Abstract
Public awareness has long focused on the risks of the transmission of viral agents through blood product transfusion. This risk, however, pales in comparison to the less publicized danger associated with the transfusion of blood products contaminated with bacteria, in particular, platelet concentrates. Up to 1,000 cases of clinical sepsis after the transfusion of platelet concentrates are reported annually in the United States. The condition is characterized by acute reaction symptoms and the rapid onset of septicemia and carries a 20 to 40% mortality rate. The urgent need for a method for the routine screening of platelet concentrates to improve patient safety has long been recognized. We describe the development of a rapid and highly sensitive method for screening for bacteria in platelet concentrates for transfusion. No culture period is required; and the entire procedure, from the time of sampling to the time that the final result is obtained, takes less than 90 min. The method involves three basic stages: the selective removal of platelets by filtration following activation with a monoclonal antibody, DNA-specific fluorescent labeling of bacteria, and concentration of the bacteria on a membrane surface for enumeration by solid-phase cytometry. The method offers a universal means of detection of live, nondividing, or dead gram-negative and gram-positive bacteria in complex cellular blood products. The sensitivity is higher than those of the culture-based methods available at present, with a detection limit of 10 to 102 CFU/ml, depending upon the bacterial strain.
In the field of blood transfusion, blood products are now routinely screened by ultrasensitive techniques to minimize the risk of transmitting viruses to recipients. The risk of bacterial contamination remains, however, and is now the most common transfusion-related infectious adverse event (1). Preparations of platelet concentrates (PCs) are particularly at risk, as they must be stored at 20 to 24°C to maintain optimal viability and functional properties. The risk of bacterial contamination in PC transfusion, estimated to be 1 in 1,000 to 1 in 2,000, is 50 to 250 times higher than the combined risk of human immunodeficiency virus, hepatitis B and C virus, and human T-cell leukemia virus type 1 and 2 contamination (4-6, 18, 26). Unlike with the transmission of a viral component, bacterial infections are associated with acute reactions, rapid onset of sepsis, and high rates of mortality in the period immediately following transfusion (24). In many countries the only bacterial screening technique currently routinely used to avoid this is platelet concentrate swirling (29), the detection limit of which is 107 to 108 bacteria/ml, well above the threshold of clinical significance, which is taken to be approximately 105 CFU/ml (21). This value is, however, arbitrary, as it is influenced by several factors, including the bacterial strain, the patient's existing medical condition, promptness in recognition of a septic reaction, identification of the bacterial strain, and the remedial action taken (6, 11, 24). Another method for screening for bacteria in PCs is that of liquid culture, which, although very sensitive, requires prolonged culture periods (2 to 4 days).
With the recognition of the limitations of these screening methods and the increasing risk of clinically significant levels of bacteria with the time stored prior to transfusion, in vitro storage of platelets has been reduced from 7 days to 5 days and has even been reduced to 3 days in some countries (14).
We have developed a highly sensitive system to screen for bacterial contamination of PCs by solid-phase cytometry. The system has previously been described for the detection of fluorescence-labeled cells (20) and for the detection of bacteria in simple filterable solutions (17, 30) and acellular clinical samples (3), but the screening of cellular biological fluids was not previously feasible. The two main problems were the selective removal of cellular material, which would have interfered with the analysis and masked any bacteria present, and the development of a method capable of labeling bacteria in complex solutions.
In the method described here, to selectively remove platelets, platelet aggregation is induced by a monoclonal antibody (MAb) and the large aggregates are removed by filtration. The gram-positive and gram-negative bacteria present in the eluate are permeabilized, and the DNA is labeled with a DNA-specific fluorescent marker before the sample is filtered through a 0.4-μm-pore-size black membrane, which retains the bacteria on its surface. The membrane is then transferred to a solid-phase laser scanning cytometer (Scansystem; Hemosystem, Marseille, France). Based on the measurement of different parameters (fluorescence, size, and shape), fluorescence-labeled microorganisms can be detected with a high sensitivity and can be discriminated from cellular debris and background material. Finally, the use of an epifluorescence microscope linked to the cytometer allows confirmation of the result and preliminary identification of the class of bacteria.
MATERIALS AND METHODS
Bacterial propagation.
All bacterial strains used in this study originated from the bacterial collection of the Pasteur Institute, Paris, France: Bacillus cereus (ATCC 7064), Enterobacter aerogenes (ATCC 13048), Escherichia coli (ATCC 25922), Klebsiella pneumoniae (CIP 79.21), Serratia marcescens (ATCC 43862), Staphylococcus aureus (ATCC 6538), Staphylococcus epidermidis (ATCC 12228), Streptococcus agalactiae (ATCC 12403), and Streptococcus bovis type 1 (ATCC 49475). Following overnight culture (16 to 18 h) in Trypticase soy broth (TSB), the turbidity of the culture was adjusted to match a 0.5 McFarland standard and dilutions were prepared with phosphate-buffered saline (PBS; pH 7.4). In all experiments, the concentrations of bacteria spiked were confirmed by quantitative culture on Mueller-Hinton agar plates or esterase labeling (17).
