nonadherence.
Our study sought to describe the current posture of private healthcare insurers in the United States relative to orphan drug–specific coverage issues, and their approaches to CEA for this class of drugs, and to investigate the application of cost-effectiveness analytic methods as a potential means to generate insights into the cost of orphan drug delivery and patient care for payers.
This study is intended as an exploratory investigation, in that cost-effectiveness methods are relatively nascent in the health benefits environment, especially as they apply to rare diseases. Given the unprecedented nature of cost escalations in this category, our research explored the potential application of these methods to the impending wave of orphan drug costs facing the US healthcare industry.
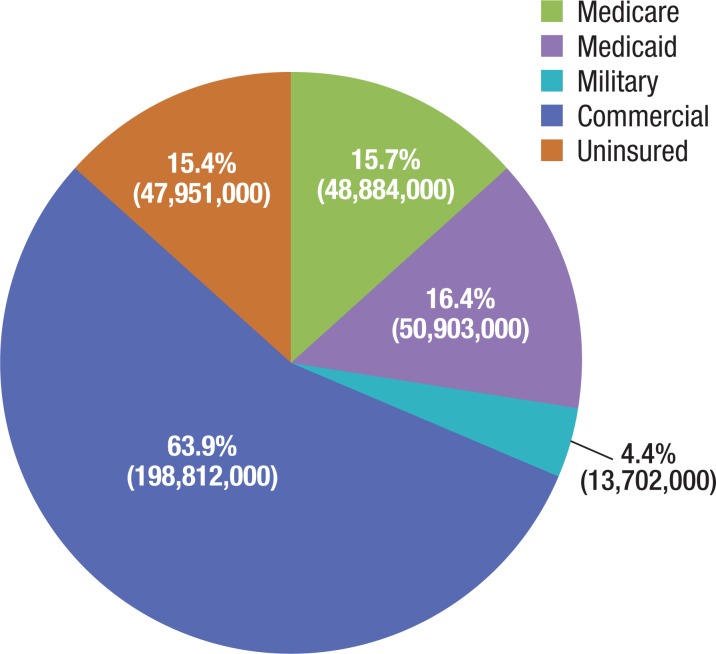
The US healthcare population consists of approximately 311 million lives, of which approximately 60% are covered by commercial insurance, approximately 31% are covered by Medicare or Medicaid, approximately 15% are uninsured, and approximately 4% are covered by military insurance (Figure 2).15
Figure 2. US Medically Insured Population, % of Total.
Medicaid enrollment is net of Medicare/Medicaid dual-eligibles.
Source: DeNavas-Walt C, Proctor BD, Smith JC; for the US Census Bureau. Income, poverty, and health insurance coverage in the United States: 2012. Current population reports. September 2013. www.census.gov/prod/2013pubs/p60–245.pdf. Accessed November 14, 2013.
Given the changes resulting from the ACA, Medicare policy for orphan drugs is not yet fully developed and remains a work in progress. Consequently, the research summarized in this article focuses on the views of private insurance carriers, covering approximately 161 million individuals in a managed care environment.
Methods
This study involved 4 in-depth, qualitative interviews with a representative sample of vice presidents of pharmacy programs, pharmacy strategy, and pharmacoeconomic think tanks, and chief medical directors from a sample of the largest private US health insurance companies. These were complemented by 3 completed online surveys from other major insurers as well, including 1 vice president of clinical pharmacy strategy, 1 chief pharmacy director, and 1 medical director. All contacts were made through America's Health Insurance Plans, a trade organization that represents the combined majority of private medical insurance carriers in the United States.
As shown in Table 2, the majority of the data were collected through interviews, with a small number of responses collected through surveys. Because of the exploratory nature of the study, we sought to clarify insights into the current state of cost-effectiveness measures being applied by private insurance companies to the case of rare diseases. All respondents were guaranteed confidentiality of their responses, and have therefore not been attributed to any specific insurance company.
Table 2.
