Abstract
The feasibility of detecting Candida albicans mRNA in formalin-fixed paraffin-embedded archival human histopathology specimens by reverse transcription-PCR (RT-PCR) was investigated. RT with gene-specific primers was used to detect five single-copy C. albicans gene transcripts, including those of two housekeeping genes, in oral candidiasis samples up to 8 years of age.
As well as causing pseudomembranous candidiasis (PC), the common commensal yeast Candida albicans can also be the etiological agent of a variety of oral mucosal lesions, including chronic hyperplastic candidiasis (CHC) (23). CHC presents as an adherent white patch on the oral mucosa, which on histological examination shows epithelial hyperplasia with Candida hyphae in the surface keratin. The epithelium may also show features of dysplasia and have the potential to progress to oral squamous cell carcinoma (OSSC) (1, 19). Levels of oral carriage of C. albicans have recently been shown to be significantly higher in patients presenting with epithelial dysplasia or OSSC (13) and to be associated with potentially precancerous oral leukoplakia lesions (15). No evidence has been reported, however, for the increased prevalence of OSSC in patients diagnosed with other manifestations of candidiasis, such as PC and denture stomatitis, leaving any potential role for candidiasis in OSSC oncogenesis as yet unclear. Differential levels of expression of C. albicans genes between CHC and other forms of candidiasis may be of use in clarifying such apparent inconsistencies. There is evidence, for example, that secreted aspartyl protease (SAP) genes are differentially expressed in oral candidiasis compared to asymptomatic carriage (7).
Clinical diagnosis of oral diseases usually involves histopathological examination of biopsies, and extensive archives of formalin-fixed paraffin-embedded (FFPE) samples are available for retrospective examination. The successful recovery of RNA species from FFPE samples has enabled the analysis of human gene expression in these samples by reverse transcription-PCR (RT-PCR) (9). The investigation of C. albicans gene expression in FFPE histopathology samples is significantly more difficult, as the microorganism composes only a very minor fraction of the biological material. One recent study successfully used nested RT-PCR to detect C. albicans translation elongation factor 1β (EFB1) and secreted aspartic proteinase 9 (SAP9) mRNA in FFPE samples prepared from an immunocompromised mouse oroesophageal candidiasis model (22). No studies reported to date, however, have detected single-copy gene transcripts for any cellular microorganism, including C. albicans, in archival FFPE clinical samples. In this study we developed an improved RT-PCR technique and used it to detect five single-copy C. albicans gene transcripts in archival human FFPE samples of candidiasis.
Optimization of two-step RT-PCR; sensitivity of detection and use of multiplex RT reactions.
Six C. albicans genes were selected for the study: the multiple-copy-number 18S rRNA gene, the actin (ACT1) gene (10), the EFB1 gene (11), the secreted aspartic proteinase 2 (SAP2) gene (26), and the alcohol dehydrogenase (Adh) 1 and 2 (ADH1 and ADH2) genes (2, 24). PCR primers for detection of cDNA (Table 1) were designed to amplify PCR products smaller than 200 bp because of the reported degradation and chemical modification of RNA recovered from FFPE samples (12). All primer pairs specifically amplified PCR products of the predicted size from C. albicans cDNA species and gave no PCR product when tested with either human genomic DNA or human oral mucosa cDNA. The ACT1 forward primer spans the sole exon-exon boundary of the processed transcript so as to exclude amplification of genomic C. albicans DNA (16). The primer pair for EFB1 spans an intron so that amplification yields a 97-bp product from cDNA and a 462-bp product from any contaminating genomic DNA (22). For the other four genes investigated, the control for contaminating genomic DNA was a mock RT-PCR performed without reverse transcriptase. All PCRs (unless otherwise stated) were undertaken in a 20-μl volume containing 1 U of Taq polymerase (HotStarTaq; Qiagen, Valencia, Calif.) with the manufacturer's buffer and the addition of MgCl2 to a final concentration of 2.0 mM, 6 pmol each of the forward and reverse primers, and 2 μl of the template. Thermal cycling was performed using a Mastercycler-Gradient instrument (Eppendorf AG, Hamburg, Germany) with an initial enzyme activation step of 95°C for 15 min, 45 cycles of denaturation at 94°C for 15 s, annealing at the appropriate temperature for the specific primer pair (Table 1) for 15 s, and extension at 72°C for 15 s followed by a final extension step of 72°C for 5 min. These PCR conditions were found to reliably amplify a plasmid template (the ACT1 PCR product cloned into pGEM-T) at concentrations as low as 20 copies per assay (data not shown).
