Abstract
Human papillomavirus type 16 (HPV-16) viral load in cervicovaginal lavage samples collected from 66 human immunodeficiency virus-seropositive women was inversely correlated with blood CD4 count (P = 0.002). HPV-16 viral load was 81-fold higher in women with cervical smears suggestive of high-grade lesions (median, 4,425,883 copies/μg of DNA) than in women with normal smears (median, 54,576), controlling for age (P = 0.006).
Oncogenic human papillomaviruses (HPV) cause squamous intraepithelial lesions (SIL) and cancer of the uterine cervix in human immunodeficiency virus (HIV)-seronegative and -seropositive women (2). HIV-seropositive women are at increased risk compared to HIV-seronegative women for cervical HPV infection and SIL (9). Although HPV infects the genital tract of most HIV-seropositive women, only a minority of HPV-infected women develop persistent HPV infection that may progress into SIL and cancer (2). Biomarkers of HPV infection that could identify HPV-infected women at high risk for persistent infection and SIL would improve screening algorithms for management of HPV-induced cervical lesions.
Several studies suggested that an increased HPV DNA viral load could be a candidate marker for the presence of cervical SIL in HIV-seropositive women (12, 17, 18). Similar studies with HIV-seronegative women on the predictive value of HPV viral load for high-grade-SIL (HSIL) detection yielded conflicting results (4, 8, 10, 13, 14, 19). Some of the assays used in the latter studies were not HPV type specific or were semiquantitative, while assays using PCR did not take into account the presence of amplification inhibitors or did not estimate the quantity of cellular DNA tested. The latter two factors have been shown to influence HPV viral load determinations (7, 14).
A recent study validated a real-time PCR assay that measures the quantity of human cells and HPV DNA contained in biological fluids and uses internal controls to screen for the presence of PCR inhibitors (7). That study also reported that samples could selectively inhibit HPV-16 amplification (7). We present here cross-sectional results of cervical HPV-16 viral loads measured in a cohort of Canadian HIV-seropositive women. HPV-16 viral loads measured in cervicovaginal lavage (CVL) samples correlated with blood CD4+ counts and were associated with cervical SIL.
Women infected with HPV-16 were selected from participants recruited in The Canadian Women's HIV Study, a cross-sectional and cohort study on the relationships between HPV and HIV infection and development of cervical SIL (3). Participants in the study were recruited from 1993 to 2000 across Canada from clinics involved in the care of HIV-infected patients and gave written informed consent (3). Ethics committees of each participating institution approved the study protocol. The demographic characteristics of the HIV-seropositive women have been described elsewhere (3). CVL samples from 732 HIV-seropositive women were screened at inclusion and at 6-month intervals for HPV infection using the MY09-MY11-HMB01 consensus L1 PCR as previously described (1), concurrently with a cervical Pap smear obtained with a Cytobrush and Ayre spatula, and blood CD4+ count. Cytology smears were interpreted in one central pathology laboratory and confirmed by one pathologist dedicated to this study. A standardized questionnaire was completed at each visit for all patients (3).
CVL samples were lysed with 0.8% (vol/vol) Tween 20 and digested with 250 μg of proteinase K/ml as previously described (1). Only cross-sectional results were considered for the present report. Sixty-six women were shown to be infected with HPV-16 and were selected for the evaluation of the HPV-16 viral load. The first HPV-16-positive CVL lysates from the 66 women were further screened for the presence of inhibitors by real-time PCR. One thousand copies of HPV-16 internal control and 1,000 copies of β-globin internal control were mixed in separate capillaries with 2 μl of CVL lysate and tested in a Light Cycler PCR and detection system (Roche Molecular Systems), as previously described (6, 7). The presence of PCR inhibitors was suspected when the 1,000-copy internal control generated a signal corresponding to less than 700 copies for one or both internal controls, as explained in detail in previous work (7). Six samples containing inhibitors (both internal controls were inhibited by one sample, and the HPV-16 internal control only was inhibited by five samples) were retested with both internal controls after a 1/10 dilution of lysate (n = 4) or after DNA purification (n = 2) with Master Pure. Two microliters of processed samples without inhibition was then tested in duplicate with a 50 nM concentration of probe U6862 and a 0.3 μM concentration of each HPV-16 primer (U6564 [5′-CCTTATTGGTTACAACGAGCAC] and L7012 [5′-GCGTCCTAAAGGAAACTGATCTA]) for HPV-16 quantitation and in duplicate with probe U62049 and primers U61992 and L62240 for β-globin quantitation (7). Cycle threshold values were compared to that of external standards from a titration curve of HPV-16 DNA that were obtained by serial 10-fold dilutions of an HPV-16 plasmid that had been kindly provided by H. zur Hausen, in a fixed amount of 150 ng of human fibroblast DNA in 10 mM Tris-HCl (pH 8.2). Titration curves of human DNA were obtained by serial dilutions of a stock of human placental DNA D4642 (Sigma, St. Louis, Mo.) in 10 mM Tris-HCl (pH 8.2).