Respondents to Interviews and/or Surveys
| Insurance company | Medical lives covered, in millions | Position | Data collection |
|---|---|---|---|
| A | 84 | Senior Vice President, Pharmacy Programs | Interviewed |
| B | 36 | Staff Vice President, Clinical Pharmacy Strategy | Survey |
| C | 9 | Chief Pharmacy Director | Survey |
| D | 18.3 | Head, Pharmacoeconomic Comparative Effectiveness Research | Interviewed |
| E | 4.4 | Medical Director | Survey |
| F | 3.8 | Medical Director | Interviewed |
| G | 2 | Senior Vice President, National Pharmacy Programs | Interviewed |
| Total | 157.5 | ||
| Total private lives covered | 210 |
The total number sampled included 7 major private US health insurance companies representing approximately 157 million medical covered lives. The interviews were conducted with 1 medical director, 2 senior vice presidents of pharmacy programs, and 1 head of pharmacoeconomics via telephone.
Although this is a small number of data points, the sample represents a large proportion of health plan member lives (based on data obtained from each company's website).
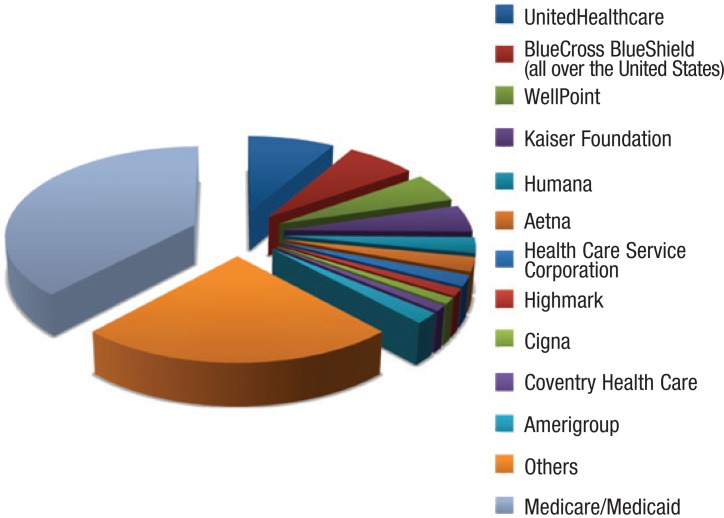
The representative nature of our sample is shown in Figure 3, which demonstrates how the US health insurance market is relatively fragmented, with no commercial provider having a majority market share. This is because of the regional positioning of many commercial US insurers. Our sample covers the smaller number of health insurance companies that represent the largest share of privately insured individuals, and provides a representative indicator of current practices relative to orphan drug coverage.
Figure 3. Breakdown of Private Insurer Market.
Source: Data were collected from each company's site at America's Health Insurance Plans. 2012. www.ahip.org/AHIPResearch/. Accessed November 11, 2012.
The questions in the interviews and survey were:
How do US health insurance companies view orphan drugs?
What are US payers’ strategies to better manage drug access and coverage claims for patients with rare diseases who need orphan drugs?
What steps, if any, are private insurance companies taking to reduce the coverage of rare diseases?
What impact will such payer initiatives have on healthcare providers when treating patients with rare diseases?
What tools or strategies are currently in use among US payers to assess the cost-effectiveness of orphan drugs?
What measurable clinical and economic benefit are they concluding?
What data are required or available to support the CEA of orphan drugs?
The responses were reviewed, coded, analyzed, and summarized based on these questions.