Blood products.
Apheresis PCs, fresh frozen plasma (FFP), and pooled PCs (PPCs) were obtained from the French blood transfusion service, Etablissement Français du Sang. PCs were prepared according to established French regulatory guidelines (Journal Officiel Français, Vol. 123, p. 9109, 28 May 2003). All experiments were carried out with platelets between 2 and 4 days old.
Determination of the optimal test sample volume.
FFP was spiked with 2-μm-diameter fluorescent beads (Standard-G; Chemunex) at a final concentration of 1, 2, 5, or 10 beads/ml. The resulting samples were mixed for 30 min on a flatbed rocker; and 1, 2, 3, 4, or 5 ml was filtered through a 0.4-μm-pore-size black membrane (Whatman). The number of fluorescent beads per membrane was then determined on a Scansystem solid-phase cytometer.
Removal of platelets and bacterial recovery from PCs.
PBS and PCs were inoculated with 102 or 103 CFU of E. coli or S. epidermidis per ml. Three milliliters of the spiked sample was immediately mixed with 1 ml of purified MAb CD9 6B1 (INSERM U268, Villejuif, France) at 7.5 μg/ml. After incubation for 30 min at 22°C on a flatbed rocker, the samples were passed through a 5-μm-pore-size filter (Pall Inc.), which retained the platelet aggregates. Purified MAb CD9 SN4 (Ancell) was used as a reference standard for platelet aggregation. Prior to the addition of the CD9 MAb and after filtration, (i) the platelets were enumerated with a cell counter (ABX Micro-60) to calculate the percentage of aggregated platelets, and (ii) aliquots were plated on solid medium for quantitative culture. The bacterial colonies were counted at 24 and 48 h.
Platelet aggregation and labeling of bacteria with BLS1 and BLS2 solutions.
Three milliliters of PC was mixed with 1 ml of BLS1 platelet aggregation solution (polyethylenimine [PEI], 60 mg/liter; MAb CD9 6B1, 30 mg/liter; 1:2,000 dilution of Picogreen nucleic acid-binding dye [Molecular Probes]) in distilled H2O, and the mixture was incubated for 40 min at room temperature on a flatbed rocker. The platelet aggregates, visible as opaque flocculates, were removed by passing the sample through a 5-μm-pore-size filter (Pall Inc.). The resulting platelet-depleted sample was incubated for 20 min at room temperature with 7 ml of permeabilizing and labeling reagent BLS2 (EDTA, 1.86 g/liter; nisin, 8 mg/liter; N-octyl-β-d-glucopyranoside [NOG], 2.5 g/liter; chlorhexidine diacetate, 150 mg/liter) in distilled H2O. The sample was then filtered through a 0.4 μm-pore-size black membrane (Whatman), which retains the bacteria on its surface. The black membrane was transferred to a Scansystem solid-phase cytometer.
The performances of the BLS1 and BLS2 solutions were further assessed by comparison with the performance of the reference method with EDTA (27). E. coli and S. epidermidis cells were spiked into water, PBS, TSB, or FFP at concentrations of 104 and 103 CFU/ml, respectively. Three milliliters of each spiked sample was processed either with 8 ml of BLS1 or BLS2 solution, as described above, or with 7 ml of EDTA reagent (5 mM EDTA, Picogreen [1:2,000]) by the reference method (27). The number of labeled bacteria was determined with a Scansystem analyzer.
Resulting design of kit for routine screening of PCs.