Categorical variables were compared with the Fisher exact test. Age, CD4 counts, and HPV-16 loads were compared with a Mann-Whitney U test. Correlation was measured with the Spearman rank correlation coefficient. The effect of age on the association between HPV-16 viral load and cervical SIL was controlled for by multivariate analysis using stepwise logistic regression. Markers of sexual activity are considered remote variables upstream of HPV in the causal pathway under study and were therefore not considered in final models.
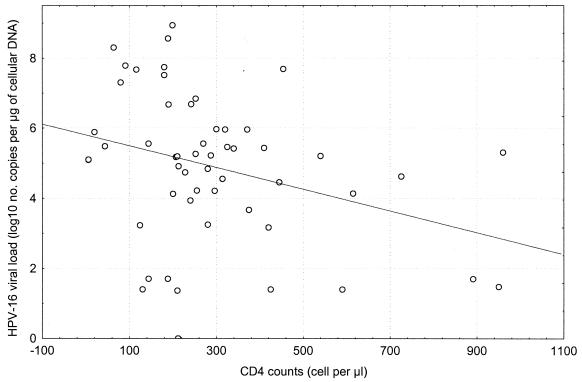
We first investigated in a cross-sectional fashion the association between HPV-16 viral load and blood CD4+ counts measured at the same visit for 63 of 66 women. The median CD4 cell counts reached 262 cells/mm3 (range of 6 to 960 cells/mm3). The median HPV-16 viral load was 208,291 HPV-16 DNA copies per μg of cellular DNA (range of 0 to 862,586,250 copies). As shown in Fig. 1, HPV-16 viral load increased with decreases in CD4 count. Forty-six (69.7%) women were infected with at least one HPV type other than 16. The number of types other than 16 per sample (median, 2; range, 0 to 7) did not correlate with CD4 cell counts (r = −0.13; P = 0.16).
FIG. 1.
Correlation between HPV-16 viral load measured in CVL samples obtained from 63 HIV-seropositive women and blood CD4 cell counts. The Shapiro-Wilks W test indicated that the CD4 cell counts and the HPV-16 viral loads were not normally distributed (P < 0.002). The Spearman rank correlation coefficient was −0.39 (P = 0.002).
Fifty-six of the 66 HIV-seropositive women had cervical smears that could be interpreted and that had been obtained concurrently with CVL samples (Table 1). HPV-16 viral load correlated with SIL grade (r = 0.34; P = 0.01). HPV-16 viral load measured in CVL samples was 81-fold greater in women with cervical smears suggestive of HSIL than in women with normal smears (Table 1). HPV types other than 16 were detected in 25 (61%) of 41 women with normal smears and 5 (63%) of 8 women with smears suggestive of HSIL (P = 1.0). CD4 counts (P = 0.11) and age (P = 0.18) were not associated with HSIL. However, age was associated with low-grade SIL (LSIL) (P = 0.02) and correlated with HPV-16 viral load (r = −0.279; P = 0.002). Controlling for age in multivariate analysis, HPV-16 viral load remained significantly associated with HSIL (P = 0.008). There was no significant difference between women with smears suggestive of LSIL and women with normal smears, but there was a tendency for women with HSIL to have a greater HPV-16 viral load than women with LSIL (P = 0.07). We could not correlate HPV-16 viral load with plasma HIV viral load, since most participants had been recruited before the advent of HIV viral load testing.
