Abstract
Blood glucose monitoring has evolved over the last century. The concept of adequate glycemic control and minimum glycemic variability requires an ideal, accurate and reliable glucose monitoring system. The search for an ideal blood glucose monitoring system still continues. This review explains the various blood glucose monitoring systems with special focus on the monitoring systems like self- monitored blood glucose (SMBG) and continuous glucose monitoring system (CGMS). It also focuses on the newer concepts of blood glucose monitoring and their incorporation in routine clinical management of diabetes mellitus.
Keywords: Blood glucose monitoring system, continuous glucose monitoring, glycated haemoglobin, glycemic variability, self-monitored blood glucose
INTRODUCTION
The effect of adequate glycemic control on the progression of micro-vascular and macro-vascular complications have been well described by the Diabetes Control and Complications Trial (DCCT)[1] and the United Kingdom Prospective Diabetes Study (UKPDS)[2] trials. The concept of adequate glycemic control and minimum glycemic variability requires an ideal, accurate and reliable glucose monitoring system. This quest to achieve an adequate glycemic control has led to the development of science of blood glucose monitoring systems.
HISTORY AND EVOLUTION OF BLOOD GLUCOSE MONITORING SYSTEMS
Blood glucose monitoring has evolved from obscure methods like urine tasting to colorimetric blood glucose strips. Then came the era of the glucose sensors and manually calibrated glucometers. Presently, we are in the modern era with auto-calibrated accurate glucometers with biosensors for SMBG. Estimation of glycated hemoglobin (HBA1c) remains the gold standard of glucose monitoring as an end point for drug intervention trials. It is postulated that glycemic variability and glycemic excursions are the basis for early development of complications through the development of oxidative stress and free radical injury.[3] To achieve minimum glycemic variability the technique of CGMS was developed making the dream of artificial pancreas a much possible realty.
Hemoglobin A1c (HBA1c)
In 1968, Rahbar first showed that hemoglobin A1 represented a glycated form of hemoglobin which was increased in diabetes.[4] HBA1c measures a physiologic process of non-enzymatic glycation, which is a surrogate for glycation of other proteins in the body and a precursor of diabetes complications. Therefore, the HBA1c represents a measurable indirect estimate of complications of diabetes.[5] It gives an average estimate of plasma glucose over the preceding three months (equal to the lifespan of red blood cells). However, 50% contribution is of the last one month.
Monnier et al., have described an important concept of relative contributions of the fasting and the post-prandial blood glucose levels to the HBA1c.[6] For HBA1c less than 8.4% is the post-prandial glucose values, which are more contributory and as the HBA1c increases, the relative contribution of fasting plasma glucose values increases.
The Diabetes Control and Complications Trial (DCCT)[1] and UK Prospective Diabetes Study (UKPDS),[2] both long-term studies had HBA1c as the primary index of glycemic control. Since then, utility of HBA1c has been well validated as an end point in therapeutic diabetes trials.
HBA1c for diagnosis of diabetes
For many years the idea that HBA1c could be used as an objective measurement of diabetes control was studied. The A1C-Derived Average Glucose (ADAG) study which included 643 participants established a validated relationship between A1C and average glucose across a range of diabetes types and patient populations.[7]
HBA1c has the least biologic variability as compared to fasting and post-prandial plasma glucose values albeit the assay is certified by NGSP.[8] It has the added flexibility of not requiring a fasting state. An International Expert Committee in 2009 reported on the diagnostic role of HBA1c proposing a cut off of >6.5%.[9] Presently the American Diabetes Association (ADA) has suggested 5.7-6.4% as pre-diabetes and proposed diabetes prevention interventions in this group.[10]
Racial and ethnic variability
Racial and ethnic differences are also known to occur in HBA1c levels as shown by two studies from Indian subcontinent. Padala et al., in a regional study from North India showed that for Indian population if the ADA cutoffs are used, 38% of the patients are underdiagnosed.[11] He has proposed cutoff of 6.1% for Indian population which is also validated by V. Mohan et al., in a similar study from South India.[12]
Limitations
HBA1c is assay dependent and hence to achieve reliability the assays have to be certified by NGSP (National Glycosylation Standardisation Program).[8] Without standardization, reported results between laboratories may not be comparable, even if both laboratories use the same assay method.