Results
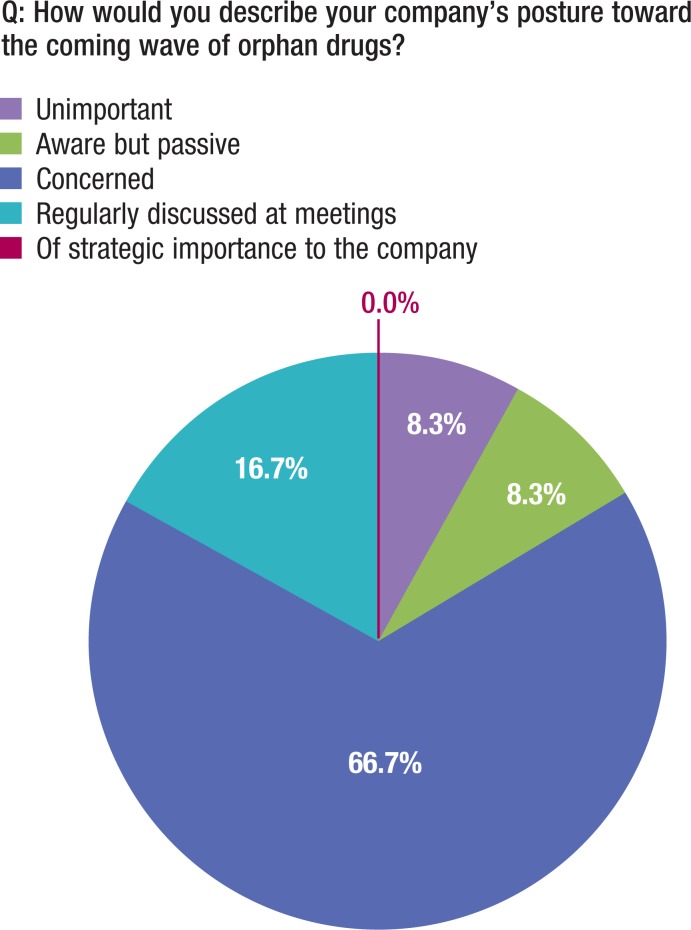
The US health insurance companies studied in this analysis view the ODA as well-intentioned and designed with sympathetic intent, and appropriately written into law to assist the patients with greatest need to address critical medical challenges. Although almost 67% of the payers in this study are concerned with the growing impact of orphan drugs, almost 17% of payers do not even have orphan drugs on their radar or are passive toward them (Figure 4, page 593).
Figure 4. Insurance Company Concern toward Orphan Drugs.
In their view, however, the ODA has been adopted as a source for revenue growth and is protected under the shield of the ODA's original incentives, with payers generally unclear on what steps to take to better manage the impending FDA approval of many new orphan drugs (Figure 4).
One insurance company noted that some medical conditions are now being labeled as rare diseases that never used to be classified as such, in part because of the improvements in research and DNA coding. Consequently, pharmaceutical and biotechnology companies can have conditions named as rare diseases with an International Classification of Diseases, Ninth Revision code, but no such label existed in the past. This gap in regulatory governance is only one of the key issues within the debate concerning the ODA.13
None of the payers who were interviewed provided evidence of a definitive orphan drug management plan (Figure 5). Even in the survey, responses to questions in the “open-ended” section were answered with “we don't have a strategy for orphan drugs.” Based on our study, 3 insurance companies simply approach orphan drugs as any other specialty drug, and have no special provisions or policies for them.
Figure 5. Insurance Company Plans to Manage Orphan Drugs.
Approximately 33% of the respondents believe that rare diseases are an important policy issue, but they noted that they do not know what to do. At least 50% of the insurance companies in this study are in dialogues with physicians and other healthcare providers about rare diseases (Figure 5), but only 1 leading payer (Company A) claimed to be actively developing a strategic plan, which has not yet launched, to manage the growing number of orphan drugs.
That particular payer stated, “There is no data pool, no pharmacoeconomics analysis, and it is becoming more and more of an issue. We are on the alert for alarming new drugs with outrageous list prices on a daily basis. We already have a tier-3 status, and it is very likely that there will be a tier-4 status developed with a substantial coinsurance. But we will be bumping up against health reform, and we will be compelled to make these high-cost drugs available. We know that we need to begin doing our own modeling, but if it is a life-threatening disease, the last thing we want to do is make it less accessible. This always trumps the affordability issue.”
This comment underscores the key challenge that faces insurance companies regarding orphan drugs—being required to provide access and coverage for pharmacotherapies that treat severe or life-threatening rare diseases, even in cases when the value of the therapy cannot be ascertained. Although this is true of many of the specialty drugs that are currently approved, the sheer number of pending orphan drugs is raising growing concern.