TABLE 1.
HPV-16 viral load and cervical SIL
| Cytology results (no. of women) | HPV-16 viral load (103)
|
CD4+ counts
|
||||||
|---|---|---|---|---|---|---|---|---|
| Median | Range | P value | r (P value) | Median | Range | P value | r (P value) | |
| HSIL (8) | 4,426 | 14-199,526 | 0.006 | 0.33 (0.013) | 122 | 6-860 | 0.11 | −0.13 (0.32) |
| LSIL (9) | 724 | 0-46,774 | 0.48 | 275 | 140-420 | 0.70 | ||
| Normal (41) | 55 | 0.02-870,963 | Reference | 283 | 6-960 | Reference | ||
This is the first report on the role of HPV-16 viral load in cervical HPV infection and SIL in HIV-infected women combining real-time PCR and screening for PCR inhibition. The assay we used was demonstrated to be specific for HPV-16 and does not cross-react with other HPV types (6, 7, 14). Several studies reported a higher quantity of HPV DNA in HIV-seropositive than HIV-seronegative women but did not evaluate the impact of HIV-induced immunosuppression on HPV viral load (5, 11, 12, 16, 18). One of these studies demonstrated that the amount of HPV DNA increased in HIV-negative and HIV-seropositive women with the presence and grade of cervical lesions (18). HIV-seropositive women who were colposcopically normal had a sevenfold-greater amount of HPV DNA than HIV-seronegative women (18). This conclusion was confirmed by a third group measuring HPV DNA in self-obtained vaginal swabs (11) but was not reached by a fourth group (16). HPV viral load also varied with age (11). One of these studies, which used Hybrid Capture and a semiquantitative PCR assay in a cross-sectional study of 150 HIV-seropositive women (12), illustrates the difficulty in estimating the HPV viral DNA copy number in clinical samples. A high signal obtained with PCR but not with Hybrid Capture was associated with a higher risk of lesion (12). A recent study using real-time PCR assays against several HPV types reported that higher HPV viral loads were measured in women with CD4+ counts below 200 cells/μl (17). A significant correlation between the level of immunodeficiency and HPV-16 viral load was demonstrated in our cohort, with women with lower CD4+-cell counts having higher HPV-16 viral loads.
To date, results on the value of HPV DNA viral load in identifying women with cervical SIL remain controversial, and the role of viral load in disease progression is still debated (4, 5, 8, 10, 12, 14, 15, 18, 19). While cervical carcinoma in situ has been found in women with consistently high HPV-16 viral loads, HSIL is not always predicted by high viral loads (8, 14, 19). To be truly quantitative, a real-time PCR assay has to measure the cell content of samples and screen for the presence of inhibitors. By combining real-time PCR and the use of internal controls to screen for PCR inhibitors, we demonstrated here that high HPV-16 viral loads were associated with HSIL. Since women included in this study had been screened initially as positive for HPV-16, the differences in HPV-16 viral load between women with normal smears and women with LSIL or HSIL have probably been underestimated. The small number of participants in this study did not allow us to establish an association of HSIL with blood CD4 counts. Further follow-up of HPV-16-infected women in our cohort to determine whether HPV-16 viral load testing could identify women with persistent infection at highest risk for SIL is warranted. However, the important overlap of HPV-16 viral load values between women with normal smears and those with SIL could be a limitation for the usefulness in clinical practice of viral load measurements at one visit.
Acknowledgments
We thank Diane Gaudreault and Diane Bronsard for processing genital samples.
This study was supported by the Canadian Foundation for AIDS Research. The Canadian Institutes for Health Research supports The Canadian Women's HIV Study cohort. F.C. is a Chercheur National supported by the Fonds de la Recherche en Santé du Québec (FRSQ) and by le Réseau FRSQ Maladies Infectieuses-SIDA.