An important limitation is that it does not depict the short-term glycemic variability and hence is of no value for acute or short-term decision making. Hence it may not be suitable in cases where adequate glycemic control has to be achieved and maintained in a short time like gestational diabetes mellitus.
All conditions which qualitatively and quantitatively affect the lifespan of red blood cells as well as the hemoglobinopathies result in non-glycemic variations and unreliable HBA1c readings. Hence HBA1c has to be read with caution in conditions like anemia and chronic kidney disease.[13] Glycemia independent increases in HBA1c is also known to occur with increasing age of the patient though studies are lacking.[13]
HBA1c as point of care measurement
Usage of HBA1c as a point of care device is now available as the home HBA1c kits; however, this method is fraught with pre-analytical and analytical difficulties resulting in errors and inaccuracies.[14,15,16]
Recommendations for HBA1c ADA 2013[10]
Perform at least twice yearly in patients meeting treatment goals and have stable glycemic control
Perform quarterly in patients whose therapy has changed or who are not meeting glycemic goals.
USE OF OTHER BIOMARKERS FOR GLUCOSE MONITORING
Fructosamine assays
Measurement of glycated serum proteins (fructosamine) depicts relatively short-term changes (1-2 weeks) in glycemic status. Hence they may be utilised in certain situations like gestational diabetes or in those where HBA1c is unreliable.[17] However, further studies are needed to determine if the test provides useful clinical information in these situations. Unlike the HBA1c, fructosamine assay has not yet been validated with progression of diabetes complications.
1,5 Anhydroglucitol assay (1,5-AG)
1,5 anhydroglucitol is reabsorbed in the renal tubule with competitive inhibition by glucose.[18] Low serum levels of 1,5 anhydroglucitol reflect glycemic excursions. 1,5-AG may be useful as a complementary marker to A1C to assess glycemic control in moderately controlled patients with diabetes but requires validation in long term studies assessing complications of diabetes.[18]
Self- monitored blood glucose (SMBG)
Self-monitored blood glucose is the easiest and the most widely used method of short-term glucose monitoring throughout the world. Fingerstick glucose testing using a glucometer is the prototype of SMBG. These points of care devices have revolutionized the concept of home-based glucose monitoring.
It has been 40 years since Anton Clemens at Ames research Division, Indiana USA developed the first glucose meter which combined dry chemistry test strips with reflectance photometry to measure blood glucose.[19] Presently there are a variety of glucose meters available in the market; however, clinicians and patients should be aware of the features as well as the disadvantages of each one of them.
Types of glucometers
Glucometers can be broadly classified into two types depending on the enzymes used: Glucose oxidase and glucose dehydrogenase (GDH) with various cofactors like (FAD/NAD/Pyrroloquinoline). Each of these types has their own advantages and disadvantages.
Glucometers based on glucose oxidase method have high specificity and do not cross react with other sugars. However, these glucometers are affected by oxygen concentration in the blood with hypoxia resulting in overestimation of blood glucose. Hence these glucometers have to be used with caution in intensive care units, high altitude and conditions associated with hypoxia.[20]
Glucometers based on glucose dehydrogenase method are not affected by oxygen concentration but they cross-react with other sugars like maltose resulting in overestimation of blood glucose. The Food and Drug Administration (FDA) in 2009 issued a warning for use of GDH-based glucometers in patients on peritoneal dialysis. Pyrroloquinoline, used as a cofactor in these meters cross-reacts with maltose produced by conversion of icodextrin, a content of peritoneal dialysis fluid. These cross-reactions have resulted in fatal overestimations of blood glucose.[21]
Technology
Enzymatic reactions convert glucose into either electrons or free radicals like hydrogen peroxide which are then measured either by an electrode (amperometric method) or by colorimetric reaction (photometric).[20] Modern glucometers work on biosensor technology and require small sample size as low as 0.3 μl of blood as compared to the 50 μl of blood used in the earlier obsolete glucometers. Ideally sides of the fingers of the hands are to be used for SMBG and only a single lancet prick to be used.