TABLE 1.
Primer pairs used to detect C. albicans RNA transcripts by RT-PCR
| Gene | Accession no. | PCR forward primer sequence | PCR reverse and RT primer sequence | PCR product size(s) (bp) | Annealing temp (°C) |
|---|---|---|---|---|---|
| 18S rRNA | AF114470 | GGATTTACTGAAGACTAACTACTG | GAACAACAACCGATCCCTAGT | 131 | 60 |
| ACT1 | X16377 | ATGGACGGGAAGAAGTTGC | CCCATACCAACCATGATACC | 146 | 62 |
| EFB1a | X96517 | TCAGATTTCTCTAAAGTCG | TGACATCAGCTTGAGTGG | 97 and 462 | 58 |
| SAP2b | M83663 | AACAACAACCCACTAGACATCACC | TGACCATTAGTAACTGGGAATGCTTTAGGA | 178 | 60 |
| ADH1 | X81694 | CACTCACGATGGTTCATTCG | AAGATGGTGCGACATTGG | 90 | 60 |
| ADH2 | AL033501 | CACCCACGATGGTTCTTTCC | AAGATTGGTGCAACATGGG | 90 | 60 |
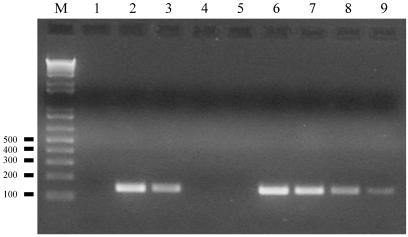
To optimize RT of rare C. albicans transcripts in RNA samples we compared the use of random hexamers as RT primers to the use of a specific (reverse) primer for the amplification of ACT1 mRNA from C. albicans ATCC 10261 total RNA. RNA was extracted from C. albicans cells grown in glucose, salts, and biotin (GSB) medium (6) with a hot-phenol method (21). RT reactions were performed in a 20-μl volume with Superscript III enzyme (Invitrogen, Carlsbad, Calif.) in accordance to the manufacturer's instructions except that when specific primer(s) were used the reaction mix was kept at 55°C, following the initial template denaturation-primer annealing step, to reduce any potential for template mispriming. We found that the use of a specific-RT primer to detect the ACT1 mRNA gave between a 10- and a 100-fold increase in sensitivity compared to the results seen with random hexamers (Fig. 1).
FIG. 1.
Sensitivity of detection of C. albicans ACT1 mRNA by RT-PCR using either random hexamer primers (lanes 2 to 5) or a gene-specific reverse primer (lanes 6 to 9) for cDNA preparation. PCR amplification was performed on cDNA prepared from 10 ng (lanes 2 and 6), 1 ng (lanes 3 and 7), 0.1 ng (lanes 4 and 8), and 10 pg (lanes 5 and 9) of C. albicans total RNA. Lane 1, negative-control PCR (no cDNA added). Lane M: DNA molecular size markers (bp).