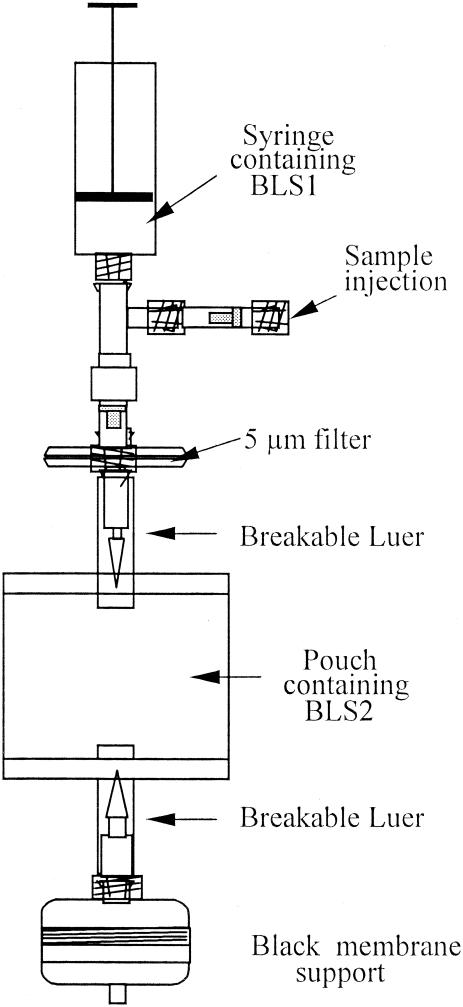
The method developed was adapted to a kit form (Scansystem Platelet kit; Hemosystem) for screening of PCs for bacteria prior to transfusion (Fig. 1). The kit is a closed device with the BLS1 solution (1 ml) in a 10-ml syringe and BLS2 solution (7 ml) in a 15-ml pouch, with the two solutions separated by the 5-μm-pore-size filter (Pall Inc.).
FIG. 1.
Diagram of the Scansystem Platelet kit (Hemosystem) design.
The 0.4-μm-pore-size black membrane is contained in a plastic membrane support (Millipore) to be connected to a vacuum pump for final filtration. Any bacteria present in the sample are then retained on the surface of the membrane, which is transferred for analysis to the Scansystem.
Scansystem solid-phase cytometry.
The solid-phase scanning cytometry method has already been described (20). Briefly, the laser scanning analyzer includes four modules: (i) a scan module, in which the black membrane is introduced and entirely scanned within 3 min; (ii) an argon laser module (488 nm excitation light), which is connected directly to the scan module; (iii) an epifluorescence microscope, which has an automated motor-driven stage; and (iv) a computer, whose proprietary software monitors all the other modules. After scanning of the membrane, all fluorescent signals detected are discriminated by the software on the basis of their sizes and fluorescence intensities. The result is then displayed on a computer screen as a map showing the number of discriminated spots and their positions on the scanned membrane. The membrane is finally transferred to the epifluorescence microscope for computer-assisted visual confirmation. In the experiments described here, 25 to 50 spots on the computer map were chosen and were confirmed to be microorganisms or particles. Spots were characterized by their relative fluorescence level (analog-to-digital convertor [ADC] count).
RESULTS
Determining the minimum test volume for reliable PC representation.
The minimum sample volume necessary to ensure the representation of very low levels of bacteria in PC units (1 to 10 CFU/ml) was determined by using fluorescence-labeled beads and the Scansystem method. FFP was spiked with increasing amounts of fluorescent beads and tested as described in Materials and Methods. When 3 ml of PCs with 2 beads/ml was used, 1 to 10 fluorescent spots were detected (n = 30). In all subsequent experiments, 3 ml was then taken as the optimal sample volume.
Removal of platelets and bacterial recovery.
Purified MAb CD9 6B1 was used at a final concentration of 7.5 μg/ml to induce platelet aggregation in PCs. The mean proportion of residual platelets in the filtrate was 5.5% (standard deviation, 3.4% [n = 36]). The maximal platelet aggregation was achieved by 15 min, with no loss of aggregate quality for up to 40 min. To determine the effects of platelet aggregates on the recovery of bacteria, PBS and PCs were inoculated with E. coli and S. epidermidis. Aggregation was performed as described above, and the bacteria were enumerated (as numbers of CFU per milliliter) on culture plates before and after sample filtration (n = 3). The number of bacteria recovered from spiked PCs was equivalent to and lower than the number recovered from spiked PBS for high (103 CFU/ml) and low (102 CFU/ml) concentrations of bacteria, respectively (Table 1). Furthermore, subsequent experiments showed no impact of bacterial forms (individuals, pairs, or clusters) on recovery rates (data not shown).
TABLE 1.
Effects of platelet aggregation and sample filtration on bacterial recoverya
| Sample conditions | Mean (SD) no. of CFU of the following bacteria spiked at the indicated concn (CFU/ml):
|
|||
|---|---|---|---|---|
|
E. coli
|
S. epidermidis
|
|||
| 102 | 103 | 102 | 103 | |
| PBS | ||||
| Prefiltration | 59 (2) | 630 (104) | 68 (24) | 780 (137) |
| Postfiltration | 42 (0) | 510 (30) | 61 (30) | 390 (216) |
| % Recovery | 71.2 | 81.0 | 89.7 | 50.0 |
| PCs | ||||
| Prefiltration | 69 (15.9) | 680 (45.8) | 102 (26.2) | 960 (0) |
| Postfiltration | 35 (10.5) | 460 (75.5) | 43 (19.3) | 540 (150) |
| % Recovery | 50.7 | 67.6 | 42.1 | 56.3 |
E. coli and S. epidermidis were spiked in PBS and PC at 102 and 103 CFU/ml, respectively, in triplicate. Bacterial recovery was determined after platelet aggregation and filtration through a 5-μm-pore-size filter. Aliquots sampled prior to and after filtration were incubated on agar plates, and the numbers of CFU were counted 24 to 48 h later. The mean numbers of CFU counted before and after filtration were compared (standard deviations are given in parentheses), and the relative percentages of recovery are reported.