Participating members of The Canadian Women's HIV Study Group include the following: Janet Conners, Lynn Johnston, Wally Schlech, and Arlo Yuzicappi-Fayant, Halifax, Canada; Ted Ralph, London, Canada; François Coutlée, Catherine Hankins, Marina Klein, Grégoire Noël, and Chantal Perron, Montréal, Canada; Garry Victor, Ottawa, Canada; Kurt Williams, Saskatoon, Canada; Alain Piché, Sherbrooke, Canada; Louise Binder, Anita Rachlis, and Sharon Walmsley, Toronto, Canada; and Paula Braitstein, David Burdge, Deborah Money, and Julio Montaner, Vancouver, Canada.
REFERENCES
- 1.Coutlée, F., C. Hankins, N. Lapointe, J. Gill, B. Romanowski, S. Shafran, R. Grimshaw, D. Haase, W. Schlech, J. Sellors, F. Smaill, M. Boucher, M. Chateauvert, J. Falutz, R. Lalonde, J. Macleod, G. Noel, J. P. Routy, E. Toma, G. Garber, G. Victor, S. Trottier, P. Berger, L. Friedland, and D. Keystone. 1997. Comparison between vaginal tampon and cervicovaginal lavage specimen collection for detection of human papillomavirus DNA by the polymerase chain reaction. J. Med. Virol. 51:42-47. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjose, S., and J. Palefsky. 2002. Cervical and anal HPV infections in HIV positive women and men. Virus Res. 89:201-211. [DOI] [PubMed] [Google Scholar]
- 3.Hankins, C., F. Coutlee, N. Lapointe, P. Simard, T. Tran, J. Samson, L. Hum, and The Canadian Women's HIV Study Group. 1999. Prevalence of risk factors associated with human papillomavirus infection in women living with HIV. Can. Med. Assoc. J. 160:185-191. [PMC free article] [PubMed] [Google Scholar]
- 4.Josefsson, A. M., P. K. E. Magnusson, N. Ylitalo, P. Sorensen, P. Qwarforth-Tubbin, P. K. Anderson, M. Melbye, H. O. Adami, and U. B. Gyllensten. 2000. Viral load of human papilloma virus 16 as a determinant for development of cervical carcinoma in situ: a nested case-control study. Lancet 355:2189-2193. [DOI] [PubMed] [Google Scholar]
- 5.Klein, R. S., G. Y. F. Ho, S. H. Vermund, I. Fleming, and R. D. Burk. 1994. Risk factors for squamous intraepithelial lesions on pap smear in women at risk for human immunodeficiency virus infection. J. Infect. Dis. 170:404-409. [DOI] [PubMed] [Google Scholar]
- 6.Lefevre, J., C. Hankins, K. Pourreaux, The Canadian Women's HIV Study Group, and F. Coutlée. 2004. Prevalence of selective inhibition of HPV-16 DNA amplification in cervicovaginal lavages. J. Med. Virol. 72:132-137. [DOI] [PubMed] [Google Scholar]
- 7.Lefevre, J., C. Hankins, K. Pourreaux, H. Voyer, The Canadian Women's HIV Study Group, and F. Coutlée. 2003. Internal controls for quantitation of HPV-16 and β-globin DNA in cervicovaginal lavages. J. Virol. Methods 114:135-144. [DOI] [PubMed] [Google Scholar]
- 8.Lorincz, A. T., P. E. Castle, M. E. Sherman, D. R. Scott, A. G. Glass, S. Wacholder, B. B. Rush, P. E. Gravitt, J. E. Schussler, and M. Schiffman. 2002. Viral load of human papillomavirus and risk of CIN3 or cervical cancer. Lancet 360:228-229. [DOI] [PubMed] [Google Scholar]
- 9.Moscicki, A. B., J. H. Ellenberg, S. H. Vermund, C. A. Holland, T. Darragh, P. A. Crowley-Nowick, L. Levin, and C. M. Wilson. 2000. Prevalence of and risks for cervical human papillomavirus infection and squamous intraepithelial lesions in adolescent girls—impact of infection with human immunodeficiency virus. Arch. Pediatr. Adolescent Med. 154:127-134. [DOI] [PubMed] [Google Scholar]