Frequency and pattern of SMBG
A European expert recommendation for SMBG in type-2 diabetes patients issued in 2011 recommended two patterns of SMBG depending on the therapy and the basal control of the patient.[22]
Less intensive pattern
It involves paired meal testing (pre- and post-prandial) once in a day to identify the dynamics of glycemia in response to a meal. The duration of testing is one paired meal testing per month, 1 week/month, 3-7 days/week, continuous paired testing depending on individual case. It is to be used in patients on medical nutrition therapy or a single oral hypoglycemic agent (OHA).[22]
Intensive pattern
Intensive testing involves seven tests per day over a minimum of 3 days up to 7 days. It focuses on the dynamics of glucose levels per day and tries to identify the variability of glucose levels. The duration of testing is a minimum of 3 days/week to 1 week/month, with continuous SMBG.[22] It is mainly used in those with poor metabolic control and those on multi-dose insulin injections or multiple OHA with basal insulin.[22]
Errors and accuracy
Accuracy of SMBG depends on the reliability and accuracy of the glucometers. The errors are classified as pre-analytical, analytical and post-analytical errors. A simple example of pre-analytical error is not washing ones hands before glucose testing. Analytical errors can be traced to instrument errors like use of GDH POQ enzyme glucometers and their interference with maltose as explained earlier which resulted in overestimation.[21] Post-analytical errors involve erroneous use of the data provided in terms of therapy.
Another important concept is use of alternative sites like palms, forearms or even arms for SMBG. A comparative study by Fedele et al., concluded that there was a higher patient preference and satisfaction for alternate site testing. However, in the hypoglycemia range it is advisable to use the conventional fingertip site.[23]
In lieu of these errors, accuracy is always a concern when using glucometers. The ISO: 15197 standards discuss quality standards in blood glucose monitoring devices and the accuracy requirements for glucometers.[24] This standard states that 95% of comparative results between a laboratory reference and the glucometer must fall within a bias of 15% for results greater than 100 mg%. Total of 99% of the results should fall in Zone A of the Parkes Error grid. The rationale is that this permissible error would not cause significant deviations during therapeutic decision making.[25] [Figure 1].
Figure 1.
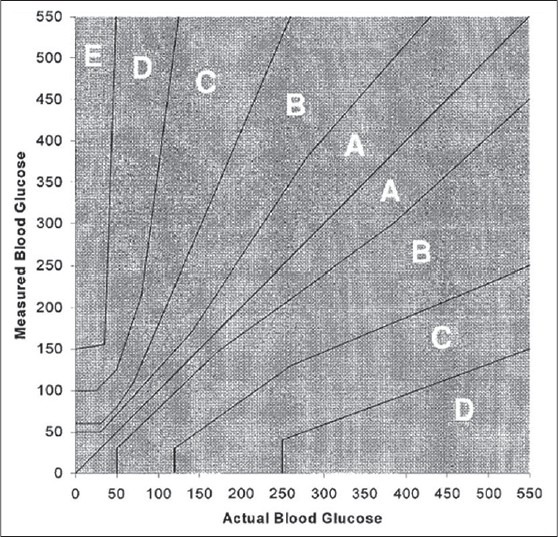
Parkes error grid
Evidence
There is no disagreement on the utility of SMBG in type-1 diabetes and type- 2 diabetes on insulin therapy.[26,27] However, there is conflicting evidence on the clinical benefits of SMBG for the type-2 patients who are not on insulin therapy. Positive effects include decreased hospital admissions and morbidity,[28] whereas negative results include no improvement in glycemic control especially that the control is not well sustained.[29] A nocebo effect is also described by few studies which show that SMBG in non-insulin treated diabetes patients result in increased anxiety and depression.[30]
Cochrane analysis of the utility of SMBG in non-insulin treated diabetes population concluded that when diabetes duration is over one year, the overall effect of SMBG on glycemic control in this group is limited only up to six months after initiation and subsides after 12 months. There is no evidence that SMBG affects patient satisfaction and general health-related quality of life. More research is required to explore the psychological impact as well as the impact of on hypoglycemia and diabetic complications.[31] A review by Kolb et al. published in 2010 also shows that the evidence base for better outcome with use of SMBG is insufficient with regard to results from RCTs, for all three diabetes types: Type 1, Type 2 and gestational diabetes.[32] The consensus for this group still remains elusive.