Bacterial DNA labeling solutions.
Picogreen was chosen from among several other nucleic acid-binding dyes for labeling and detection of the bacteria. When it was used at a dilution of 1:2,000, it gave good bacterial DNA labeling, with no interference with the black membrane or nonspecific labeling of platelets and a low background. In the adaptation of the method for kit form, it was therefore incorporated into the BLS1 solution (the platelet aggregation-stage solution).
A variety of reagents and chemicals that potentially influence permeabilization and labeling of the DNA of bacteria were also tested with the bacteria. PEI was found to augment efficient labeling of both gram-negative and gram-positive bacteria. Although gram-positive bacteria are very sensitive to lysis, they were found to tolerate PEI up to a final concentration of 15 mg/liter. This PEI concentration enhanced the labeling of gram-negative bacteria when the incubation period was beyond the 20 min required for the optimal efficiencies of the other reagents. By incorporating PEI into the aggregation solution and extending the incubation period to 40 min, good labeling of gram-negative bacteria was achieved without compromising the labeling of gram-positive bacteria (data not shown).
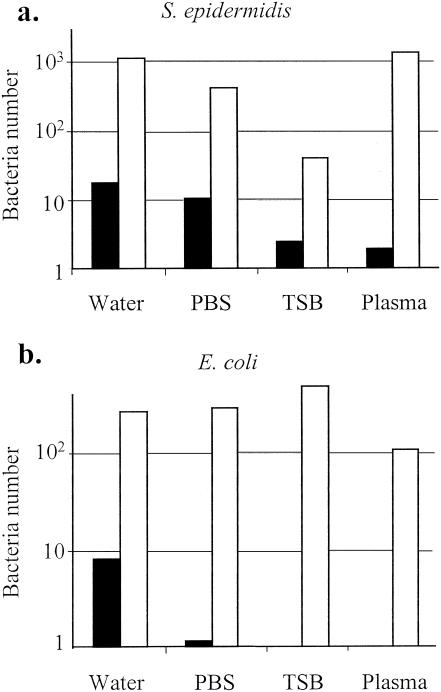
The BLS1 and BLS2 solutions used in the Scansystem method were compared to the standard EDTA permeabilizing solution (27) for the labeling of gram-positive and gram-negative bacteria present in different solutions (Fig. 2a and b, respectively). Detection of S. epidermidis was enhanced 65-, 42-, 16-, and 728-fold in water, PBS, TSB, and plasma, respectively, when the BLS1 and BLS2 solutions were used. The gram-negative bacterium E. coli was better detected after labeling with the BLS1 and BLS2 solutions than after preparation with the EDTA solution. The levels of detection were improved 35- and 274-fold for bacterial suspensions in water and PBS, respectively. TSB and plasma did not interfere with the labeling of E. coli by the BLS1 and BLS2 solutions, while detection of the bacteria was totally impaired when the EDTA solution was used.
FIG. 2.
Comparison of BLS1-BLS2 and EDTA reagents for bacteria permeabilization and labeling. S. epidermidis (a gram-positive organism) (a) and E. coli (a gram-negative organism) (b) were spiked at 103 and 104 CFU/ml, respectively, in water, PBS, TSB, or plasma. The samples were immediately processed with BLS1-BLS2 (□) or EDTA (▪) solution. Bacteria were detected and counted with the Scansystem analyzer. The numbers of bacteria obtained by both methods were compared.
Validation of the kit for sensitivity and linearity of detection of bacteria.
To validate the method with the Scansystem kit, a broad spectrum of bacteria associated with blood product contamination (23), gram-positive aerobic cocci (S. aureus, Streptococcus pyogenes, S. epidermidis, S. agalactiae), rods (B. cereus), and gram-negative aerobic rods (E. coli, S. marcescens, Pseudomonas aeruginosa, K. pneumoniae), were inoculated into PCs and tested by the Scansystem method. Bacteria were detected in all spiked platelet samples down to concentrations between 10 and 103 CFU/ml, depending on the strain (Table 2).
TABLE 2.