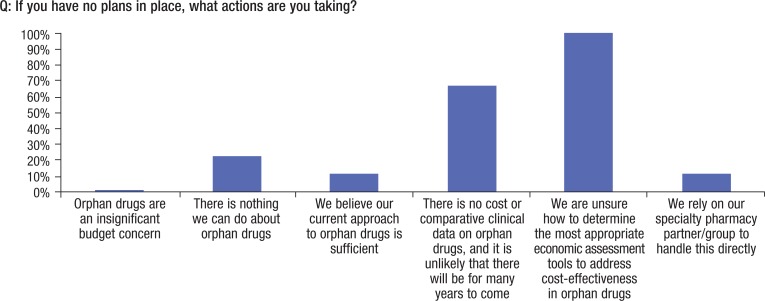
Approximately 85% of the payers in this study observed that some action must be taken (although 20% noted that “doing nothing” is part of the risk-pooling process). For many insurance companies, a risk pool is a function of the types of members the plan has, and doing nothing is a perfectly appropriate plan of action given the level of risk involved. As shown in Figure 6, 67% of the payers believe that with no comparative clinical or cost data available on orphan drugs, it is unlikely that any meaningful action can occur.
Figure 6. Actions Taken for Orphan Drugs.
All of the payers who responded to this question expressed uncertainty regarding what constitutes the right tools to be used for such a CEA. One company (Company F), however, deferred policy decisions to its specialty pharmacy partner to address the issue, stating, “Unequivocally across the many companies I've worked with, there are not a lot of [effective] methods being used. Approaches vary from sophisticated prior authorizations, to manual review of prior authorizations (providing they can pull them out of their spend analysis), to simply paying the bill. There is somewhat of a shift, however. Five years ago, 80% were just paying the bill, and 20% were on some continuum of prior authorizations. Today that has flipped, with about 80% now realizing they have operational challenges with orphan diseases on their radar, but have no clue what to do beyond placing prior authorizations on them. To be perfectly blunt, they are not looking at this in a sophisticated manner.”
Every insurance company we interviewed is struggling to identify evidence to assess comparative drug efficacy (Figure 6). This is even more of an issue in the realm of data access and analytic methodologies to evaluate the cost-effectiveness of a drug, which becomes still more complex when rare diseases are involved.
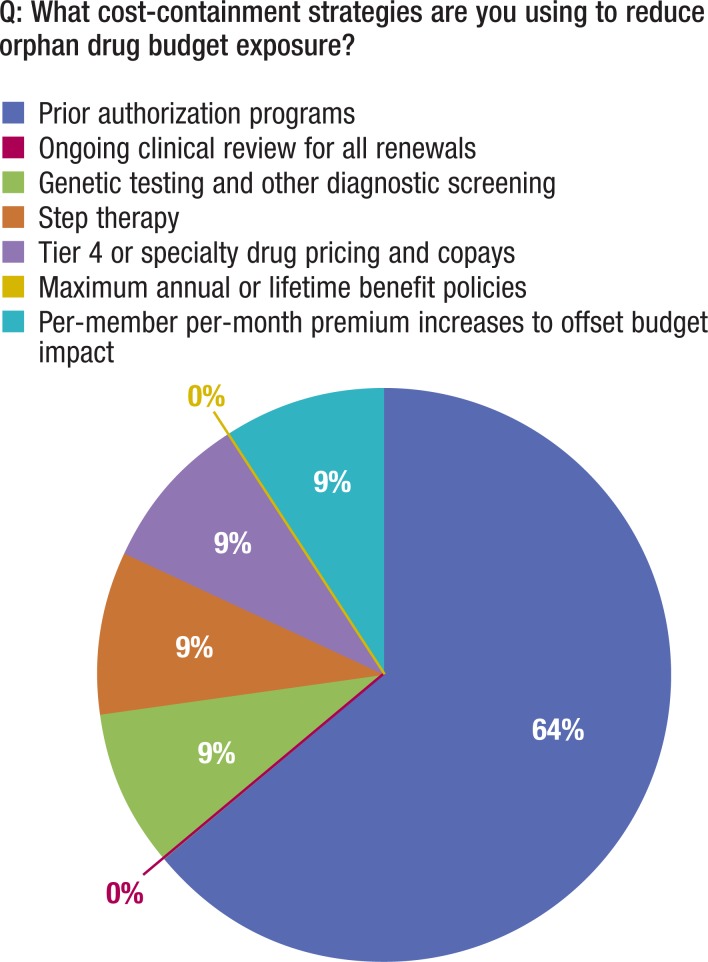
As shown in Figure 7, the primary approach used to render decisions on orphan disease budget exposure was prior authorizations (for high-prevalence medications), which involve determining if a drug will save “outpatient days,” or the number of days that a patient will miss work; however, assessing quality-of-life changes is typically avoided because of the subjective nature of the assessment.