RECOMMENDATIONS
ADA 2013
Patients on multiple-dose insulin or insulin pump therapy should do SMBG especially prior to critical tasks like driving and exercise as well as post-meals and suspected hypoglycemia.[10]
In non-insulin treated type-2 diabetes patients also SMBG is useful but is not clearly defined by ADA 2013.
Limitations of SMBG
Calibration and accuracy of glucometers is a very important limitation of SMBG and has to be standardized as described earlier. SMBG cannot predict the future trends of blood glucose and its efficacy is dependent on adherence and compliance. Repeated lancet injuries are a major cause of poor compliance and non-adherence. Concerns of contamination and possible spread of blood borne pathogens like hepatitis are also being studied.[33] Center for disease control (CDC) has also issued timely statements recognizing the importance of universal precautions in SMBG, especially the assisted blood glucose monitoring.[34]
Continuous glucose monitoring systems (CGMS)
Through the understanding of the limitations of SMBG and the concept of glycemic variability emerged the technology of continuous glucose monitoring systems. The first CGMS device was approved by FDA in June 1999 and manufactured by Medtronic Minimed.[35]
Technology
The principle of CGMS is based on the continuous measurement of interstitial glucose levels. Hence it has the ability to provide information about the direction, magnitude, duration, frequency of fluctuations in blood glucose levels. It is an effective tool to measure the glycemic variability and glycemic excursions. There are two main types of CGMS devices: Retrospective also called as ‘Professional CGMS’ and ‘Real time or Personal CGMS’. The Retrospective CGMS gives a retrospective data of 3-5 days depending on the duration of use. It records readings every 5 minutes giving about 288 readings every day. The recorded data is downloaded in the physician's office and hence this type of CGMS does not give us real-time values and cannot be linked with an insulin pump. On the contrary, Real time CGMS gives continuous real time results and has built-in alarm system which provides warnings in rapid fluctuations of blood glucose. The monitor shows trends and predicts future glucose readings. The real time readings help in immediate feed-back and appropriate therapeutic action and can be linked to an insulin pump.
Every CGMS device has a sensor which measures interstitial fluid glucose levels and is inserted using an inserter. The real-time CGMS has a monitor which displays the glucose readings and predicts future trends. In case of retrospective CGMS other components include a docking site which helps download the data from the sensor.
Currently approved CGM devices utilize glucose oxidase based electrochemical subcutaneous sensors. Electric current generated by the sensor as the glucose is oxidized is transmitted to the receiver or monitor.[36]
Evidence
In multiple clinical trials, adults as well as children with T1DM have shown improved glycemic control in the form of lowering of HBA1c using CGMS compared with routine SMBG.[37,38] The STAR 3 study showed improved HBA1c without increased rates of severe hypoglycemia in both adults and children with CGMS.[39]
Murphy et al., showed that CGMS with routine ante-natal care resulted in better glycemic control and reduced risk of macrosomia.[40] Further studies are required for validation of CGMS in pregnancy.