Sensitivity of Scansystem screening methoda
| Bacterium | No. of samples with the following spiking concn (CFU/ml) in which bacteria were detected:
|
||||
|---|---|---|---|---|---|
| Total | 10 | 102 | 103 | 104 | |
| Gram-positive bacteria | |||||
| S. epidermidis | 25 | 16 | 21 | 25 | 25 |
| S. aureus | 2 | 1 | 2 | 2 | 2 |
| S. pyogenes | 2 | 2 | 2 | 2 | 2 |
| S. agalactiae | 2 | 2 | 2 | 2 | 2 |
| B. cereus | 3 | 0 | 3 | 3 | 3 |
| Gram-negative bacteria | |||||
| E. coli | 25 | 20 | 23 | 25 | 25 |
| S. marcescens | 2 | 0 | 1 | 2 | 2 |
| K. pneumoniae | 2 | NDb | ND | 2 | 2 |
| P. aeruginosa | 2 | ND | ND | 2 | 2 |
| Total | 65 | 41 | 54 | 65 | 65 |
| % Positive | 67 | 89 | 100 | 100 | |
Nine different strains, including five gram-positive strains and four gram-negative strains, at each spiking concentration, were spiked in PCs at concentrations ranging from 10 to 104 CFU/ml. Three samples of 3 ml were processed with the kit and analyzed with the Scansystem solid-phase cytometer. The number of samples in which bacteria were detected is reported for each strain at each spiking concentration.
ND, not determined.
For all experiments, PC units were preliminarily checked for bacterial contamination (by the Scansystem method and on culture plates) and were shown to be negative (n = 20).
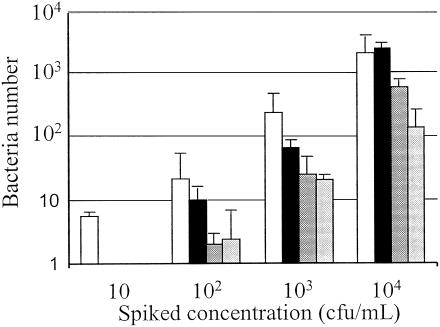
To determine the linearity of the number of bacteria detected by the bacterial screening system, PCs were inoculated with four different bacterial strains at concentrations ranging from 10 to 104 CFU/ml (Fig. 3). There was a close linear correlation with the bacterial spiking concentration and bacterial detection, with sensitivities down to 100 CFU/ml for E. coli, B. cereus, and S. marcescens and 10 CFU/ml for S. epidermidis.
FIG. 3.
Linearity of Scansystem method. Increasing concentrations ranging from 10 to 104 CFU of S. epidermidis (white bars; n = 5), B. cereus (black bars; n = 3), S. marcescens (heavily shaded bars; n = 2), and E. coli (lightly shaded bars; n = 3) per ml were spiked into PCs and immediately analyzed by the Scansystem method. The mean numbers of bacteria detected ± standard deviations are reported.
DISCUSSION
Different approaches have been taken in an attempt to reduce the risk of transfusion-associated bacterial sepsis, including stricter donor selection criteria, improved puncture site disinfection (13), use of apheresis PCs from a single donor instead of pooled random PCs prepared from several donors (22), reduction of the platelet shelf life from 7 to 5 days (8), in vitro treatment with bactericidal products (12, 19), and the use of bacterial screening systems. The screening methods used by different centers are visual inspection, microscopic examination of stained smears, pH and glucose dipstick tests (29), and tests with automated culture systems (7, 28). No screening method, however, satisfies the main requirements of high sensitivity, high specificity, and rapidity.
The most common pathogens isolated from PCs are skin flora, including S. epidermidis and Propionibacterium acnes (5, 26). The majority of cases of bacterial contamination of PCs will therefore have occurred at the time of donation due to inadequate skin disinfection. The initial levels of bacteria in the PC unit are usually exceedingly low, below the detection limits of current screening systems and below the level considered clinically significant (105 CFU/ml) (21). With longer times between sampling and testing, there is a higher probability that all contaminated units will be identified. The short shelf life of platelets, however, means that a delay of testing for 3 to 4 days is not an option. Another factor that influences the probability that units infected with bacteria will be detected is the lag phase before the bacteria start to actively proliferate. Some bacteria remain in a nondividing state for up to 6 days (15).
Liquid culture systems are suitable for most cellular samples, such as whole blood and blood products. They are automatable and have a high level of sensitivity (2), with the presence of a single microorganism theoretically being sufficient to obtain a positive result. Long incubation periods, however, are necessary, in particular for PCs with low bacterial concentrations and slowly growing strains. The detection of P. acnes by the automated blood culture method requires more than 4 days (7, 9), and the detection of Mycobacterium is reported to require 20 days (25).