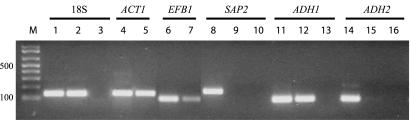
While the use of a specific primer for the RT reaction was useful for increasing the sensitivity of detection for a single-gene transcript, the amount of RNA obtained from an archival FFPE sample was still extremely limited. We therefore examined the use of a multiplex RT reaction including six specific primers to produce PCR-detectable cDNA of all the genes of interest in a single reaction. To simulate the complexity of an archival RNA sample we tested the multiplex RT reaction with a mixture of 1 ng of C. albicans total RNA (as described above) with 100 ng of mouse liver total RNA (Ambion, Austin, Tex.) and 1.5 pmol of each of the reverse primers for the 18S, ACT1, EFB1, SAP2, ADH1, and ADH2 genes. This RT reaction product was used as a template in separate PCRs for each gene of interest. Using this technique we were able to detect C. albicans 18S rRNA and ACT1, EFB1, and ADH1 mRNAs (Fig. 2). While no SAP2 or ADH2 cDNA was detected in this experiment, this probably reflects reduced expression of these genes under the culture conditions used to produce the C. albicans RNA (GSB medium). Sap expression has been shown to be repressed in medium containing ammonium (20). Multiplex RT reactions were used for all subsequent analyses of archival RNA samples.
FIG. 2.
Use of multiplex RT for simultaneous preparation of gene-specific cDNAs in a single reaction. The RT reaction used a mixture of C. albicans and mouse (1:100) total RNA and the reverse primers for C. albicans 18S, ACT1, EFB1, SAP2, ADH1, and ADH2 transcripts. The cDNA template was used in individual PCRs with the primer pairs for the 18S (lane 2), ACT1 (lane 5), EFB1 (lane 7), SAP2 (lane 9), ADH1 (lane 12), and ADH2 (lane 15) gene transcripts. Positive PCR control experiments were performed using either 0.1 ng of C. albicans cDNA preparations (ACT1 and EFB1 [lanes 4 and 6, respectively]) or 0.1 ng of C. albicans genomic DNA (18S, SAP2, ADH1, and ADH2 [lanes 1, 8, 11, and 14, respectively]) as a template. As a control for contamination by C. albicans genomic DNA, a mock-RT reaction (without enzyme) was performed and the reaction mixture was used in PCRs with the 18S (lane 3), SAP2 (lane 10), ADH1 (lane 13), and ADH2 (lane 16) primer pairs. Lane M: DNA molecular size markers (bp).
FFPE biopsy samples, RNA extraction, and assessment of RNA integrity.
All FFPE samples were obtained from the University of Otago Oral Pathology Diagnostic Service (Medlab Dental) archives and had been stored in darkness at room temperature. CHC or PC samples had been diagnosed according to standard criteria (18). Three 20-μm sections were excised from each FFPE block, and RNA from the sections was extracted using an Ambion paraffin block RNA isolation kit according to the manufacturer's instructions except for the following additional step to digest C. albicans cell walls. After deparaffinizing, samples were incubated in 1 ml of SK buffer (1 M sorbitol, 50 mM KH2PO4 [pH 8.0]) containing 0.5 mg of Zymolyase (Seikagaku Corp., Tokyo, Japan) and 0.1% (vol/vol) β-mercaptoethanol at 37°C for 1 h. Sample material was then pelleted for 5 min in a microcentrifuge, as much supernatant as possible was carefully aspirated, and then the manufacturer's (Ambion) protocol was continued. The protocol included treatment with DNase I to eliminate residual traces of DNA in the RNA samples.
One of the most challenging problems faced in using archival FFPE material is the enormous potential for variation in both the quality and the quantity of the RNA extracted. The samples we investigated differed considerably in the amounts of biological material present, in their ages (between a few months and over 10 years), and, perhaps most importantly, in their fixation periods. While all the samples examined were fixed using neutrally buffered formalin, the length of this fixation period was not standardized and was estimated by pathology staff to have ranged between 1 and 5 days. An increased duration of fixation has been shown to decrease the ability to subsequently extract nucleic acids suitable for PCR analysis (9, 17, 22). It was, therefore, necessary to carefully assess each sample for the integrity of the RNA extracted.