Figure 7. Cost-Containment Strategies Used.
Furthermore, based on our interviews, 100% of insurance companies that have a plan for orphan drugs are relying on prior authorizations as a cost-containment strategy to screen patients for the drug coverage. Approximately 27% of the respondents are experimenting with approaches that rely on the ongoing monitoring of an insured's clinical progress, step therapy, and diagnostic and genetic screening—which are all approached in an ad hoc manner and not as an organized strategy aimed at data collection and CEA (Figure 7).
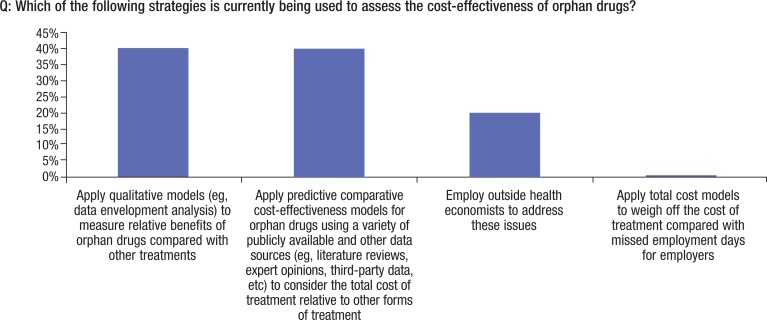
As shown in Figure 8, approximately 40% of the payers are using some form of analytical tool to measure the clinical effectiveness of orphan drugs. One carrier cited the use of data envelopment analysis, a qualitative assessment that essentially examines comparative efficiency between drugs. Data envelopment analysis techniques have been used to benchmark other treatments, such as medical ventilation treatments16 and physician practice behavior.17 Other payers cited the use of expert opinion in their analyses. Another more promising comparative approach that payers are beginning to explore is cost-effectiveness modeling, which aggregates a variety of clinical and economic data sources on comparative drugs as a means of gaining insight into the head-to-head comparative value of a drug.
Figure 8. Strategies for Cost-Effectiveness for Orphan Drugs.
Discussion
One health insurance company in the study cited reliance on its specialty pharmacy department, whereas another cited pushing decision-making to its pharmacy benefits organization. Two companies rely on a Pharmacy & Therapeutics committee, and another relies on a combination of medical affairs and its pharmacy department. Based on this small sample size, no common denominator emerged regarding how US insurance companies best handle decisions regarding medications for rare diseases.
A large percentage of Medicaid state plans do not treat the coverage of orphan drugs differently from that of drugs for prevalent diseases.18 States often have little or no basis on which to make cost-effectiveness decisions, because they are required to cover drugs for which they receive a rebate. The Medicaid Drug Rebate Program requires a drug manufacturer to provide a rebate of at least 13% of the drug's cost for it to be covered by a state Medicaid program.18 Therefore, any manufacturer that provides a rebate has a drug that Medicaid providers must cover, automatically putting the medication onto the state formulary list. This drug access and coverage policy is in turn pushed onto managed care organizations (MCOs), requiring the MCOs to cover any drug that a given state covers.