Solid-phase scanning cytometry has long been recognized as a highly sensitive bacterial screening system, as very low levels of bacteria can be concentrated from a large volume by filtration. This, however, limits the method to use with simple filterable fluids. PCs contain, on average, 5 × 109 platelets/ml, with the platelets having an average size of 2 to 3 μm. The similarity of the sizes of platelets and bacteria exclude the possibility of preremoval of bacteria by size-selective filtration, and the sheer mass of platelets would overwhelm the membrane retaining the bacteria. To overcome this problem, aggregation of the platelets was induced by using an MAb to the glycoprotein CD9, which is expressed at high levels on the surfaces of platelets. Although its physiological role is not yet known, binding of MAbs to different CD9 antigen epitopes has been shown to induce platelet aggregation through at least one platelet activation pathway (10, 31). The aggregates range in size from 20 to greater than 200 μm, which enables their selective removal by filtration. Filtration of the aggregated platelet samples was shown to influence bacterial retention in the presence of a low concentration (102 CFU/ml) of bacteria. However, the bacterial recovery rate remains acceptable for the detection of bacteria in PCs by the Scansystem method.
Although the permeabilizing method with EDTA solution has long been accepted as adequate for detection of bacterial contamination in simple solutions (27), it is less suitable when more complex solutions, in particular, biological fluids, are tested. To develop a permeabilizing solution more suitable for use in the screening of blood products, a variety of different reagents reported to have effects on membrane permeability and stability were tested. These include enzymes (lysozyme, nisin), chelating agents (EDTA, EGTA), fixatives (formaldehyde, glutaraldehyde, ethanol), permeabilizing components (PEI, digitonin, chlorhexidine diacetate, monesin, NOG, sodium hexametaphosphate, benzalkonium chloride, cetrimide), antibiotics (polymyxin B, rifampin), salts (NaCl, KCl), and other agents (potassium citrate, streptolysin O, urea, sucrose) (16, 27). The reagent composition was chosen by focusing on achieving a universal method for the labeling of bacteria that was sensitive for the detection of a broad spectrum of bacterial strains and that was not inhibited by biologically complex fluids.
As described in the literature (16), PEI was found to be essential for permeabilization of gram-negative strains. At the concentrations optimal for labeling of gram-negative bacteria, all gram-positive bacteria were lost due to lysis. This problem was overcome by reducing the PEI concentration and incorporating it into an extended platelet aggregation stage solution in order to increase the incubation time.
Since PCs do not contain any nucleated cells, other than a low number of residual white blood cells, we chose to use a nucleic acid-binding dye to label the bacteria. Picogreen (Molecular Probes) is a DNA-specific dye commonly used for cellular labeling. Picogreen was compared to several other fluorescent dyes, SYTO13, YOPRO1, and SYBR Green I (Molecular Probes), and was selected for the establishment of the Scansystem screening method. SYBR Green I (dilution, 1:10,000) allowed efficient labeling of the bacteria, with a mean peak intensity of 1,400 ADC counts, whereas Picogreen (diluted 1:2,000) provided a mean peak intensity of 600 ADC counts; however, SYBR Green I induced too many nonspecific fluorescent particles on the membrane surface to be considered further. As Picogreen was found to be unstable when it was stored for long periods in combination with the other reagents in the BLS2 solution (lysis solution), it was incorporated into the BLS1 solution (aggregation solution), in which its stability is maintained at 4°C for long periods (>9 months).
Our method allows labeling and detection of bacteria in complex biological fluids with a high degree of sensitivity. The detection limit of the Scansystem is 10 to 102 CFU/ml for gram-negative and gram-positive bacteria, with a linearity of detection in the range of 10 to 105 CFU/ml. When spiked PCs were stored under usual conditions for several hours (up to 72 h) prior to analysis, the sensitivities of bacterial detection were similar, with 100% of samples containing 103 CFU/ml being positive (P. Morel, M. Dechaseaux, X. Bertrand, C. Naegelen, and D. Talon, Abstr. Ann. Transfusion, vol. 43, abstr. SP13, 2003). When PCs without bacteria (n = 20) were tested by the Scansystem method, they were always confirmed to be negative by the culture plate method. PCs found to be positive by testing by the Scansystem method were always confirmed to be positive as well, emphasizing the high degree of specificity of the method. The method does not depend on bacterial growth and does not require any culture phase. The entire analysis, from the time of sampling to the time that the final result is obtained, actually requires less than 90 min, while culture methods require incubation periods of 24 to 48 h and give results representative of the levels of bacteria in the samples 1 or 2 days earlier. Furthermore, our screening method allows the detection of not only living bacteria but also moribund or nondividing microorganisms, preventing the recipient from being potentially exposed to the effects of bacterial endotoxins.