- 10.Schlecht, N. F., A. Trevisan, E. Duarte-Franco, T. E. Rohan, A. Ferenczy, Villa, L. L., and E. L. Franco. 2003. Viral load as a predictor of the risk of cervical intraepithelial neoplasia. Int. J. Cancer 103:519-524. [DOI] [PubMed] [Google Scholar]
- 11.Serwadda, D., M. J. Wawer, K. V. Shah, N. K. Sewankambo, R. Daniel, C. Li, A. Lorincz, M. P. Meehan, F. Wabwire-Mangen, and R. H. Gray. 1999. Use of a hybrid capture assay of self-collected vaginal swabs in rural Uganda for detection of human papillomavirus. J. Infect. Dis. 180:1316-1319. [DOI] [PubMed] [Google Scholar]
- 12.Shah, K. V., L. Solomon, R. Daniel, S. Cohn, and D. Vlahov. 1997. Comparison of PCR and hybrid capture methods for detection of human papillomavirus in injection drug-using women at high risk of human immunodeficiency virus infection. J. Clin. Microbiol. 35:517-519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sun, X. W., A. Ferenczy, D. Johnson, J. P. Koulos, O. Lungu, R. M. Richart, and T. C. Wright, Jr. 1995. Evaluation of the hybrid capture human papillomavirus deoxyribonucleic acid detection test. Am. J. Obstet. Gynecol. 173:1432-1437. [DOI] [PubMed] [Google Scholar]
- 14.Swan, D. C., R. A. Tucker, G. Tortolero-Luna, M. F. Mitchell, L. Wideroff, E. R. Unger, R. A. Nisenbaum, W. C. Reeves, and J. P. Icenogle. 1999. Human papillomavirus (HPV) DNA copy number is dependent on grade of cervical disease and HPV type. J. Clin. Microbiol. 37:1030-1034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.van Duin, M., P. J. F. Snijders, H. F. J. Schrijnemakers, F. J. Voorhorst, L. Rozendaal, M. A. E. Nobbenhuis, A. J. C. van den Brule, R. H. M. Verheijen, T. J. Helmerhorst, and C. J. L. M. Meijer. 2002. Human papillomavirus 16 load in normal and abnormal cervical scrapes: an indicator of CIN II/III and viral clearance. Int. J. Cancer 98:590-595. [DOI] [PubMed] [Google Scholar]
- 16.Vernon, S. D., W. C. Reeves, K. A. Clancy, M. Laga, M. St. Louis, H. E. Gary, R. W. Ryder, A. T. Manoka, and J. P. Icenogle. 1994. A longitudinal study of human papillomavirus DNA detection in human immunodeficiency virus type 1-seropositive and -seronegative women. J. Infect. Dis. 169:1108-1112. [DOI] [PubMed] [Google Scholar]
- 17.Weissenborn, S. J., A. M. Funke, M. Hellmich, P. Mallmann, P. G. Fuschs, H. J. Pfister, and U. Wieland. 2003. Oncogenic human papillomavirus DNA loads in human immunodeficiency virus-positive women with high-grade cervical lesions are strongly elevated. J. Clin. Microbiol. 41:2763-2767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Womack, S. D., Z. M. Chirenje, L. Gaffikin, P. D. Blumenthal, J. A. McGrath, T. Chipato, S. Ngwalle, M. Munjoma, and K. V. Shah. 2000. HPV-based cervical cancer screening in a population at high risk for HIV infection. Int. J. Cancer 85:206-210. [PubMed] [Google Scholar]
- 19.Ylitalo, N., P. Sorensen, A. M. Josefsson, P. K. E. Magnusson, P. K. Anderson, J. Ponten, H. O. Adami, U. B. Gyllensten, and M. Melbye. 2000. Consistent high viral load of human papillomavirus 16 and risk of cervical carcinoma in situ: a nested case-control study. Lancet 355:2194-2198. [DOI] [PubMed] [Google Scholar]