Like state Medicaid plans, several private insurers in this study pointed out that they do not have resources for conducting cost-benefit or cost-effectiveness analyses, citing thin margins and insufficient budgets for studies. Consequently, payers often rely on what is published and on what is preferred by healthcare providers, together with assessments based on drug monographs, and, at times, input from external consulting firms and market intelligence. Company G's representative noted, “We know that some manufacturers are labeling more and more medicines as having unique indications that allow them to price drugs at 20 times what they should be.”
Because the pharmaceutical industry has sharpened its commercial focus on rare diseases in the “postblock-buster” era, the fair balance of the US ODA among patients, providers, and healthcare insurance companies has been eclipsed, moving beyond the ODA's original intent of 3 decades ago. Aligning the ODA more closely with orphan drug incentivization and market access policies of other global markets will help the ODA to more appropriately meet the needs of the entire US healthcare system.
Like all pharmaceuticals, orphan drugs have specific indications. Physicians are responsible for verifying the needs of patients with a rare disease and for correctly directing them to novel therapies for rare diseases. Then insurance companies screen the claims and can refuse payment unless the screening criteria are met. This critical element—evidence of substantiation—is the leverage point on which the decision is made. The FDA neither calls into question the cost nor the appropriate level of cost associated with a drug, only whether it is effective or better than nothing.
Limitations
There are several limitations to this study. The primary limitation is the small sample size of insurance companies identified in this study. Even so, the small number of companies represents a sizable proportion of the private insurance industry. Within this sample, there is a possibility of selection bias, and it is possible that a broader representation of organizations would reveal some isolated strategies that have not been captured here.
There is also a possibility of respondent bias, in that we interviewed a single individual within each company. However, we sought to identify the highest-level executive responsible for strategic planning on orphan drug policy development, and we have assumed that this individual was willing to provide a clear picture based on the guaranteed anonymity of the study.
There is also a possibility of misrepresentation of the results through the survey data collection, although most of the statements are self-evident in nature.
Conclusions
Although the ODA has shown tremendous success in advancing the development and commercialization of life-saving and life-enhancing medications for patients with rare diseases, there remains a singular lack of cost-effectiveness measures. Measures that take into consideration a far more comprehensive array of issues, data, and CEA, as well as a sliding scale of orphan drug pricing policies, standards, and commercial incentives for industry, are needed to provide a more equitable set of guidelines for payers and for patients.
The results of this study suggest little in the way of cost-effectiveness methods that align with pricing policies. We believe that work is needed to construct the design of a sliding scale of orphan drug pricing policies subject not only to a compound's total research and development and commercialization demand, but also whether and to what degree its cost-effectiveness can be ascertained through the application of new cost-effectiveness methodologies. Current research provides limited accurate data on the prices, sales, and profit margins.13 Health economists, together with providers and payers, must work together to design rational methodologies for considering the value of orphan drugs, vis-à-vis other interventions when available (which is uncommon, given the nature of rare diseases) or in relation to the cost in overall medical utilization and quality of life versus the natural progression of rare diseases.
A new series of rare disease–specific incremental cost-effectiveness ratio (ICER) modeling may help to provide greater clarity into the true costs versus benefits of a drug. Although the expectation should not be perfection for any such novel orphan disease–specific ICER, intellectual resources need to be deployed in this direction to address the orphan disease comparative effectiveness research gap, given the considerable number of orphan drugs now under FDA review, as well as the payer and commercial challenges.
Author Disclosure Statement
Dr Handfield is a consultant to Caterpillar, Thomson Reuters, and Enbridge Gas Distribution. Mr Feldstein is a consultant to QLT, Inc.
Contributor Information
Robert Handfield, Bank of America University Distinguished Professor of Supply Chain Management, and Codirector, Supply Chain Resource Cooperative, Poole College of Management, North Carolina State University, Raleigh.
Josh Feldstein, Chief Executive Officer, Center for Applied Value Analysis, New York, NY.
References
- 1.Pollack A. States seek curb on patient bills for costly drugs. New York Times. April 12, 2012. www.nytimes.com/2012/04/13/health/states-seek-to-curb-exorbitant-drug-costs-incurred-by-patients.html?pagewanted=all&_r=0 Accessed November 11, 2012.