Poolsup et al., in 2013 in a meta-analysis showed that in type-1 pediatric diabetic patients CGMS overall was not more effective than SMBG. However real- time CGMS performed better as compared to retrospective CGMS and SMBG. In type-2 diabetics, significant reduction in HBA1c was observed in patients with CGMS as compared to SMBG.[41]
Limitations
CGMS, though a promising technology, it is still not an ideal glucose monitoring system. Interference of certain substances like glutathione, ascorbic acid and salicylates results in inaccurate readings. Limitation of using interstitial fluid glucose is the lag time between serum glucose levels to interstitial glucose which is up to 15 minutes especially during rapid glucose fluctuations.[42]
Current sensors are generally less accurate in the first 24 hours due to local tissue inflammation following tissue trauma at the time of insertion. The highest accuracy is by the second day. To improve accuracy both the types of CGMS devices require calibration by SMBG at least 4 times every day resulting in reduced compliance and added inaccuracies due to dependency on glucometers.[42]
Importance of adherence is shown by the ONSET study where the improved glycemic control was lost in children with non-adherence to CGMS devices. Reasons for non-adherence include pain and discomfort, device inaccuracy and issues with insurance approval.[43]
RECOMMENDATIONS
ADA 2013
Continuous glucose monitoring (CGM) in conjunction with intensive insulin regimens can be a useful tool to lower a1c in selected adults (<25 years) with type 1 diabetes. (Level A Evidence).[10]
Glucose monitoring in gestational diabetes mellitus
Controversies exist about the intensity of glucose monitoring in gestational diabetes as well as about the monitoring systems to be used.[44] SMBG through many studied has shown benefit but uncertainty exists about the optimal frequency and timing of self-monitoring.[45] The utility of HBA1c is presently limited to periconceptual period.[46] In some studies, however, weekly HBA1c has shown to be beneficial. Through few studies, CGMs has shown to be beneficial in insulin-treated gestational diabetes, especially for those whose blood sugars are difficult to control or may have nocturnal hypoglycemia, but still this technology needs additional evaluation with larger randomized controlled trials.[45]
Future of glucose monitoring
Non-invasive glucose monitoring forms the future of glucose monitoring systems. Raman spectroscopy, optical coherence tomography, photo-acoustic spectroscopy and fluorescence show the greatest promise in achieving the goal of an ideal glucose sensor.[47] However, at present none of these devices meet the criteria for the ideal sensor and an ideal/accurate biosensor of a closed-loop system remains elusive.
Concept of artificial pancreas: Closing the loop?
CGMS connected to continuous insulin infusion systems in a closed loop forms the basic structure of an artificial pancreas.[48] Thus CGMS forms the key link towards realization of the unrealistic dream of artificial pancreas.
CONCLUSIONS
To summarise, the options of glucose monitoring are varied and each option has its own merits and flaws. We have to bear in mind that at the end of the day the goal of adequate glycemic control has to be achieved in every diabetic patient with minimum hypoglycemia and with utilization of available resources of monitoring.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
References
- 1.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–86. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 2.Intensive blood glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in type 2 diabetes (UKPDS33) United Kingdom Prospective Diabetes study (UKPDS) group. Lancet. 1998;352:837–53. [PubMed] [Google Scholar]