As the cellular structures of the microorganisms are conserved through the process, microscopic confirmation and preliminary bacterial class identification (rods or cocci) are also possible. At this development stage, microscopic confirmation is necessary to validate the presence of bacteria detected by the Scansystem analyzer software. Software discrimination parameters will be set up precisely after positive PC units are tested in routine studies. This will allow the use of the microscope as an option.
In conclusion, Scansystem is a rapid, specific, and sensitive bacterial screening system that can be used to ensure the increased safety of PCs destined for transfusion. It will certainly permit the possible extension of the shelf life of PCs stored in vitro to their full functional potential of 7 days (8), alleviating shortage problems and reducing wastage. Progress is now being made to extend this screening system to other blood products and for use of this system in clinical situations to test the blood of patients with suspected septicemia. Early diagnosis would improve patient prognosis and reduce the risk of the more serious and debilitating outcomes of this disease.
Acknowledgments
This work was supported by Hemosystem.
We thank N. Goncalves for critical reading of the manuscript and useful comments.
REFERENCES
- 1.Andreu, G., P. Morel, F. Forestier, J. Debeir, D. Rebibo, G. Janvier, and P. Herve. 2002. Hemovigilance network in France: organization and analysis of immediate transfusion incident reports from 1994 to 1998. Transfusion 42:1356-1364. [DOI] [PubMed] [Google Scholar]
- 2.Aubert, G., A. C. Vautin, V. P. Michel, and G. Dorche. 1993. Evaluation of three automated blood culture systems. Bio Argod, Bact T/Alert, Bactec NR-860. Pathol. Biol. 41:434-440. [PubMed] [Google Scholar]
- 3.Bauters, T. G. M., D. Swinne, V. Stove, and H. J. Nelis. 2003. Detection of single cells of Cryptococcus neoformans in clinical samples by solid-phase cytometry. J. Clin. Microbiol. 41:1736-1737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Blajchman, M. A. 2002. Incidence and significance of the bacterial contamination of blood components. Dev. Biol. 108:59-67. [PubMed] [Google Scholar]
- 5.Blajchman, M. A., and M. Goldman. 1991. Blood product-associated bacterial sepsis. Transfus. Med. Rev. 5:73-83. [DOI] [PubMed] [Google Scholar]
- 6.Blajchman, M. A., and M. Goldman. 2001. Bacterial contamination of platelet concentrates: incidence, significance, and prevention. Semin. Hematol. 38:4. [DOI] [PubMed] [Google Scholar]
- 7.Brecher, M. E., D. J. Heath, S. N. Hay, S. J. Rothenberg, and L. C. Stutzman. 2002. Evaluation of a new generation of culture bottle using an automated bacterial culture system for detecting nine common contaminating organisms found in platelet components. Transfusion 42:774-779. [DOI] [PubMed] [Google Scholar]
- 8.Brecher, M. E., P. V. Holland, A. A. Pineda, G. E. Tegtmeier, and R. Yomtovian. 2000. Growth of bacteria in inoculated platelets: implications for bacteria detection and the extension of platelet storage. Transfusion 40:1308-1312. [DOI] [PubMed] [Google Scholar]
- 9.Brecher, M. E., N. Means, C. S. Jere, D. Heath, S. Rothenberg, and L. C. Stutzman. 2001. Evaluation of an automated culture system for detecting bacterial contamination of platelets: an analysis with 15 contaminating organisms. Transfusion 41:477-482. [DOI] [PubMed] [Google Scholar]
- 10.Carroll, R. C., R. E. Worthington, and C. Boucheix. 2002. Stimulus-response coupling in human platelets activated by monoclonal antibodies to the CD9 antigen, a 24 kDa surface-membrane glycoprotein. Biochem. J. 266:527-535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chiu, E. K., K. Y. Yuen, A. K. Lie, R. Liang, Y. L. Lau, A. C. Lee, Y. L. Kwong, S. Wong, M. H. Ng, and T. K. Chan. 1994. A prospective study of symptomatic bacteremia following platelet transfusion and of its management. Transfusion 34:950-954. [DOI] [PubMed] [Google Scholar]
- 12.Corbin, F. 2002. Pathogen inactivation of blood components: current status and introduction of an approach using riboflavin as a photosensitizer. Int. J. Hematol. 76:253-257. [DOI] [PubMed] [Google Scholar]