Initially we investigated the ability of RT-PCR to detect C. albicans 18S rRNA due to its relatively high cellular abundance in comparison to that of the mRNA of single-copy genes. In seven out of nine CHC samples and four out of four PC samples, the 131-bp product expected for amplification of the C. albicans 18S rRNA was detected whereas no PCR product was seen in the control RT reactions (data not shown). As an additional negative control we extracted RNA from a 6-month-old FFPE sample of a periapical granuloma known to have been formalin fixed for less than 24 h and which was considered highly unlikely to contain C. albicans cells. To test the integrity of the negative-control RNA it was reverse transcribed with random hexamers and a 346-bp fragment of the human 18S rRNA transcript amplified using Ambion primers (PCR conditions: 35 cycles; annealing temperature, 57°C). No C. albicans 18S rRNA transcript could be detected in this sample with the previously described RT-PCR protocol (data not shown). Only FFPE CHC and PC samples in which the C. albicans 18S rRNA transcript was successfully detected were further investigated.
Detection of single-copy C. albicans genes.
To investigate whether C. albicans mRNA transcripts could also be detected in FFPE archival samples we attempted to amplify transcripts of the constitutively expressed ACT1 and EFB1 housekeeping genes. Both transcripts could be detected in five out of seven of the CHC samples investigated and two out of four of the PC samples investigated; again, neither transcript was detected in the FFPE periapical granuloma negative control (data not shown). Only samples in which both the ACT1 and EFB1 transcripts had been detected were further investigated for the expression of other genes of interest. The oldest of these biopsy samples was taken in 1996, demonstrating that samples up to 8 years old (at least) contained C. albicans mRNA that could be detected with our techniques.
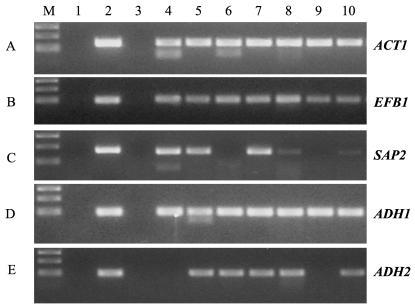
Nine members of the C. albicans SAP gene family have been extensively investigated, as their products are considered important virulence factors (4, 7, 25). While there appears to be considerable differential expression of the SAP genes, SAP2 mRNA has been shown to be one of the predominant proteinase gene transcripts in both oral candidiasis and asymptomatic oral carriage (14). Using a primer pair specific for the SAP2 gene transcript (14) we examined RNA extracted from FFPE CHC and PC samples. We successfully detected SAP2 mRNA in four out of the five CHC samples and one of the two CMC samples (Fig. 3), confirming that the SAP2 transcript is expressed in both forms of oral candidiasis. The inability to detect the SAP2 transcript in two samples could be due to specific repression of SAP2 transcription or to insufficient C. albicans RNA being extracted from these samples.
FIG. 3.
Detection of single-copy C. albicans gene mRNAs in FFPE human archival biopsies. PCR amplification was performed using primer pairs for the ACT1 (A), EFB1 (B), SAP2 (C), ADH1 (D), and ADH2 (E) genes. Template cDNA was prepared by multiplex RT with RNA extracted from FFPE samples of CHC biopsies (lanes 4 to 8), PC biopsies (lanes 9 to 10), and a periapical granuloma biopsy (lane 3) as a negative control. Lane 1, negative control of PCR without cDNA template; lane 2, positive control of PCR using either 0.1 ng of C. albicans cDNA (ACT1 and EFB1) or 0.1 ng of C. albicans genomic DNA (SAP2, ADH1, and ADH2). Lane M: DNA molecular weight markers.
Adh is an important intracellular enzyme in C. albicans and an immunodominant antigen with several isoforms, and Adh can also be found among cell wall proteins (5). Using primer pairs specific for the ADH1 and ADH2 gene transcripts we looked for their presence in RNA extracted from FFPE CHC and PC samples. The ADH1 mRNA was detected in all samples (Fig. 3), but the ADH2 transcript was detected in only four out of five CHC samples and one of two PC samples. Although ADH2 mRNA was not detected in RNA extracted from two samples, ACT1, EFB1, and ADH1 mRNAs were, indicating the possible differential expression of ADH2 in the samples.