- 3.Monnier L, Mas E, Ginet C, Michel F, Villon L, Cristol JP, et al. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–7. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 4.Rahbar S. An abnormal haemoglobin in red cells of diabetics. Clin Chem Acta. 1968;22:296–8. doi: 10.1016/0009-8981(68)90372-0. [DOI] [PubMed] [Google Scholar]
- 5.Kowalski AJ, Dutta S. It's time to move from the A1c to better metrics for diabetes control. Diabetes Technol Ther. 2013;15:194–6. doi: 10.1089/dia.2013.0060. [DOI] [PubMed] [Google Scholar]
- 6.Monnier L, Lapinski H, Colette C. Contributions of fasting and post-prandial plasma glucose increments to the overall diurnal hyperglycemia of type 2 diabetic patients. Diabetes Care. 2003;26:881–5. doi: 10.2337/diacare.26.3.881. [DOI] [PubMed] [Google Scholar]
- 7.Nathan DM, Kuenen J, Borg R, Zheng H, Schoenfeld D, Heine RJ, A1c-Derived Average Glucose Study Group. Translating the A1C assay into estimated average glucose values. Diabetes Care. 2008;31:1473–8. doi: 10.2337/dc08-0545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.The International Expert Committee. International Expert Committee report on the role of the A1C assay in the diagnosis of diabetes. Diabetes Care. 2009;32:1327–34. doi: 10.2337/dc09-9033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little RR. Glycated hemoglobin standardization--National Glycohemoglobin Standardization Program (NGSP) perspective. Clin Chem Lab Med. 2003;41:1191–8. doi: 10.1515/CCLM.2003.183. [DOI] [PubMed] [Google Scholar]
- 10.American Diabetes Association. Standards of Medical Care in Diabetes--2013 Diabetes Care. 2013;36 Suppl 1:11–S66. [Google Scholar]
- 11.Kumar PR, Bhansali A, Ravikiran M, Bhansali S, Dutta P, Thakur JS, et al. Utility of glycated haemoglobin in diagnosing type 2 diabetes mellitus: A community-based study. J Clin Endocrinol Metab. 2010;95:2832–5. doi: 10.1210/jc.2009-2433. [DOI] [PubMed] [Google Scholar]
- 12.Mohan V, Vijayachandrika V, Gokulakrishnan K, Anjana RM, Ganesan A, Weber MB, et al. A1C cut points to define various glucose intolerance groups in Asian Indians. Diabetes Care. 2010;33:515–9. doi: 10.2337/dc09-1694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Saudek CD, Brick JC. The clinical use of hemoglobin A1c. J Diabetes Sci Technol. 2009;3:629–34. doi: 10.1177/193229680900300402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bode BW, Irvin BR, Pierce JA, Allen M, Clark AL. Advances in hemoglobin A1c point of care technology. J Diabetes Sci Technol. 2007;1:405–11. doi: 10.1177/193229680700100314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Al-Ansary L, Farmer A, Hirst J, Roberts N, Glasziou P, Perera R, et al. Point-of-care testing for Hb A1c in the management of diabetes: A systematic review and metaanalysis. Clin Chem. 2011;57:568–76. doi: 10.1373/clinchem.2010.157586. [DOI] [PubMed] [Google Scholar]
- 16.Handelsman Y, Mechanick JI, Blonde L, Grunberger G, Bloomgarden ZT, Bray GA, et al. AACE Task Force for Developing a Diabetes Comprehensive Care Plan. American Association of Clinical Endocrinologists Medical Guidelines for clinical practice for developing a diabetes mellitus comprehensive care plan: Executive summary. Endocr Pract. 2011;17:287–302. doi: 10.4158/ep.17.2.287. [DOI] [PubMed] [Google Scholar]
- 17.Lindsey CC, Carter AW, Mangum S, Greene D, Richardson A, Brown SJ, et al. A prospective, randomized, multicentered controlled trial to compare the annual glycemic and quality outcomes of patients with diabetes mellitus monitored with weekly fructosamine testing versus usual care. Diabetes Technol Ther. 2004;6:370–7. doi: 10.1089/152091504774198070. [DOI] [PubMed] [Google Scholar]