- 2.Denis A, Mergaert L, Foster C, et al. Orphan diseases and orphan medicines: a Belgian and European study. J Pharm Belg. 2009;(4):131–137 [PubMed]
- 3.Pharmaceutical Research and Manufacturers of America. Orphan drugs in development for rare diseases. Report. February 2011. www.phrma.org/sites/default/files/pdf/rarediseases2011.pdf Accessed November 11, 2012.
- 4.Brewer GJ. Drug development for orphan diseases in the context of personalized medicine. Transl Res. 2009; 154: 314–322 [DOI] [PubMed] [Google Scholar]
- 5.National Institutes of Health; National Center for Advancing Translational Sciences. FAQ about rare diseases. www.ncats.nih.gov/about/faq/rare/rare-faq.html Accessed November 11, 2013.
- 6.National Institute for Clinical Excellence. Ultra orphan drugs. NICE Citizens Council Report. November 2004. www.nice.org.uk/media/065/85/Citizens_Council_Ultraorphan.pdf Accessed November 11, 2013.
- 7.US Food and Drug Administration. Total number of orphan drug designation reviews completed in the month. Fiscal year 2013. Current as of June 30, 2013. www.accessdata.fda.gov/FDATrack/track?program=osmp&id=OSMP-OOPD-Total-number-orphan-drug-designation-reviews-completed&fy=2013 Accessed November 11, 2013.
- 8.Braun MM, Faraq-El-Massah S, Xu K, Coté TR. Emergence of orphan drugs in the United States: a quantitative assessment of the first 25 years. Nat Rev Drug Discov. 2010; 9: 519–522 [DOI] [PubMed] [Google Scholar]
- 9.Denis A, Mergaert L, Fostier C, et al. A comparative study of European rare disease and orphan drug markets. Health Policy. 2010; 97: 173–179 [DOI] [PubMed] [Google Scholar]
- 10.Brewer GJ. Fundamental problems lie ahead in the drug discovery and commercialization process: restructuring of the pharmaceutical industry and an improved partnership with academia are required. J Investig Med. 2006; 54: 291–302 [DOI] [PubMed] [Google Scholar]
- 11.Whalen J. Gene-therapy approval marks major milestone. Wall Street Journal. Updated November 2, 2012. http://online.wsj.com/article/SB10001424052970203707604578095091940871524.html Accessed November 11, 2012.
- 12.Hughes D. Rationing of drugs for rare diseases. Pharmacoeconomics. 2006; 24: 315–316 [DOI] [PubMed] [Google Scholar]
- 13.Hyde R, Dobrovolny D. Orphan drug pricing and payer management in the United States: are we approaching the tipping point? Am Health Drug Benefits. 2010; 3(1): 15–23 [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Clinical Excellence. Appraising orphan drugs. Draft v3. March 16, 2006. www.nice.org.uk/niceMedia/pdf/smt/120705item4.pdf Accessed November 11, 2012.
- 15.DeNavas-Walt C, Proctor BD, Smith JC; for the US Census Bureau. Income, poverty, and health insurance coverage in the United States: 2012. Current population reports; September 2013. www.census.gov/prod/2013pubs/p60-245.pdf Accessed November 14, 2013. [Google Scholar]
- 16.O'Neal PV, Ozcan YA, Ma Y. Benchmarking mechanical ventilation services in teaching hospitals. J Med Syst. 2002; 26: 227–240 [DOI] [PubMed] [Google Scholar]
- 17.Ozcan YA. Physician benchmarking: measuring variation in practice behavior in treatment of otitis media. Health Care Manag Sci. 1998;1:5–17 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Medicare & Medicaid Services. Medicaid Drug Rebate Program. November 5, 2013. www.medicaid.gov/Medicaid-CHIP-Program-Information/By-Topics/Benefits/Prescription-Drugs/Medicaid-Drug-Rebate-Program.html Accessed November 14, 2013.