To our knowledge this is the first reported detection of C. albicans mRNA in archival human biopsies. The transcript detection techniques described in this study also appear to be significantly more sensitive than those previously described for an investigation of FFPE samples from a mouse oral infection (22). Future studies could use real-time RT-PCR techniques to accurately measure mRNA levels (3, 8) in biopsy samples. The ability to correlate differential levels of C. albicans gene expression to disease outcomes could provide new diagnostic markers for OSSC and elucidate any potential role of C. albicans in oncogenesis.
Acknowledgments
This work was approved by the University of Otago Human Ethics Committee and supported by University of Otago research grant 200200608.
We gratefully acknowledge the technical assistance provided by Lynda Horne.
REFERENCES
- 1.Barrett, A. W., V. J. Kingsmill, and P. M. Speight. 1998. The frequency of fungal infection in biopsies of oral mucosal lesions. Oral Dis. 4:26-31. [DOI] [PubMed] [Google Scholar]
- 2.Bertram, G., R. K. Swoboda, G. W. Gooday, N. A. Gow, and A. J. Brown. 1996. Structure and regulation of the Candida albicans ADH1 gene encoding an immunogenic alcohol dehydrogenase. Yeast 12:115-127. [DOI] [PubMed] [Google Scholar]
- 3.Bustin, S. A. 2002. Quantification of mRNA using real-time reverse transcription PCR (RT-PCR): trends and problems. J. Mol. Endocrinol. 29:23-39. [DOI] [PubMed] [Google Scholar]
- 4.Calderone, R. A., and W. A. Fonzi. 2001. Virulence factors of Candida albicans. Trends Microbiol. 9:327-335. [DOI] [PubMed] [Google Scholar]
- 5.Crowe, J. D., I. K. Sievwright, G. C. Auld, N. R. Moore, N. A. Gow, and N. A. Booth. 2003. Candida albicans binds human plasminogen: identification of eight plasminogen-binding proteins. Mol. Microbiol. 47:1637-1651. [DOI] [PubMed] [Google Scholar]
- 6.Holmes, A. R., and M. G. Shepherd. 1988. Nutritional factors determine germ tube formation in Candida albicans. J. Med. Vet. Mycol. 26:127-131. [PubMed] [Google Scholar]
- 7.Hube, B., and J. Naglik. 2001. Candida albicans proteinases: resolving the mystery of a gene family. Microbiology 147:1997-2005. [DOI] [PubMed] [Google Scholar]
- 8.Lehmann, U., and H. Kreipe. 2001. Real-time PCR analysis of DNA and RNA extracted from formalin-fixed and paraffin-embedded biopsies. Methods 25:409-418. [DOI] [PubMed] [Google Scholar]
- 9.Lewis, F., N. J. Maughan, V. Smith, K. Hillan, and P. Quirke. 2001. Unlocking the archive—gene expression in paraffin-embedded tissue. J. Pathol. 195:66-71. [DOI] [PubMed] [Google Scholar]
- 10.Losberger, C., and J. F. Ernst. 1989. Sequence of the Candida albicans gene encoding actin. Nucleic Acids Res. 17:9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Maneu, V., A. M. Cervera, J. P. Martinez, and D. Gozalbo. 1996. Molecular cloning and characterization of a Candida albicans gene (EFB1) coding for the elongation factor EF-1 beta. FEMS Microbiol. Lett. 145:157-162. [DOI] [PubMed] [Google Scholar]
- 12.Masuda, N., T. Ohnishi, S. Kawamoto, M. Monden, and K. Okubo. 1999. Analysis of chemical modification of RNA from formalin-fixed samples and optimization of molecular biology applications for such samples. Nucleic Acids Res. 27:4436-4443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McCullough, M., M. Jaber, A. W. Barrett, L. Bain, P. M. Speight, and S. R. Porter. 2002. Oral yeast carriage correlates with presence of oral epithelial dysplasia. Oral Oncol. 38:391-393. [DOI] [PubMed] [Google Scholar]