- 18.Dungan KM. 1,5-anhydroglucitol (GlycoMark) as a marker of short-term glycemic control and glycemic excursions. Expert Rev Mol Diagn. 2008;8:9–19. doi: 10.1586/14737159.8.1.9. [DOI] [PubMed] [Google Scholar]
- 19.Clarke SF, Foster JR. A history of blood glucose meters and their role in self-monitoring of diabetes mellitus. Br J Biomed Sci. 2012;69:83–93. [PubMed] [Google Scholar]
- 20.Rebel A, Rice MA, Fahy BG. Accuracy of point-of-care glucose measurements. Diabetes Sci Technol. 2012;6:396–411. doi: 10.1177/193229681200600228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.U S. Food and Drug administration: FDA public health notification: Potentiallty fatal errors with GDH-POQ glucose monitoring technology August, 9 S Food and Drug administration: FDA public health notification: Potentiallty fatal errors with GDH-POQ glucose monitoring technology August 13, 2009. [Google Scholar]
- 22.Schnell O, Alawi H, Battelino T, Ceriello A, Diem P, Felton A, et al. Addressing schemes of self-monitoring of blood glucose in type 2 diabetes: A European perspective and expert recommendation. Diabetes Technol Ther. 2011;13:959–65. doi: 10.1089/dia.2011.0028. [DOI] [PubMed] [Google Scholar]
- 23.Fedele D, Corsi A, Noacco C, Prisco F, Squatrito S, Torre E, et al. Alternative site blood glucose testing: A multicenter study. Diabetes Technol Ther. 2003;5:983–9. doi: 10.1089/152091503322641033. [DOI] [PubMed] [Google Scholar]
- 24.Freckmann G, Schmid C, Baumstark A, Pleus S, Link M, Haug C. System accuracy evaluation of 43 blood glucose monitoring systems for self-monitoring of blood glucose according to DIN EN ISO 15197. J Diabetes Sci Technol. 2012;6:1060–75. doi: 10.1177/193229681200600510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Parkes JL, Slatin SL, Pardo S, Ginsberg BH. A new consensus error grid to evaluate the clinical significance of inaccuracies in the measurement of blood glucose. Diabetes Care. 2000;23:1143–8. doi: 10.2337/diacare.23.8.1143. [DOI] [PubMed] [Google Scholar]
- 26.Strowig SM, Raskin P. Improved glycemic control in intensively treated type 1 diabetic patients using blood glucose meters with storage capability and computer-assisted data analyses. Diabetes Care. 1998;21:1694–8. doi: 10.2337/diacare.21.10.1694. [DOI] [PubMed] [Google Scholar]
- 27.National Institute for Clinical Excellence. Type 1 diabetes: Diagnosis and management of type 1 diabetes in adults. 2004 [Google Scholar]
- 28.Burge MR. Lack of compliance with home blood glucose monitoring predicts hospitalization in diabetes. Diabetes Care. 2001;24:1502–3. doi: 10.2337/diacare.24.8.1502. [DOI] [PubMed] [Google Scholar]
- 29.Farmer AJ, Wade AN, French DP, Simon J, Yudkin P, Gray A, et al. , DiGEM Trial Group.Blood glucose self monitoring in type 2 diabetes: A randomised controlled trial. Health Technol Assess. 2009;13:iii. doi: 10.3310/hta13150. [DOI] [PubMed] [Google Scholar]
- 30.Fisher L, Polonsky W, Parkin CG, Jelsovsky Z, Amstutz L, Wagner RS. The impact of blood glucose monitoring on depression and distress in insulin-naïve patients with type 2 diabetes. Curr Med Res Opin. 2011;27 Suppl 3:39–46. doi: 10.1185/03007995.2011.619176. [DOI] [PubMed] [Google Scholar]
- 31.Malanda UL, Welschen LM, Riphagen II, Dekker JM, Nijpels G, Bot SD. Self-monitoring of blood glucose in patients with type 2 diabetes mellitus who are not using insulin. Cochrane Database Syst Rev. 2012;1:CD005060. doi: 10.1002/14651858.CD005060.pub3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kolb H, Kempf K, Martin S, Stumvoll M, Landgraf R. On what evidence-base do we recommend self-monitoring of blood glucose? Diabetes Res Clin Pract. 2010;87:150–6. doi: 10.1016/j.diabres.2009.10.014. [DOI] [PubMed] [Google Scholar]