- 13.Dumont, L. J., and J. P. Aubuchon. 2002. Making risk statements stick. Vox Sang. 83:199-203. [DOI] [PubMed] [Google Scholar]
- 14.Dumont, L. J., J. P. Aubuchon, P. Whitley, L. H. Herschel, A. Johnson, D. McNeil, S. Sawyer, and J. C. Roger. 2002. Seven-day storage of single-donor platelets: recovery and survival in an autologous transfusion study. Transfusion 42:847-854. [DOI] [PubMed] [Google Scholar]
- 15.Heal, J. M., S. Singal, E. Sardisco, and T. Mayer. 1986. Bacterial proliferation in platelet concentrates. Transfusion 26:388-390. [DOI] [PubMed] [Google Scholar]
- 16.Helander, I. M., H. I. Alakomi, K. Latva-Kala, and P. Kosi. 1997. Polyethyleneimine is an effective permeabilizer of gram-negative bacteria. Microbiology 143:3193-3199. [DOI] [PubMed] [Google Scholar]
- 17.Jones, D. L., M. A. Brailsford, and J.-L. Drocourt. 1999. Solid-phase, laser-scanning cytometry: a new two-hour method for the enumeration of microorganisms in pharmaceutical water. Pharmacop. Forum 25:7626-7645. [Google Scholar]
- 18.Kuehnert, M. J., V. R. Roth, N. R. Haley, K. R. Gregory, K. V. Elder, G. B. Schreiber, M. J. Arduino, S. C. Holt, L. A. Carson, S. N. Banerjee, and W. R. Jarvis. 2002. Transfusion-transmitted bacterial infection in the United States, 1998 through 2000. Transfusion 41:1493-1499. [DOI] [PubMed] [Google Scholar]
- 19.Lin, L., H. Londe, J. M. Janda, C. V. Hanson, and L. Corash. 1994. Photochemical inactivation of pathogenic bacteria in human platelet concentrates. Blood 83:2698-2706. [PubMed] [Google Scholar]
- 20.Mignon-Godefroy, K., J.-G. Guillet, and C. Butor. 1997. Solid phase cytometry for detection of rare events. Cytometry 27:336-344. [PubMed] [Google Scholar]
- 21.Muder, R. R., Y. C. Yee, J. D. Rihs, and M. Bunker. 1992. Staphylococcus epidermidis bacteremia from transfusion of contaminated platelets: application of bacterial DNA analysis. Transfusion 32:771-774. [DOI] [PubMed] [Google Scholar]
- 22.Ness, P., H. Braine, K. King, C. Barrasso, T. Kickler, A. Fuller, and N. Blades. 2001. Single-donor platelets reduce the risk of septic platelet transfusion reactions. Transfusion 41:857-861. [DOI] [PubMed] [Google Scholar]
- 23.Perez, P., L. R. Salmi, G. Follea, J. L. Schmit, B. de Barbeyrac, P. Sudre, and R. Salamon. 2001. Determinants of transfusion-associated bacterial contamination: results of the French BACTHEM Case-Control Study. Transfusion 41:862-872. [DOI] [PubMed] [Google Scholar]
- 24.Roth, V. R., M. J. Kuehnert, N. R. Haley, K. R. Gregory, G. B. Schreiber, M. J. Arduino, S. C. Holt, L. A. Carson, K. V. Elder, and W. R. Jarvis. 2001. Evaluation of a reporting system for bacterial contamination of blood components in the United States. Transfusion 41:1486-1492. [DOI] [PubMed] [Google Scholar]
- 25.Saitoh, H., N. Yamane, C. Miyagi, and I. Nakasone. 2000. Comparative evaluation of two different formulae of Middlebrook 7H9 broth in a fully automated mycobacteria culture system, MB/BacT; the effect of Tween 80. Rinsho Biseibutshu Jinsoku Shindan Kenkyukai Shi 11:79-85. [PubMed] [Google Scholar]
- 26.Sazama, K. 1994. Bacteria in blood for transfusion. A review. Arch. Pathol. Lab. Med. 18:350-365. [PubMed] [Google Scholar]
- 27.Vaara, M. 1992. Agents that increase the permeability of the outer membrane. Microbiol. Rev. 56:395-411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Wagner, S. J., and D. Robinette. 1998. Evaluation of an automated microbiologic blood culture device for detection of bacteria in platelet components. Transfusion 38:674-679. [DOI] [PubMed] [Google Scholar]
- 29.Wagner, S. J., and D. Robinette. 2003. Evaluation of swirling, pH, and glucose tests for the detection of bacterial contamination in platelet concentrates. Transfusion 36:989-993. [DOI] [PubMed] [Google Scholar]
- 30.Wallner, G., D. Tillmann, and K. Haberer. 1999. Evaluation of the ChemScan system for rapid microbiological analysis of pharmaceutical water. PDA J. Pharm. Sci. Technol. 53:70-74. [PubMed] [Google Scholar]
- 31.Worthington, R. E., R. C. Carroll, and C. Boucheix. 1990. Platelet activation by CD9 monoclonal antibodies is mediated by FcgammaII receptor. Br. J. Haematol. 74:216-222. [DOI] [PubMed] [Google Scholar]