- 14.Naglik, J. R., G. Newport, T. C. White, L. L. Fernandes-Naglik, J. S. Greenspan, D. Greenspan, S. P. Sweet, S. J. Challacombe, and N. Agabian. 1999. In vivo analysis of secreted aspartyl proteinase expression in human oral candidiasis. Infect. Immun. 67:2482-2490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nagy, K. N., I. Sonkodi, I. Szoke, E. Nagy, and H. N. Newman. 1998. The microflora associated with human oral carcinomas. Oral Oncol. 34:304-308. [PubMed] [Google Scholar]
- 16.Okeke, C. N., R. Tsuboi, and H. Ogawa. 2001. Quantification of Candida albicans actin mRNA by the LightCycler system as a means of assessing viability in a model of cutaneous candidiasis. J. Clin. Microbiol. 39:3491-3494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.O'Leary, J. J., G. Browne, R. J. Landers, M. Crowley, I. B. Healy, J. T. Street, A. M. Pollock, J. Murphy, M. I. Johnson, F. A. Lewis, et al. 1994. The importance of fixation procedures on DNA template and its suitability for solution-phase polymerase chain reaction and PCR in situ hybridization. Histochem. J. 26:337-346. [DOI] [PubMed] [Google Scholar]
- 18.Pindborg, J. J., P. A. Reichart, C. J. Smith, and I. van der Waal. 1997. Histological typing of cancer and precancer of the oral mucosa. World Health Organization international histological classification of tumours, 2nd ed. Springer, Berlin, Germany.
- 19.Renstrup, G. 1970. Occurrence of candida in oral leukoplakias. Acta Pathol. Microbiol. Scand. Sect. B Microbiol. Immunol. 78:421-424. [DOI] [PubMed] [Google Scholar]
- 20.Ross, I. K., F. De Bernardis, G. W. Emerson, A. Cassone, and P. A. Sullivan. 1990. The secreted aspartate proteinase of Candida albicans: physiology of secretion and virulence of a proteinase-deficient mutant. J. Gen. Microbiol. 136:687-694. [DOI] [PubMed] [Google Scholar]
- 21.Schmitt, M. E., T. A. Brown, and B. L. Trumpower. 1990. A rapid and simple method for preparation of RNA from Saccharomyces cerevisiae. Nucleic Acids Res. 18:3091-3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Schofield, D. A., C. Westwater, E. E. Paulling, P. J. Nicholas, and E. Balish. 2003. Detection of Candida albicans mRNA from formalin-fixed, paraffin-embedded mouse tissues by nested reverse transcription-PCR. J. Clin. Microbiol. 41:831-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sitheeque, M. A., and L. P. Samaranayake. 2003. Chronic hyperplastic candidosis/candidiasis (candidal leukoplakia). Crit. Rev. Oral Biol. Med. 14:253-267. [DOI] [PubMed] [Google Scholar]
- 24.Tait, E., M. C. Simon, S. King, A. J. Brown, N. A. Gow, and D. J. Shaw. 1997. A Candida albicans genome project: cosmid contigs, physical mapping, and gene isolation. Fungal Genet. Biol. 21:308-314. [DOI] [PubMed] [Google Scholar]
- 25.White, T. C., and N. Agabian. 1995. Candida albicans secreted aspartyl proteinases: isoenzyme pattern is determined by cell type, and levels are determined by environmental factors. J. Bacteriol. 177:5215-5221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wright, R. J., A. Carne, A. D. Hieber, I. L. Lamont, G. W. Emerson, and P. A. Sullivan. 1992. A second gene for a secreted aspartate proteinase in Candida albicans. J. Bacteriol. 174:7848-7853. [DOI] [PMC free article] [PubMed] [Google Scholar]