- 33.Louie RF, Lau MJ, Lee JH, Tang ZM, Kost GJ. Multicenter study of the prevalence of blood contamination on point-of-care glucose meters and recommendations for controlling contamination. Point Care J Near Patient Test Technol. 2005;4:158–63. [Google Scholar]
- 34.Centers for Disease Control and Prevention. Notes from the Field: Deaths from Acute Hepatitis B Virus Infection Associated with Assisted Blood Glucose Monitoring in an Assisted-Living Facility - North Carolina, August-October 2010. MMWR. 2011;60:182. [PubMed] [Google Scholar]
- 35.Kim HS, Shin JA, Chang JS, Cho JH, Son HY, Yoon KH. Continuous glucose monitoring: Current clinical use. Diabetes Metab Res Rev. 2012;28 Suppl 2:73–8. doi: 10.1002/dmrr.2346. [DOI] [PubMed] [Google Scholar]
- 36.DeSalvo D, Buckingham B. Continuous glucose monitoring: Current use and future directions. Curr Diab Rep. 2013;13:657–62. doi: 10.1007/s11892-013-0398-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group, Tamborlane WV, Beck RW, Bode BW, Buckingham B, Chase HP, Clemons R, et al. Continuous glucose monitoring and type 1 Diabetes. N Engl J Med. 2008;359:1464–76. doi: 10.1056/NEJMoa0805017. [DOI] [PubMed] [Google Scholar]
- 38.Deiss D, Bolinder J, Riveline JP, Battelino T, Bosi E, Tubiana-Rufi N, et al. Improved glycemic control in poorly controlled patients with type 1 diabetes using real-time continuous glucose monitoring. Diabetes Care. 2006;29:2730–2. doi: 10.2337/dc06-1134. [DOI] [PubMed] [Google Scholar]
- 39.Bergenstal RM, Tamborlane WV, Ahmann A, Buse JB, Dailey G, Davis SN, et al. STAR 3 Study Group.Effectiveness of sensor-augmented insulin-pump therapy in type 1 diabetes. N Engl J Med. 2010;363:311–20. doi: 10.1056/NEJMoa1002853. [DOI] [PubMed] [Google Scholar]
- 40.Murphy HR, Rayman G, Lewis K, Kelly S, Johal B, Duffield K, et al. Effectiveness of continuous glucose monitoring in pregnant women with diabetes: Randomised clinical trial. BMJ. 2008;337:A1680. doi: 10.1136/bmj.a1680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Poolsup N, Suksomboon N, Kyaw AM. Systematic review and meta-analysis of the effectiveness of continuous glucose monitoring (CGM) on glucose control in diabetes. Diabetol Metab Syndr. 2013;5:39. doi: 10.1186/1758-5996-5-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rebrin K, Sheppard NF Jr, Steil GM. Use of subcutaneous interstitial fluid glucose to estimate blood glucose: Revisiting delay and sensor offset. J Diabetes Sci Technol. 2010;4:1087–98. doi: 10.1177/193229681000400507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kordonouri O, Pankowska E, Kami B, Kapellen T, Coutant R, Hartmann R, et al. Sensor-augmented pump therapy from the diagnosis of childhood type 1 diabetes: Results of the Paediatric Onset Study (ONSET) after 12 months of treatment. Diabetologia. 2010;53:2487–95. doi: 10.1007/s00125-010-1878-6. [DOI] [PubMed] [Google Scholar]
- 44.Buchanan TA, Kjos SL. Counterpoint: Glucose monitoring in gestational diabetes: Lots of heat, not much light. Diabetes Care. 2003;26:948–9. doi: 10.2337/diacare.26.3.948. [DOI] [PubMed] [Google Scholar]
- 45.Hawkins JS. Glucose monitoring during pregnancy. Curr Diab Rep. 2010;10:229–34. doi: 10.1007/s11892-010-0111-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Jovanovic L, Savas H, Mehta M, Trujillo A, Pettitt DJ. Frequent monitoring of A1C during pregnancy as a treatment tool to guide therapy. Diabetes Care. 2011;34:53–4. doi: 10.2337/dc10-1455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Oliver NS, Toumazou C, Cass AE, Johnston DG. Glucose sensors: A review of current and emerging technology. Diabet Med. 2009;26:197–210. doi: 10.1111/j.1464-5491.2008.02642.x. [DOI] [PubMed] [Google Scholar]
- 48.Weinstock R. Closing the loop: Another step forward. Diabetes Care. 2011;34:2136–7. doi: 10.2337/dc11-1217. [DOI] [PMC free article] [PubMed] [Google Scholar]


